The Emergence of the Next-Generation Vaccines
Article Information
Léa Monet1, Nicolas Grandchamp2*
1Biology department, Ecole Polytechnique, Institut Polytechnique de Paris, France
2Biosource / GEG Tech, Paris, France
*Corresponding author: Nicolas Grandchamp, Biosource / GEG Tech, Paris, France
Received: 10 July 2020; Accepted: 17 July 2020; Published: 27 July 2020
Citation: Léa Monet, Nicolas Grandchamp. The Emergence of the Next-Generation Vaccines. Archives of Clinical and Biomedical Research 4 (2020): 325-348.
Share at FacebookKeywords
Next-Generation Vaccines
Article Details
Introduction
Vaccination is a medical technique that was first scientifically experimented by the physician Edward Jenner in the 18th century [1]. At that time, smallpox, a disease of viral origin, killed those infected and disfigured the survivors. Edward Jenner observed that farmers did not contract smallpox. However, they were often infected with vaccinia, a virus that induces a disease in cattle similar to smallpox but is beguiling to humans. E. Jenner hypothesizes that inoculating the contents of a vaccinia pustule taken from a female farmer would induce an immune response in humans that would protect them from subsequent infection by viruses similar to vaccinia such as the smallpox virus. He therefore carried out this experiment on a child and then voluntarily infected him with smallpox. As hoped, the child did not contract smallpox [2]. This discovery has been a breakthrough in science and became known as "vaccination".
Today, vaccination is a type of therapy that involves stimulating the immune system by injecting an antigen to produce a specific response [3]. The antigen injected is often based on viral or bacterial elements and should induce protective immunity and immune memory. Vaccination can either be preventive, in which case its aim is to prepare the individual's immune system for an encounter with a pathogen, or it can be therapeutic, in which case its aim is to redirect the patient's immune response to help defend against a pathogen already present in the body [4,5].
The injection of a preventive vaccine induces the activation of the innate and adaptive immune response [6]. During the adaptive immune response, a proliferation of T and B cells specific to the injected antigen takes place and leads to the formation of memory lymphocytes. The B cells and their differentiation into plasma cells enable the production of antigen-specific antibodies. When the vaccine injection is successful, subsequent exposure to the pathogen induces a strong immune response that either eliminates the pathogen or deactivates the toxin responsible for the disease, including a large and rapid production of antibodies due to the presence of memory lymphocytes [4]. Today, there are a variety of vaccine types. One type of vaccine is based on live attenuated microorganisms. It is based on the use of microorganisms that have lost their pathogenicity. This type of vaccination makes it possible to expose several specific epitopes of the pathogen to the immune system and reproduces the characteristics of an infection like the active pathogen, without developing the disease. These vaccines often require only one immunization because they induce a very strong immune response and the production of memory lymphocytes [7,8]. Moreover, one of its limitations is the possibility of reversion to a form that is dangerous to the body through a mechanism called reversion [9]. This type of vaccine is, however, gradually being replaced by vaccines based on killed or inactivated microorganisms. The purpose of inactivating the pathogen using heat or chemicals is to induce in the virus the inability to replicate within its host. Vaccines based on killed or inactivated microorganisms are frequently used because they have low toxicity and are stable. However, upon first injection, a weaker immune response is (removed weakly) induced because the microorganism is unable to replicate [10]. Several booster shots of the vaccine are therefore necessary. A second injection of the same pathogen leads to a greater immune response than that induced after the first injection.
With the advent of genetic engineering, new types of vaccines are being developed to avoid some of the risks of vaccines being based on whole microorganisms. These new vaccines contain only one or more molecules specific to the pathogen. They are called subunit vaccines [11]. They can be used against toxins or specific protein elements of microorganisms which are isolated and cloned to generate subunit vaccines [12,13]. However, some antigens do not elicit a sufficient immune response when injected alone. It is then necessary to fuse the weakly immunogenic antigen with a highly immunogenic protein. In this case, the vaccines are called conjugates [10].
The advent of genetic engineering has also made it possible to design another type of vaccine, the recombinant vaccine. They are based on the use of genetically modified living microorganisms to eliminate the genes responsible for their pathogenicity and to render them harmless. The interest of this type of vaccine is that it is based on genetically attenuate microorganisms without losing its immunogenicity effect and without reversion mechanism [10].
Furthermore, the recent development of sequencing techniques opens the door to new developments in vaccination processes. Pathogen genome sequencing enables access to all the specific epitopes of each microorganism, even those that cannot be grown in the laboratory and therefore cannot be attenuated or modified. The development of vaccines based on the use of new sequencing techniques and the identification of new antigens is called reverse vaccinology [14].
The various preventive vaccination approaches described are based on the same principle which consists of injecting a whole microorganism or a specific antigen to stimulate the immune system and prepare it for subsequent infection.
Another approach of vaccination is explored since several years and has recently done a technology leap thanks to the advances in genetic engineering. This type of vaccine is based on the use of nucleic acids coding the antigens [15].
Nucleic acid vaccines:
Principle and mode of operation
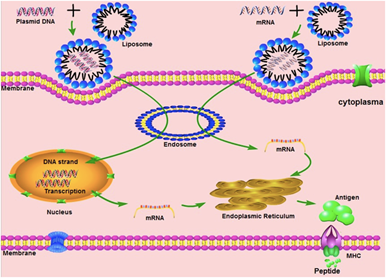
Nucleic acid-based vaccines used cDNA (coding DNA) or messenger RNA (mRNA) coding an antigen [16–18]. The DNA form is a plasmid, containing the cDNA of an antigen under the control of a suitable promoter. For mRNA, there are two possibilities, either vaccines are based on a standard mRNA or on a self-amplifying mRNA system. Non-replicating mRNAs have a simple open reading frame with untranslated regions (UTR). These are transcribed in vitro from a linear DNA template using a phage RNA polymerase [19]. A 5' cap that protects it and allows ribosome recruitment and a 3' polyadenosine tail, which increases stability, are added to form a mature mRNA. The self-amplifying mRNAs are based on a modified viral genome containing the genes coding for the RNA replication mechanism and the structural protein sequences are replaced by the gene of interest. Once the DNA or RNA is internalized in the cell, the cellular transcription or translation mechanism produces an endogenous protein that undergoes post-translational modifications, resulting in a correctly folded and functional protein.
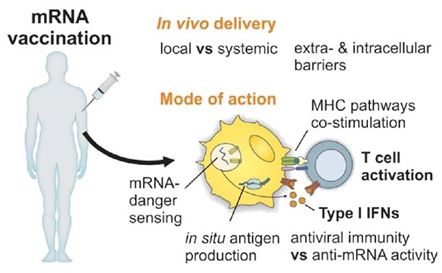
After a patient has been injected, plasmid DNA or mRNA enters the cells and the synthesis of the coding antigens and the production of antibodies playing a key role in protecting against further infection [20,21]. To do this, DNA or RNA vaccines must generate the antigen in sufficient quantities but must also produce it either within professional antigen presenting cells (APCs), such as dendritic cells (CDs), or in a cell from which the antigen can be presented to APCs. APCs strongly activate CD4+ and CD8+ T cells by presenting peptide antigens on class I and II major histocompatibility complex (MHC) molecules and induce the release of co-stimulating molecules [4,6]. Activation of APCs results in the production of antigen-specific antibodies by B cells and plasma cells. However, it is difficult to target these types of cells in vivo with RNA or DNA vectors. The most efficient way to transmit an antigen to professional APCs is to transduce or transfect them in vitro before administration to a patient, conferring a high level of complexity of the therapeutic protocol [22].
DNA and mRNA vaccines induce transient expression of the antigen in a manner that mimics the in vivo antigen production of viral pathogens [23–25]. These types of vaccines trigger humoral and cellular immune responses [26–31]. However, self-amplifying RNA vaccines systems occur the RNA replication through the synthesis of the RNA-dependent RNA polymerase complex. This type of vaccine generates multiple copies of the mRNA by encoding the antigen and expressing high levels of the antigen. Compared to the rapid expression of classical RNA vaccines, published results have shown that vaccination with self-amplifying RNA vaccines results in higher, albeit delayed, levels of antigen expression that persist for several days in vivo. Equivalent protection of a non-replicating RNA vaccine is conferred but at a much lower dose of mRNA [32].
*Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 1 oct 2019 ; 28:100766.
Advantages of DNA and RNA vaccines
The major advantages of DNA and RNA vaccines are their speed and low cost production, ease of manufacture and lower handling risks compared to current vaccines [33]. Synthesizing the sequence of a given antigen is much simpler and faster than trying to inactivate or attenuate the pathogenicity of a microorganism or making recombinant proteins. The manufacturing technique for DNA or RNA vaccines also avoids the potential risks associated with working with live pathogens [34,35]. Moreover, these vaccines induce a temporary in vivo presence of the synthesized antigen. In addition, injected DNA and mRNA molecules with a good efficiency of transfection or transduction produce more antigen molecules with a better quality compared to traditional approaches [35–38].
Furthermore, DNA and mRNA molecules have immunostimulatory properties that enable nucleic acids vaccines to induce the innate immune response [37–41] and also adaptive immune response [37,42,43]. DNA plasmids do this through their CpG motifs, which stimulate the Toll-like receptor 9, because plasmid DNA also acts on the TBK1-STING pathway through cytosolic receptors [16,38,44,45]. This results in the production of type 1 interferons (IFN type I), which act as adjuvants and generate the immune response against the antigen encoded by the plasmid DNA vaccine. IFN type I is a family of proteins, including interferon-β (IFNβ) and the multiple isoforms of IFNα, which are released by cells in response to viral infections and pathogens. Detection of IFN type I results in upregulation of genes stimulated by interferon and an antiviral cellular state [39,46]. The double-stranded structure of DNA is also considered an immune stimulant [47].
The injected mRNA stimulates the innate and adaptative response by being recognized by a variety of innate cell surfaces, endosomal and cytosolic immune receptors, notably thanks to two types of sensors: Toll-like receptors (TLR) 3, 7 and 8 which are located in the endosomal compartment of professional immune monitoring cells such as CDs, macrophages and monocytes and pattern recognition receptors (PRR) such as RIG-I and MDA5 [37,40]. Thus, activation of TLR7 leads to positive regulation of chemokines, which in turn recruit innate immune cells such as CDs and macrophages at the injection site. Plasmid DNA has fewer immunostimulatory properties than mRNA, but these are better defined [35].
At present, most of the vaccines used, except for some animal vaccines, have to be transported and stored at low temperatures. In poor rural areas of tropical countries, the cold chain may be broken, and the injected vaccine will present a potential hazard. This is why there is growing interest in the development of heat-stable vaccines. The optimization of RNA vaccines has shown that it is possible to produce heat-stable vaccines [48]. A freeze-dried RNA vaccine has been shown to be stable for 36 months at 5-25∘C and 6 months at 40∘C [49]. Moreover, a conventional rabies vaccine based on mRNA encapsulated in protamine was subjected to temperatures ranging from 4 to 56∘C for 20 cycles and exposure to 70∘C. The immunogenicity of this vaccine and its protective effects were not compromised [50].
DNA and RNA vaccines have many advantages over conventional vaccines, such as speed and ease of manufacture, low production costs, and residence at high heat. These vaccines induce an innate and adaptive immune response, thanks to the immunostimulant properties of plasmid DNA or mRNA. Although these two vaccines share some common advantages and characteristics, they differ in some respects.
Differences between DNA and RNA vaccines:
The main difference between DNA and mRNA vaccines is the cell compartment to which the different molecules are delivered.
The injected DNA is in the nucleus of the cell while the injected mRNA is in the cytoplasm. To be correctly located, the plasmid DNA must cross the plasma and nuclear membrane and the mRNA must cross the plasma and endosome membranes. These vaccines must deliver the antigen sequence through the injection of plasmid DNA or mRNA and they must also activate the danger signals of the innate immune response in order for the immune response to occur correctly [34,51,52]. The DNA and mRNA danger sensors are not the same and are located in different parts of the cell. Sensors of a DNA fragment, such as cyclic GMP-AMP synthase-stimulator of interferon genes, DEAH box nucleic acid helicase and AIM2 absent in melanoma 2, are located in the cytoplasm. RNA sensors, such as Toll-like receptors 7 and 8, are located in the endosomes to avoid chronic inflammatory reactions [53,54].
MH: Major histocompatibility complex
*Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol [Internet]. March 27th 2019
Furthermore, mRNA is synthesized in vitro from plasmid DNA. The production of RNA vaccines therefore has an additional step compared to that of DNA vaccines. In addition, DNA vaccines have been shown to produce the given antigen for a longer period of time than RNA vaccines due to the greater stability of plasmid DNA compared to mRNA [23,55]. However, unlike plasmid DNA, both classical and self-amplifying RNAs cannot integrate into the host genome and will be naturally degraded during the antigen expression process [52]. During the use of DNA vaccines, plasmid DNA integrate events have been observed but at a very low rate and is not considered as a biosafety problem [55], namely all measures to ensure the safe use of biological resources are respected. However, these characteristics indicate that RNA vaccines therefore have a higher level of biosecurity.
Although DNA and mRNA vaccines show differences and use different ways and mechanisms of action, these two types of nucleic acid vaccines have several advantages compared to traditional vaccines and seems very promising. However, advancements in nucleic acid vaccine development are still hindered by several technical points which could be addressed by the recent advancements in genetic engineering.
Limitations of DNA and RNA vaccines
The major difficulty to DNA and RNA vaccines is that nucleic acids must cross biological barriers to enter the desired cells. Furthermore, although sequencing techniques have made it possible to synthesize plasmid DNA or mRNA bearing the coding sequence of an antigen, for many diseases, scientists do not know which is the best immune response/combination of immune responses, and which are the most immunogenic antigenic targets [16,39,56].
The optimal protocol for administering the DNA or RNA vaccine in humans can be difficult to find because we cannot predict whether the vaccine administered will respond in the same way as in the animal model [49,57]. Despite preclinical studies that have shown the efficacy of DNA vaccines for various diseases in animal models, the human doses of several milligrams of DNA can produce an insufficient antigen expression and the efficacy of the vaccine has been disappointing [58]. In humans, DNA vaccines have not yet shown sufficient protection in Phase III [59]. The major limitation of these technologies is related to their in vivo administration. Indeed, DNA and RNA can be rapidly degraded after in vivo administrations, the genic vectors used so far showed a poor efficiency of transfection or transduction and they are not able to target the specific cell populations inducing the immune response [52,60,60–63]. Consequently, the quantity of DNA or RNA coding an antigen which penetrates in the APCs is not enough to induce a strong immune response conferring an immune protection.
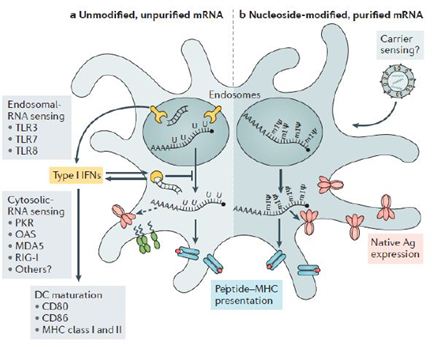
In addition, during the manufacture of RNA vaccines, other molecules such as contaminating nucleoside triphosphate remnants, DNA matrices and dsRNA, which are introduced and then remain, are also highly immunostimulatory [64]. Recognition by dsRNA also induces activation of IRF3 and NF-kB, which then leads to an increase in the production of type I IFN [65–67] dsRNA contamination due to aberrant RNA polymerase activities, which leads to decreased translation and degradation of cellular mRNA and ribosomal RNA and thus decreases protein expression by interrupting the translation machine [68]. Suppression of dsRNA can dramatically increase translation. Vaccine mRNA should therefore be purified as much as possible from excess components and DNA matrices or dsRNA during manufacturing. Purification by Fast Protein Liquid Chromatography (FPLC) or High-Performance Liquid Chromatography (HPLC) can be used to remove any remaining product and produce purified mRNA on a large scale [68–70]. However, the mechanisms of mRNA inflammation are relevant to the efficacy of the vaccine, particularly as an adjuvant. An effective adjuvant strategy, TriMix, a combination of mRNAs encoding three immune activating proteins: CD70, CD40 ligand and the constitutively active TLR4 protein, has increased the immunogenicity of naked, unmodified and unpurified mRNA in multiple cancer vaccine studies and has been particularly associated with increased CDs maturation and cytotoxic T cell responses [57,71–73].
These new vaccines have limitations, particularly in terms of the choice of antigens to be synthesized or the constraints to cross the various cell barriers in order to deliver the DNA or mRNA molecule into the right cell compartment. However, some limitations may prove useful, for example the inflammation mechanisms of the mRNA can be used as an adjuvant.
Yellow: the RNA sensors, Red: the antigen, Green: the CD maturation factors and Light Blue and Red: the major histocompatibility complexes (MHC). An example of a lipid nanoparticle carrier is shown in the upper right. A list of the main known RNA sensors that contribute to the recognition of unmodified double-stranded and single-stranded RNA is given. Unmodified, unpurified mRNAs (part a) and nucleoside-modified, purified by rapid protein liquid chromatography (FPLC) (part b) illustrates the two formats of mRNA vaccines where known forms of mRNA detection are present and absent, respectively. The dotted arrow represents reduced expression of the antigen. Ag, antigen; PKR, interferon-induced, RNA-activated, double-stranded protein kinase; MDA5, protein 1 containing the interferon-induced helicase C domain (also known as IFIH1); IFN, interferon; m1Ψ, 1 methylpseudouridine; OAS, 2′ 5′ oligoadenylate synthetase; TLR, Toll-like receptor
|
Vaccines |
Advantages |
Disadvantages |
|
DNA Vaccines |
· Based on non-infectious pathogen. · Stable, rapid and scalable production. · Potential to trigger a strong immune response. · Stimulation of innate immune response. · Induction of T and B cell immune response. |
· Potential integration into the human genome. · Low in vivo delivery efficiency. · Need to target cells of interest. · Need for adjuvants to improve efficacy of in vivo administration. |
|
ARN Vaccines |
· Based on non-infectious pathogen · Rapid and scalable production. · Potential to trigger a strong immune response. · Stimulation of innate immune response. · Induction of T and B cell immune response. · Non-infectious, non-integrating, natural degradation. · Degraded by normal cellular processes. · Modulation of antigen half-life. |
· RNA Instability. · Low in vivo delivery efficiency. · Need to target cells of interest. · Need for adjuvants to improve efficacy of in vivo administration. |
Table 1: The main advantages and disadvantages of DNA vaccines and RNA vaccines
*Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. avr 2018;17(4):261-7
Applications, pre-clinical and clinical trials of DNA and RNA vaccines
Despite some limitations observed in these new vaccines, several laboratories initiated clinical trials [45,74,75]. Four DNA vaccines have received marketing authorization for animals. They are used to vaccinate farmed salmon against infectious hematopoietic necrosis and pancreatic disease caused by a subtype of alphavirus, chickens against avian influenza, and to treat dogs with oral melanoma [76–79]. However, for now, no DNA vaccine is currently available for humans even clinical trials on humans are underway [80–82]. In clinical trials for DNA vaccines against the Ebola and Marburg viruses or West Nile virus, individuals have produced antibodies that are considered protective [83,84]. These observations show that DNA vaccines are therefore capable of inducing enough antibodies in humans. The production of effective antibodies would therefore not be a limitation of this technology. On the other hand, the choice of the right antigenic target remains a major one. The first two Zika vaccines in human clinical trials were also DNA vaccines [85], further clinical trials of DNA vaccines are underway against cancer [80,86–90]. Plasmid DNA codes for fusion proteins, including cytotoxic tumor T cell epitopes and helper T cell stimulators. A clinical trial against cellular hepatocarcinoma using DNA vaccine is ongoing [53]. The DNA codes for the embryonic antigen α-fetoprotein, which is over-expressed in 70% of individuals with this cancer. The DNA is vectorized using a nanovector called Nanotaxi, a tetra-functional amphiphilic copolymer consisting of four hydrophobic-hybrophilic polymer blocks centered on an ionizable core. The results of the regulatory safety study demonstrated that the amphiphilic tetra-functional copolymer alone or in combination with DNA is very well tolerated, inducing neither mortality nor toxicity.
For a decade, mRNA was of much less interest to the scientific community because of its instability relative to plasmid DNA. mRNA has a number of immunostimulatory mechanisms that can be useful or harmful to vaccines [27,40]. However, the use of modified nucleosides has solved key problems concerning the stability of mRNA, increased production of the encoded protein and some decrease in innate immunogenicity [68,91–93]. The mRNA is also stable in liquid or lyophilized form [49,50]. As a result, mRNA has begun to generate a lot of interest in vaccine manufacturing and clinical trials have been conducted [49,57,94]. The delivery of mRNA to antigen-presenting cells and lymph nodes at the injection site was made possible by the lipid nanovector CHOLK (cholesterol-kanamycin), derived from a natural sugar [53,95]. It was also shown that activation of specific mRNA hazard signals and immunogen synthesis took place when this vector was used [53,95]. However, the largest number of ongoing clinical trials of mRNA vaccines, particularly for cancer immunotherapy, involve the ex vivo transfection of cells with mRNA encoding tumor antigens, followed by re-infusion of the transfected cells to the patient [35,37,39]. Cancer RNA vaccines have been designed to express tumor-associated antigens that stimulate cell-mediated immune responses to eliminate or inhibit cancer cells. Another approach that is currently under development is to move towards personalized immunotherapeutic cancer vaccine products using a library of mRNAs encoding antigens that can be combined to be personalized for an individual. A significant number of cancer trials use mRNA by ex vivo transfection of CDs that are then re-infused into the patient. In addition, a company recently announced in a press release that a clinical trial has been launched for a Chikungunya RNA vaccine where the mRNA codes for a monoclonal antibody [96]. Similarly, it has been shown that an mRNA encoding the light and heavy chains of an HIV antibody and encapsulated in lipid nanoparticles protects mice against intravenous HIV infection [97]. The rabies vaccine using a vaccine with non-adjuvanted RNA has been replaced by a vaccine using mRNA encoding the glycoprotein of the rabies virus. Following an intradermal or intramuscular injection of this rabies mRNA vaccine, effective antibodies have been obtained. However, 78% of those tested had systemic adverse events and 10% had serious but non-life-threatening (grade III) adverse events [35,49]. Nevertheless, the vaccine has been described as generally safe with a reasonable tolerance profile which shows that sometimes there is some margin of acceptance. Self-amplifying RNA vaccines have also been produced, the polyethyleneimine (PEI) formulation of self-amplifying mRNA encoding H1N1/PR8-HA resulted in significantly higher antibody titer and longer lasting antigen expression than the use of unformulated self-amplifying mRNA [32,98]. Chitosan and PEI were also used to provide the self-amplifying mRNA in nanoparticle form 98,99. Other new modified nanoparticles are currently being investigated, such as polyplexes, nanoplex and scaffold release porous polymers [100,101].
Currently, the SARS-CoV-2 coronavirus pandemic, known as Covid-19, which was declared on 16 March 2020 by the World Health Organization, is enabling start-ups that have long been working on DNA or RNA vaccines and companies to use their technology to work for health. On March 27, Sanofi Pasteur announced a collaboration with Translate Bio on an mRNA vaccine against covid-19 [102]. According to the World Health Organization, 80% of cases of Covid-19 contamination are harmless, 14% are serious and 5% are critical, causing severe respiratory problems where the lungs fill with fluids preventing oxygen from reaching the organs. Patients with mild cases recover in one to two weeks, while severe cases take an average of six weeks to recover and 1% of those infected die. The search for a vaccine against Covid-19 is ongoing in many countries. On March 16, 2020, the first phase 1 clinical trials were launched in the United States to develop a vaccine against Covid-19 by the start-up Moderna Therapeutics [103]. Its Covid-19 mRNA vaccine, mRNA-1273, was injected into 45 people at the Kaiser Permanente Washington Health Research Institute in Seattle. Modern Therapeutics developed its first vaccine in 63 days illustrating the speed of the technology. They encapsulated the mRNA encoding the Covid-19 surface antigen in lipids to enter the cytoplasm of cells by endocytosis. On April 6, 2020, Inovio Pharmaceuticals announced that the Food and Drug Administration had approved its application to test its DNA vaccine named INO-4800 against the SARS-CoV-2 coronavirus [104]. To inject DNA into the cells, Inovio Therapeutics uses an electroporation method called Cellectra. At the injection site, needles inserted into the muscle or dermis induce electrical impulses that disrupt the cell membranes and enable the DNA to enter them. However, even with the launches of these first clinical trials, the American authorities have estimated that it will still be a year and a half before a vaccine is available if all goes according to plan.
The numerous applications, preclinical and clinical trials of DNA and RNA vaccines on going show that more and more nucleic acid vaccines have tended to be developed in recent years in contrast to conventional vaccines. This illustrates that, the recent progresses in our understanding in immunology and the new technologies emerging in genetic engineering enable, step by step, to undo the technological locks of nucleic acid vaccines use.
II Approaches to improve DNA and RNA vaccines
The number of studies and clinical trials developing and using nucleic acids vaccination demonstrates the interest in this technology. However, the low number of studies leads to a marketing authorization showing that improvements of the technology are necessary. The principle issue is to achieve enough antigen level expression in the immune cells to generate a protective immune response. Improvement of this parameter is crucial to make this technology available and several approaches are developed in this purpose.
Nucleic acid sequence design
The immunogenicity level induced by nucleic acid vaccination needs to be improved by increasing protein production, abundance and stability. In this purpose, strategies to optimize the plasmid DNA backbone or by modifying rare, low-use codons and promoters must be undertaken.
Efforts are also being made to increase the potency of mRNA including sequence optimization and the use of modified nucleosides, such as pseudo uridine, 5-methylcytidine, cap-1 structure and optimized codons, which improve translation efficiency. These modified nucleosides avoid recognition by immune sensors and thus prevent degradation of the mRNA before it reaches its site of arrival [19,91,105–108]. However, the observed toxicity of RNA drugs using non-naturally occurring modified nucleotide analogues raises questions about the possible toxic effects of RNA vaccines [109–111]. Different ways to improve the stability of mRNA are underway, such as the use of circular RNA molecules that are more stable and result in a more potent protein production than linear mRNA [112–114]. In addition, mRNA can also be modified at the codon and its guanine and cytosine content, as well as at the poly-A tails [18].
Stimulants use
To increase the protective power of current nucleic acid vaccines, admiration of enhancers is used such as adjuvants, immunostimulants like cytokines and co-stimulant molecules either as recombinant proteins or encoded by plasmid DNA or mRNA. Different strategies such as prime-boost combinations, usually using plasmid DNA as a primer, followed by a heterologous booster with a viral vector or protein [35]. However, this could also increase the potential for toxicity due to inflammation mechanisms. This booster could have an impact in particular on the potency due to the inflammatory effects of the nucleic acid that would lead to a decrease in its translation [115]. The search for a better balance between inflammation and possible deleterious toxicities is therefore underway by exploiting the adjuvant activity of nucleic acids while limiting or eliminating its toxicity. This would require optimization of nucleoside substitutions, nucleic acid building blocks, immunostimulants in vaccines, delivery devices and specific ways of administration [35].
Administration mode
The route of administration of nucleic acid vaccines is also an important parameter affecting the level of antigen expression and the strength of the immune response. Nucleic acid vaccines are administered by a systemic or local method depending on the desired location of antigen expression. For example, intravenous administration results in rapid digestion of naked mRNA not modified by ribonucleases and stimulation of the innate immune response [116]. However, these detrimental effects can be overcome with appropriate delivery systems and mRNA modifications. For infectious diseases, direct intramuscular (i.m.), intradermal (i.d.) or subcutaneous injection is the main route of nucleic acids vaccines administration, while intraperitoneal (i.p.) and intravenous (i.v.) administration is more used for therapeutic applications. Several studies have shown that i.m. and i.d. injections offer the best levels and duration of effect, with protein production peaking at 4 h and remaining locally 8 to 10 days after injection, depending on the dose [117,118]. However, this may vary between vaccines. In an influenza vaccine assay, intranodal (i.n.) administration of naked mRNA triggered potent CD4 and CD8 T cell immune responses in mice, and repeated i.n. injection of modified mRNA resulted in priming of antigen-specific CD4 and CD8 T cells, whereas subcutaneous, i.d. administration did not [119]. Combination with two or more methods of administration has been studied and used in the development of mRNA cancer vaccines [120].
Targeting the cells of interest
Once DNA or RNA is administered in the body, it must enter into specific immune cells to trigger an immune response. The development of strategies that directly target immune cells such as APCs could enable to improve nucleic acid vaccines. An effective approach for DC targeting of mRNA vaccines after systemic administration has recently been described. A complex delivery platform was generated using neutral cationic lipids and auxiliary lipids and mRNA [121]. It was found that the lipid to mRNA ratio, and thus the net particle load, has a profound impact on the biodistribution of the vaccine. While a positively charged lipid particle primarily targeted the lung, a negatively charged particle targeted CDs in secondary lymphoid tissues and bone marrow. The negatively charged particle induced potent immune responses against specific tumors that were associated with impressive tumor reduction in various mouse models. As no toxic effects were observed in mice or non-human primates, trials using this approach are underway to treat patients with melanoma or breast cancer [121]. In addition, certain formulations, such as the administration of a lipoplex mRNA vaccine, have been shown to be specifically absorbed by CDs through micropinocytosis [122]. Finally, for RNA vaccines, cells absorb mRNA by endocytosis, so efforts are being made to design delivery systems that increase the endosomal release of mRNA into the cytoplasm to improve the correct delivery of mRNA [64].
Vector design
The type of the vector and techniques used to deliver nucleic acids are also critical parameters. Indeed, the vector employed needs to have a good stability in vivo and a high efficiency to deliver DNA or RNA molecules into the cells. Until now, most of the vectors used were largely degraded in vivo, limiting the quantity of DNA or RNA delivered to the cells and finally, the quantity of antigens expressed. New delivery techniques such as electrolocation are designed to improve the quantity of nucleic acids delivered in the cells in vivo [61,62,121,123–126]. Cell penetration peptides, a type of cationic peptide, represent promising tools for the mRNA delivery to intracellular target sites. Encapsulation of mRNA with a cationic liposome or cell penetrating peptide protected the mRNA from degradation by RNase. Protamine, a cationic peptide rich in arginine, can bind to the mRNA and transport it into the cytoplasm [20,73,127]. This molecule has been widely used as a delivery system for cancer vaccines and viral RNA vaccines. Another approach to improve the potency of the vaccine could be to increase the amount of protein produced through a new vector design, protecting the nucleic acids from digestion by the ribonuclease, enabling efficient absorption by the target cells and easy release of the RNA into the cytoplasm without toxic effects. Several approaches have been explored and progress has been made in the design of mRNA delivery vectors [60,61,63,91,98,99,117,121]. However, several of these delivery vectors have demonstrated in vivo toxicity, which may limit their use in humans [128]. Self-amplifying RNA vaccines with lipid nanoparticles as the delivery vector induce a potent cellular and humoral immune response through different routes of administration [97]. Although progress has been made in the development of delivery tools, further efforts in understanding the mechanism of action may be required.
The use of viral vectors could be a relevant option to design stable vectors in vivo [129,130]. Indeed, this class of vector is naturally protected from the degradation as a wild type virus thanks to its capsid or envelope. Furthermore, this class of vectors shows a high efficiency to deliver nucleic acids in cells without adding adjuvants which could induce toxicity. In addition, some of viral vectors have a high flexibility of design enabling to add specific envelopes targeting immune cells such as DCs [131]. Despite all these advantages, until now, they have been scarcely used in vaccination because their level of biosafety was low and hardly compatible with this field of application. The principal issue about the use of vectors derived from lentivirus is to avoid the potential genotoxic effects due to the integration of their genomes into cell [132–138]. To answer this challenge, new versions of lentiviral vectors were designed to be non-integrating [139–141].
This type of vector shows a better level of biosafety than the integrating version and has been successfully tested in several models of DNA vaccination142. Nevertheless, several studies have shown that a small fraction of NILVs can be integrated into the genome of transduced cells, between 0.1% and 1% [143], including within vaccination context with primate models [144]. Although these integration events could generate genotoxic effects, they are not deemed an impediment to using NILVs in vaccination [144]. However, this risk must be taken into consideration when vaccines and therapeutic strategies based on NILVs are designed, limiting the scope of application.
Recently, a new version of lentiviral vectors (LVs) has shown a higher level of biosafety than that of NILVs. This type of vector contains a mutation of the reverse transcriptase which is the protein that converts the lentiviral genome from RNA to DNA. Therefore, this version of LV is unable to conduct this step of conversion. After transduction, the genome of the vector remains in RNA form and is taken in charge by the cells as an mRNA to be translated into protein [145]. Furthermore, it has been demonstrated that the mutation entirely abolishes the activity of the reverse transcriptase and no event of pseudotranfection has been observed, demonstrating that this version of LV is fully unable to deliver DNA. This new version of LVs could be used in vaccination, opening new avenues in this field thanks to its high stability, high level of transduction, flexibility of design and biosafety.
Conclusion
Vaccination is one of the major advances in human health. The principle consists of stimulating the immune system by injecting an antigen in order to obtain a specific response. The antigen injected is often of viral or bacterial origin and should induce protective immunity and immune memory. Vaccination can either be preventive or therapeutic. Since its creation in the 18th century, it has made it possible to eradicate smallpox globally and the World Health Organization has announced the eradication of other pathogens, notably polio in 2003. In addition, in France, since 1 January 2018, 11 vaccines have become compulsory.
Over the last few years, a set of so-called “classic vaccines” have been developed. The different types of vaccines vary according to their composition. They can be composed of an entire bacterium or virus, attenuated or inactivated, or only a protein. Recombinant antigens can also be obtained by genetic engineering. These conventional vaccines have facilitated vast improvements in the sphere of human health. However, they show several limitations in terms of quality, safety, production, or scope of disease. A new class of vaccine named nucleic acid vaccine could overcome these limits. They are based on the use of cDNA (coding DNA) or mRNA (messenger RNA) molecules, carrying the coding part of an antigen. Administration of a nucleic acid vaccine results in an endogenous generation of pathogen proteins with native conformation, glycosylation profiles, and other posttranslational modifications that mimic antigen produced during natural viral infection, offering several advantages over conventional vaccines. The principle of this type of vaccination has long been recognized but is impeded by the low level of nucleic acids entering in the cells, not enabling to generate a high quantity of antigens, consequently the immune response induced is weak. Consequently, during a long time, very few studies have validated a vaccine for animals or humans due to several biological barriers limiting the quantity of DNA or RNA entering the immune cells. Until now, the vectors used for in vivo.
administrations were synthetic. This type of vector shows a high level of biosafety; however, they do not confer a high level of protection against in vivo degradation after injection, their efficiency of transfection is limited, and it is difficult to combine them with a system targeting specific cell populations. On the contrary, the vectors derived from the virus confer a high level of nucleic acids protection, they can very efficiently transduce cells in vivo and can be combined with a system that targets specific cell populations. Nevertheless, these vectors’ level of biosafety is low and have so far not been compatible for vaccination applications. The recent advances in genetic engineering enable the design of new generations of DNA or RNA vectors combining the biosafety level of synthetic vectors with the high efficiency and design flexibility of viral vectors. These new generations of vectors promise a potential breakthrough, permitting the extension of the nucleic acid vaccines scope in animal health and making their use in human health possible. More than twenty years after this approach was first described, encouraging results have finally been reported. For example, in human clinical trials, even if the immune system’s response was lower than expected, compared to those observed in animal model, the results showed good tolerance and activation of antigen-specific T and B cell immune responses.
In addition, RNA vaccines that have historically been more difficult to design due to molecule stability issues but are more promising than DNA in terms of biosafety, have also shown promising results in recent times. Several pre-clinical and clinical trials have shown that RNA vaccines provide, among other things, a safe and durable immune response in animal and human models.
Recently, a new proof of the nucleic acid vaccines development speeding is about the SARS-CoV-2 coronavirus pandemic. Indeed, the first two vaccines developed and tested in humans are nucleic acid vaccines and not conventional vaccines, illustrating its rapid design and its good level of biosafety. The first vaccine to be licensed for a clinical trial against Covid-19 is an mRNA-1273 RNA vaccine and was developed in just 63 days. Soon after, another clinical trial started, based on INO-4800 DNA vaccine.
For a long time, vaccinations based on nucleic acids has been imagined and described. However, our limited know-how in immunology and genetic engineering prevented us from uncovering its potential. Recent advancements in these areas are game-changing. The increase in the number of clinical trials in a short period of time clearly shows that nucleic acid vaccines are becoming available for more and more diseases. Furthermore, the fields of application for this type of vaccine may extend beyond prophylactically areas to other therapeutically areas such as cancer therapies.
References
- Jenner E. Dr. Jenner, on the Vaccine Inoculation. The Medical and Physical Journal 3 (1800): 502.
- Jenner E. History of the Inoculation of the Cow-Pox: Further Observations on the Variolæ Vaccinæ, or Cow-Pox. The Medical and Physical Journal 1 (1799): 313.
- Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nature Reviews Microbiology 9 (2011): 889-893.
- Moser M, Leo O. Key concepts in immunology. Vaccine 28 (2010): C2-C13.
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature Immunology 12 (2011): 509.
- Hoebe K, Janssen E, Beutler B. The interface between Innate and Adaptive Immunity. Nature Immunology 5 (2004): 971-974.
- Plotkin SA. Vaccines: the fourth century. Clinical and Vaccine Immunology 16 (2009): 1709-1719.
- Ehrenfeld E, Modlin J, Chumakov K. Future of polio vaccines. Expert Review of Vaccines 8 (2009): 899-905.
- Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514 (2014): 47-53.
- Rappuoli R, Black S, Bloom DE. Vaccines and global health: In search of a sustainable model for vaccine development and delivery. Science Translational Medicine 11 (2019): eaaw2888.
- Bertholet S, Ireton GC, Ordway DJ, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium Tuberculosis. Science translational medicine 2 (2010): 53ra74.
- Awasthi S, Hook LM, Pardi N, et al. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Science Immunology 4 (2019): eaaw7083.
- Bart SA, Hohenboken M, Della Cioppa G, et al. A cell culture–derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Science Translational Medicine 6 (2014): 234ra55.
- Reverse Vaccinology - an overview | ScienceDirect Topics. https://www.sciencedirect.com/topics/immunology-and-microbiology/reverse-vaccinology.
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 356 (1992): 152-154.
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunological Reviews 239 (2011): 62-84.
- Weissman D. mRNA transcript therapy. Expert Review of Vaccines 1 (2015) Feb: 265-281.
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nature Reviews Drug Discovery 13 (2014): 759-780.
- Pardi N, Muramatsu H, Weissman D, et al. In vitro transcription of long RNA containing modified nucleosides. InSynthetic Messenger RNA and Cell Metabolism Modulation (2013): pp. 29-42.
- Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nature Biotechnology 30 (2012): 1210-1216.
- Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543 (2017): 248-251.
- Benteyn D, Heirman C, Bonehill A, et al. mRNA-based dendritic cell vaccines. Expert Review of Vaccines 14 (2015): 161-176.
- Middaugh CR, Evans RK, Montgomery DL, et al. Analysis of plasmid DNA from a pharmaceutical perspective. Journal of Pharmaceutical Sciences 87 (1998): 130-146.
- Houseley J, Tollervey D. The many pathways of RNA degradation. Cell 136 (2009): 763-776.
- Syrengelas AD, Chen TT, Levy R. DNA immunization induces protective immunity against B–cell lymphoma. Nature Medicine 2 (1996): 1038-1041.
- Geall AJ, Verma A, Otten GR, et al. Nonviral delivery of self-amplifying RNA vaccines. Proceedings of the National Academy of Sciences 109 (2012): 14604-14609.
- Brito LA, Chan M, Shaw CA, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Molecular Therapy 22 (2014): 2118-2129.
- Deering RP, Kommareddy S, Ulmer JB, et al. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opinion on Drug Delivery 11 (2014): 885-899.
- Tews BA, Meyers G. Self-replicating RNA. InRNA Vaccines (2017): pp. 15-35.
- Lundstrom K. Replicon RNA viral vectors as vaccines. Vaccines 4 (2016): 39.
- Ljungberg K, Liljeström P. Self-replicating alphavirus RNA vaccines. Expert Review of Vaccines 14 (2015): 177-194.
- Vogel AB, Lambert L, Kinnear E, et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Molecular Therapy 26 (2018): 446-455.
- Reautschnig P, Vogel P, Stafforst T. The notorious RNA in the spotlight-drug or target for the treatment of disease. RNA Biology 14 (2017): 651-668.
- Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Frontiers in Immunology 9 (2018): 1963.
- Liu MA. A comparison of plasmid DNA and mrna as vaccine technologies. Vaccines 7 (2019): 37.
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science 247 (1990): 1465-1468.
- Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nature Reviews Materials 2 (2017): 1-7.
- Campbell JD. Development of the CpG adjuvant 1018: a case study. InVaccine Adjuvants (2017): pp. 15-27.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery 17 (2018): 261.
- Chen N, Xia P, Li S, et al. RNA sensors of the innate immune system and their detection of pathogens. IUBMB life 69 (2017): 297-304.
- Edwards DK, Jasny E, Yoon H, et al. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. Journal of Translational Medicine 15 (2017): 1-8.
- Deck RR, DeWitt CM, Donnelly JJ, et al. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine 15 (1997): 71-78.
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 356 (1992): 152-154.
- Allen A, Wang C, Caproni LJ, et al. Linear doggybone DNA vaccine induces similar immunological responses to conventional plasmid DNA independently of immune recognition by TLR9 in a pre-clinical model. Cancer Immunology, Immunotherapy 67 (2018): 627-638.
- Gottlieb P, Utz PJ, Robinson W, et al. Clinical optimization of antigen specific modulation of type 1 diabetes with the plasmid DNA platform. Clinical Immunology 149 (2013): 297-306.
- Pollard C, Rejman J, De Haes W, et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Molecular Therapy 21 (2013): 251-259.
- Coban C, Kobiyama K, Aoshi T, et al. Novel strategies to improve DNA vaccine immunogenicity. Current Gene Therapy 11 (2011): 479-484.
- Jones KL, Drane D, Gowans EJ. Long-term storage of DNA-free RNA for use in vaccine studies. Biotechniques 43 (2007): 675-681.
- Alberer M, Gnad-Vogt U, Hong HS, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. The Lancet 390 (2017): 1511-1520.
- Stitz L, Vogel A, Schnee M, et al. A thermostable messenger RNA based vaccine against rabies. PLoS Neglected Tropical Diseases 11 (2017): e0006108.
- Coban C, Kobiyama K, Jounai N, et al. DNA vaccines: a simple DNA sensing matter?. Human Vaccines & Immunotherapeutics 9 (2013): 2216-2221.
- Zhang C, Maruggi G, Shan H, et al. Advances in mRNA vaccines for infectious diseases. Frontiers in Immunology 10 (2019): 594.
- Pitard B. Nanotaxi® pour les vaccins ARN et ADN. Médecine/Sciences 35 (2019): 749-752.
- Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annual Review of Immunology 32 (2014): 461-488.
- Ledwith BJ, Manam S, Troilo PJ, et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 43 (2000): 258-272.
- Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259 (1993): 1745-1749.
- Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Molecular Therapy 25 (2017): 1316-1327.
- Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. The Lancet 372 (2008): 1881-1893.
- Mir LM, Bureau MF, Gehl J, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proceedings of the National Academy of Sciences 96 (1999): 4262-4267.
- Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. Journal of Controlled Release 240 (2016): 227-234.
- Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene therapy 24 (2017): 133-143.
- Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Human Vaccines & Immunotherapeutics 13 (2017): 2837-2848.
- Thess A, Grund S, Mui BL, et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Molecular Therapy 23 (2015): 1456-1464.
- Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Medicine 9 (2017): 60.
- Goubau D, Schlee M, Deddouche S, et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514 (2014): 372-375.
- Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413 (2001): 732-738.
- Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine 13 (2007): 460-469.
- Kariko K, Muramatsu H, Ludwig J, et al. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Research 39 (2011): e142.
- Pascolo S. Vaccination with messenger RNA. InDNA Vaccines (2006): pp. 23-40.
- Weissman D, Pardi N, Muramatsu H, et al. HPLC purification of in vitro transcribed long RNA. InSynthetic Messenger RNA and Cell Metabolism Modulation (2013): pp. 43-54.
- Van Lint S, Goyvaerts C, Maenhout S, et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Research 72 (2012): 1661-1671.
- Van Lint S, Renmans D, Broos K, et al. Intratumoral delivery of TriMix mRNA results in T-cell activation by cross-presenting dendritic cells. Cancer Immunology Research 4 (2016): 146-156.
- Kallen KJ, Heidenreich R, Schnee M, et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive® vaccines. Human Vaccines & Immunotherapeutics 9 (2013): 2263-2276.
- Doener F, Hong HS, Meyer I, et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine 37 (2019): 1819-1826.
- Garren H, Robinson WH, Krasulová E, et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Annals of Neurology 63 (2008): 611-620.
- Cany J, Barteau B, Tran L, et al. AFP-specific immunotherapy impairs growth of autochthonous hepatocellular carcinoma in mice. Journal of Hepatology 54 (2011): 115-121.
- Beilvert F, Tissot A, Langelot M, et al. DNA/amphiphilic block copolymer nanospheres reduce asthmatic response in a mouse model of allergic asthma. Human Gene Therapy 23 (2012): 597-608.
- Cambien B, Richard-Fiardo P, Karimdjee BF, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma. PloS One 6 (2011): e28842.
- Le Moigne V, Rottman M, Goulard C, et al. Bacterial phospholipases C as vaccine candidate antigens against cystic fibrosis respiratory pathogens: the Mycobacterium Abscessus Model. Vaccine. 33 (2015): 2118-2124.
- Aggarwal C, Cohen RB, Morrow MP, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clinical Cancer Research 25 (2019): 110-124.
- Geall AJ, Mandl CW, Ulmer JB. RNA: the new revolution in nucleic acid vaccines. InSeminars in Immunology 25 (2013): 152-159.
- Faurez F, Dory D, Le Moigne V, et al. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine. 28 (2010): 3888-3895.
- Sarwar UN, Costner P, Enama ME, et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. The Journal of Infectious Diseases. 211 (2015): 549-557.
- Martin JE, Pierson TC, Hubka S, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. Journal of Infectious Diseases. 196 (2007): 1732-1740.
- Gaudinski MR, Houser KV, Morabito KM, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. The Lancet. 391 (2018): 552-562.
- Gaudinski MR, Houser KV, Morabito KM, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. The Lancet 391 (2018): 552-562.
- Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. The Lancet 386 (2015): 2078-2088.
- Chudley L, McCann K, Mander A, et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunology, Immunotherapy 61 (2012): 2161-2170.
- McCann KJ, Mander A, Cazaly A, et al. Targeting carcinoembryonic antigen with DNA vaccination: on-target adverse events link with immunologic and clinical outcomes. Clinical Cancer Research 22 (2016): 4827-4836.
- Patel PM, Ottensmeier CH, Mulatero C, et al. Targeting gp100 and TRP-2 with a DNA vaccine: Incorporating T cell epitopes with a human IgG1 antibody induces potent T cell responses that are associated with favourable clinical outcome in a phase I/II trial. Oncoimmunology 7 (2018): e1433516.
- Anderson BR, Muramatsu H, Nallagatla SR, et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Research. 38 (2010): 5884-5892.
- Fotin-Mleczek M, Duchardt KM, Lorenz C, et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. Journal of Immunotherapy 34 (2011): 1-5.
- Rettig L, Haen SP, Bittermann AG, et al. Particle size and activation threshold: a new dimension of Danger Signaling Blood 115 (2010): 4533-4541.
- Routy JP, Boulassel MR, Yassine-Diab B, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clinical Immunology 134 (2010): 140-147.
- Lindsay KE, Bhosle SM, Zurla C, et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nature Biomedical Engineering 3 (2019): 371-380.
- Moderna Announces Dosing of the First Monoclonal Antibody Encoded by mRNA in a Clinical Trial. Moderna, Inc. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-dosing-first-monoclonal-antibody-encoded-mrna.
- Pardi N, Secreto AJ, Shan X, et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nature Communications 8 (2017): 1-8.
- Démoulins T, Milona P, Englezou PC, et al. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine: Nanotechnology, Biology and Medicine 12 (2016): 711-722.
- McCullough KC, Bassi I, Milona P, et al. Self-replicating replicon-RNA delivery to dendritic cells by chitosan-nanoparticles for translation in vitro and in vivo. Molecular Therapy-Nucleic Acids 3 (2014): e173.
- Lai WF, Wong WT. Design of polymeric gene carriers for effective intracellular delivery. Trends in Biotechnology 36 (2018): 713-728.
- McKinlay CJ, Benner NL, Haabeth OA, et al. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proceedings of the National Academy of Sciences115 (2018): E5859-5866.
- Sanofi: Press Releases, Friday, March 27, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-03-27-07-00-00 https://www.sanofi.com/media-room/press-releases/2020/2020-03-27 07-00-00 2007404.
- Zhang J, Zeng H, Gu J, et al. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines 8 (2020): 153.
- Hodgson J. The pandemic pipeline. Nature Biotechnology (2020).
- Andries O, Mc Cafferty S, De Smedt SC, et al. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. Journal of Controlled Release 217 (2015): 337-344.
- Meyer M, Huang E, Yuzhakov O, et al. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. The Journal of Infectious Diseases 217 (2018): 451-455.
- Van Gulck ER, Ponsaerts P, Heyndrickx L, et al. Efficient stimulation of HIV-1–specific T cells using dendritic cells electroporated with mRNA encoding autologous HIV-1 Gag and Env Proteins. Blood.107 (2006): 1818-1827.
- Pardi N, Weissman D. Nucleoside modified mRNA vaccines for infectious diseases. InRNA Vaccines (2017):109-121.
- Feng JY, Johnson AA, Johnson KA, et al. Insights into the molecular mechanism of mitochondrial toxicity by AIDS drugs. Journal of Biological Chemistry 276 (2001): 23832-23837.
- Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. Journal of Biological Chemistry 276 (2001): 40847-40857.
- Moyle G. Toxicity of antiretroviral nucleoside and nucleotide analogues. Drug Safety 23 (2000): 467-481.
- Xu Z, Li P, Fan L, et al. The potential role of circRNA in tumor immunity regulation and immunotherapy. Frontiers in Immunology 9 (2018): 9.
- Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nature Communications 9 (2018): 1-10.
- Holdt LM, Kohlmaier A, Teupser D. Circular RNAs as therapeutic agents and targets. Frontiers in Physiology 9 (2018): 1262.
- Iavarone C, O’hagan DT, Yu D, et al. Mechanism of action of mRNA-based vaccines. Expert Review of Vaccines 16 (2017): 871-881.
- Whitehead KA, Dahlman JE, Langer RS, et al. Silencing or stimulation? siRNA delivery and the immune system. Annual review of Chemical and Biomolecular Engineering 2 (2011): 77-96.
- Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. Journal of Controlled Release 217 (2015): 345-351.
- Lindgren G, Ols S, Liang F, et al. Induction of robust B cell responses after influenza mRNA vaccination is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Frontiers in Immunology 8 (2017): 1539.
- Kreiter S, Selmi A, Diken M, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Research 70 (2010): 9031-9040.
- Van Nuffel AM, Benteyn D, Wilgenhof S, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunology, Immunotherapy 61 (2012): 1033-1043.
- Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534 (2016): 396-401.
- Persano S, Guevara ML, Li Z, et al. Lipopolyplex potentiates anti-tumor immunity of mRNA-based Vaccination. Biomaterials 125 (2017): 81-89.
- Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends in Biotechnology 22 (2004): 346-353.
- Johansson DX, Ljungberg K, Kakoulidou M, et al. Intradermal electroporation of naked replicon RNA elicits strong immune responses. PloS one 7 (2012): e29732.
- Piggott JM, Sheahan BJ, Soden DM, et al. Electroporation of RNA stimulates immunity to an encoded reporter gene in mice. Molecular Medicine Reports 2 (2009): 753-756.
- Broderick KE, Humeau LM. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Review of Vaccines 14 (2015): 195-204.
- Schnee M, Vogel AB, Voss D, et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Neglected Tropical Diseases 10 (2016): e0004746.
- Lv H, Zhang S, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. Journal of Controlled Release 114 (2006): 100-109.
- Naldini L, Blömer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272 (1996): 263-267.
- Grandchamp N. Pharmacodynamic Evaluation: Gene Therapy. Drug Discovery and Evaluation: Methods in Clinical Pharmacology (2020): 361-384.
- Lopes L, Dewannieux M, Takeuchi Y, et al. A lentiviral vector pseudotype suitable for vaccine development. The Journal of Gene Medicine 13 (2011):181-187.
- Montini E, Cesana D, Schmidt M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. The Journal of Clinical Investigation 119 (2009): 964-975.
- Ranzani M, Cesana D, Bartholomae CC, et al. Lentiviral vector–based insertional mutagenesis identifies genes associated with liver cancer. Nature methods 10 (2013): 155-161.
- Almarza D, Bussadori G, Navarro M, et al. Risk assessment in skin gene therapy: viral–cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Therapy 18 (2011): 674-681.
- Cesana D, Sgualdino J, Rudilosso L, et al. Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. The Journal of Clinical Investigation 122 (2012): 1667-1676.
- Moiani A, Paleari Y, Sartori D, et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. The Journal of Clinical Investigation 122 (2012): 1653-1666.
- Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467 (2010): 318-322.
- Grandchamp N, Altémir D, Philippe S, et al. Hybrid lentivirus-phiC31-int-NLS vector allows site-specific recombination in murine and human cells but induces DNA damage. PLoS One 9 (2014): e99649.
- Philippe S, Sarkis C, Barkats M, et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proceedings of the National Academy of Sciences 103 (2006): 17684-17689.
- Grandchamp N, Henriot D, Philippe S, et al. Influence of insulators on transgene expression from integrating and non-integrating lentiviral vectors. Genetic Vaccines and Therapy 9 (2011): 1-8.
- Saeed MQ, Dufour N, Bartholmae C, et al. Comparison Between several integrase-defective lentiviral vectors reveals increased integration of an HIV vector bearing a D167H mutant. Molecular Therapy-Nucleic Acids 3 (2014): e213.
- Negri DR, Michelini Z, Baroncelli S, et al. Successful immunization with a single injection of non-integrating lentiviral vector. Molecular Therapy 15 (2007): 1716-1723.
- Sarkis C, Philippe S, Mallet J, et al. Non-integrating lentiviral vectors. Current Gene Therapy 8 (2008): 430-437.
- Blasi M, Negri D, LaBranche C, et al. IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells. Communications Biology 1 (2018): 1-3.
- Sarkis C, Altemir D, Grandchamp N, et al, inventors; NEWVECTYS, assignee. Transient expression vectors, preparation and uses thereof. United States patent US 10,308,955 (2019).