The Efficacy of Omega-3 and Cholecalciferol Supplementation On Il-23 Production in the Airways of Cystic Fibrosis Patients Infected by Pseudomonas aeruginosa – A Pilot Study
Article Information
Malgorzata Olszowiec-Chlebna*, Joanna Jerzynska, Iwona Stelmach
Department of Pediatrics and Allergy, Medical University of Lodz, Copernicus Memorial Hospital in Lodz, Poland
*Corresponding author: Malgorzata Olszowiec-Chlebna, Department of Pediatrics and Allergy, Medical University of Lodz, Poland, Copernicus Memorial Hospital, Korczak Paediatric Center, Pi?sudskiego 71 Str, 90-329 Lodz, Poland
Received: 08 January 2020; Accepted: 17 January 2020; Published: 07 February 2020
Citation: Malgorzata Olszowiec-Chlebna, Joanna Jerzynska, Iwona Stelmach. The Efficacy of Omega-3 and Cholecalciferol Supplementation On Il-23 Production in the Airways of Cystic Fibrosis Patients Infected by Pseudomonas aeruginosa – A Pilot Study. Archives of Clinical and Biomedical Research 4 (2020): 033-047.
Share at FacebookAbstract
Introduction: Polyunsaturated fatty acids (PUFA) supplementation is thought to reduce inflammation in cystic fibrosis. Likewise vitamin D has immunoregulatory properties. The aim of the study was to compare the effect of cholecalciferol supplementation to cholecalciferol with omega-3 supplementation on the inflammatory response of CF patients infected with P.aeruginosa.
Methods: This was a double-blind, cross-over trial. Twenty-three CF patients (aged 6 – 19) infected by P. aeruginosa were randomised to received: cholecalciferol 1000IU or cholecalciferol 1000IU and omega – 3 1000 mg daily for three months. The levels of IL-23 and IL-17A in the exhaled breath condensate (EBC), calcium-phosphorus balance and serum lipid balance were measured. Data were analysed using means of Stata/Special Edition, release 14.2.
Results: The level of IL-23 in EBC significantly decreased in vitamin D + omega-3 group from 9,39 pg/mL (± SD = 6,40 pg/mL) to 7,33 pg/mL (± SD = 3,88 pg/mL) (p = 0.023); there was no change in vitamin D group (p = 0.630). The blood concentration of 25OHD significantly increased in both groups: in vitamin D group form 24,64 ng/mL (± SD = 8,31 ng/mL) to 29,41 ng/mL (± SD = 7,78 ng/mL) (P < 0,001) and in vitamin D +omega-3 group from 24,31 ng/mL (± SD = 8,28 ng/mL) to 30,67 ng/mL (± SD = 8,01 ng/mL) (P < 0,001).
Conclusions: Omega-3 supplementation with cholecalciferol may provide some benefits and modulate the immunity response in airway CF patients with chronic P. aeruginosa infection reflected as reduction of the level of IL-23 in EBC.
Keywords
Cystic fibrosis; omega-3; PUFA; Cholecalciferol; Interleukin 17A; Interleukin 23
Article Details
Abbreviations
CF - cystic fibrosis
P. aeruginosa - Pseudomonas aeruginosa
IL-17A - interleukin 17A
IL-23 - interleukin 23
PUFA - polyunsaturated fatty acids
EBC - exhaled breath concentrate
25OHD - 25-hydroxycholecalciferol
PTH – parathormone
HDL - high density lipoprotein
LDL - low density lipoprotein
ALT - alanine transaminase
CRP - c-reactive protein
Introduction
Cystic fibrosis (CF) is a most common life-shortening autosomal recessive diseases in Caucasians. Opportunistic pathogens such as Pseudomonas aeruginosa (P. aeruginosa) is connected with progressive loss of lung function due to excessive inflammation. Pro-inflammatory cytokines mainly secreted by neutrophils such as interleukin 17A (IL-17A) and interleukin 23 (IL-23) are critical for neutrophil recruitment in a chronic P. aeruginosa pulmonary infection [1-6].
The anti-inflammatory role is attributed to polyunsaturated fatty acids (PUFA): linoleic acid (18:2 omega-6) and alpha-linolenic (18:3 omega-3). They are essential for normal growth and function, play an important role in the integrity of cellular membranes. Essential fatty acids deficiency was reported in more than 80% of CF patients due to poor nutritional status and its impact in tissue destruction has been well demonstrated [7-10]. Clinical trials performed on CF patients have revealed that oral supplementation with omega-3 modifies fatty acid profiles [11] and improve nutritional, clinical, spirometry and inflammatory parameters [12-14]. Some beneficial effects of omega-3 on an inflammatory disease which is CF can be explained by a decrease in the production of pro-inflammatory metabolites. Still, there is not enough evidence to draw firm conclusions or recommend the routine use of these supplements in people with CF [15].
Vitamin D insufficiency in CF patients is common due to the high prevalence of fat soluble vitamin malabsorption and impaired hydroxylation in the liver. Most of them require oral supplementation to avoid its deficiency. The role of vitamin D in maintenance of bone and mineral homeostasis is known. Simultaneously its supplementation may contribute to reduce inflammation and improved lung function in patients with CF. Vitamin D exerts a direct regulatory effect on T cells, inhibiting T cell proliferation and IL-17 production what is connected with exaggerated inflammatory response to pulmonary infection [16-20].
The aim of this pilot trail was to compare the effect of the cholecalciferol supplementation alone to the oral cholecalciferol with omega 3 supplementation on the inflammatory response of CF patients infected with P. aeruginosa. The changes in IL-17A and IL-23 levels in the exhaled breath condensate (EBC) were analyzed.
Materials and methods
This was a randomized, placebo-controlled, double-blind, cross-over trial. Twenty-three CF patients from twenty-five assessed for eligibility agreed to participated in the study. There were 11 women and 12 men between 6 and 19 years, all of them completed the intervention. All of them were attending the Cystic Fibrosis Outpatient Clinic in Copernicus Hospital in Lodz, Poland. The demographics and clinical characteristics of the study participants are shown in Table no 1. The chronic P. aeruginosa infection were defined as more than 50% of the monthly sputum samples positive for P. aeruginosa in the previous year. The subjects who had an exacerbation - deterioration of the FEV1 and/or increase in symptoms and/or documented radiological changes and were on intravenous antibiotic therapy for at least 4 weeks before the study were not included to the trial. Prior to the trail the participants used 800 IU of cholecalciferol (according to the previous valid recommendation [21]) and did not take PUFA supplementation.
|
Variables |
Characteristic |
|
Age [years], mean±SD |
16,5±4,38 |
|
Male gender, n(%) |
12(52,7) |
|
Mutation (CFTR) |
|
|
Delta F508/other , n(%) |
9(39,1) |
|
Delta F508/Delta F508 n(%) |
10(43,4) |
|
Others, n(%) |
4(17,3) |
|
BMI [kg/m2] mean±SD |
16,1 kg/m2±SD 12,28 |
|
FEV 1 [% best to predicted], mean±SD |
88,32±22,02 |
|
Table 1: Baseline characteristics |
Study design
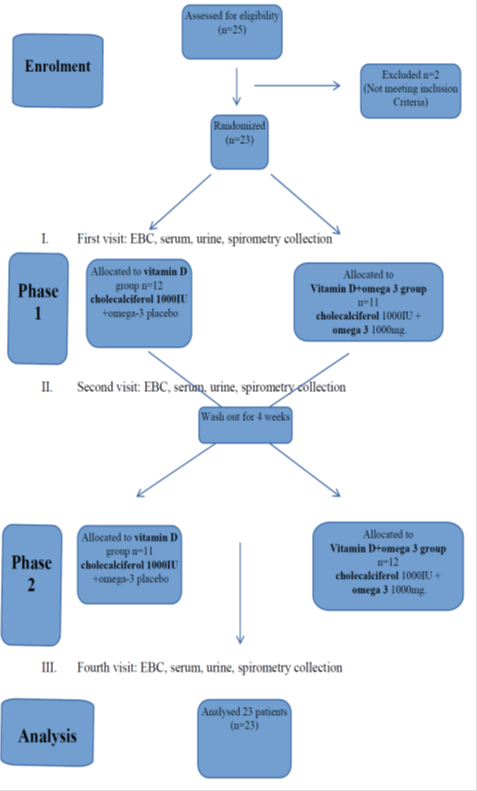
The study was carried out from May to November 2016. At all three study visits collection of EBC, serum and urine samples and spirometry took place. Informed consent was obtained from all patients and their parents. The study was approved by the local ethical committee no RNN/21/14/KE. Randomization and treatment assignment were carried out by research pharmacy by standard allocation procedures. Only blind treatment codes were noted on randomization lists provided to study staff. All study staff and participants remained blind to treatment assignment.
At the first visit all 23 participants were randomly assigned to the group:
- vitamin D + placebo group receiving: cholecalciferol 1000IU daily in a capsule and omega – 3 placebo in a capsule
or
- vitamin D + omega–3 group receiving: cholecalciferol 1000IU daily in a capsule and omega – 3 1000 mg in a capsule
The participants received cholecalciferol - 1000 IU per day alone (Bioaron D 1000, 25 mcg = 1000 IU, Phytopharm, caps.) or cholecalciferol - 1000 IU per day (Bioaron D 1000, 25 mcg = 1000 IU, Phytopharm, caps.) with omga-3 1000mg (Omega-3 1000, Naturell, caps.) per day for three months. Cholecalciferol, omega-3 and placebo were blinded by the hospital pharmacy. Patients were informed that during the trail they cannot take other vitamin D and PUFA supplements. Other CF treatment was maintained. Three months of supplementation (the second visit) were followed by 4 weeks of washout (patients returned to their previous). Then patients according to their earlier randomization group, received cholecalciferol - 1000 IU per day alone or cholecalciferol - 1000 IU with omega-3 1000 mg per day for the next three months. The third ending visit took place after 7 months from the first visit. Figure 1. At the and the participants were asked to bring all used and unused medication to check compliance.
Lung function tests
Pulmonary function testing was done by using a Master Screen unit (Erich Jaeger Gmbh, Hochberg, Germany). All pulmonary function tests were performed according to the ATS/European Respiratory Society (ERS) standards [22].
Collection and analysis of EBC
The EBC was sampled by a commercially available condenser (EcoScreen; Jaeger) according to the current ATS/ERS guidelines. Samples of the EBC were obtained from subjects during tidal breathing while wearing a nose clip. The two-way non-rebreathing valves and tubing to the condenser served as a saliva trap. After a 10-minute collection, the EBC was rapidly frozen in small plastic tubes at 80 °C by using dry ice and was stored at 80°C until analysis. All EBC samples for biochemical markers of inflammation were measured by Laboratory of Cytometry, at Cathedral of Molecular Biophysics at the University of Lodz. The level of IL-23 was conducted by using separate competitive enzyme-linked immunosorbent assay kits ELISA (ELISA Quantikine, R&D Systems) and IL-17A by using enhanced sensitivity cytometric bead array (BD™ CBAray, Biosciences). All the assays were performed according to the manufacturer’s instructions.
Laboratory tests
Blood was sampled and analyzed for 25OHD (using a specific radioimmunoassay - 25-OH-vit.D3-RIA-CT, BioSource Europe S.A., Nivelles, Belgium), calcium, phosphorus, parathormone – PTH (using a immunochemiluminescence assay -Intact PTH, Roche Diagnostic GmbH, Mannheim) and calcium/creatinine ratio in urine at baseline and after 3 months of supplementation. Total cholesterol, HDL (high density lipoprotein), LDL (low density lipoprotein), triglycerides, ALT (alanine transaminase), and CRP (c-reactive protein) were also evaluated. All the measurements were made in the hospital laboratory using standard laboratory methods.
Statistical analysis
The investigated traits were described by way of measures of location – mean, along with measures of dispersion – standard deviation, 95% confidence interval, and minimum-to-maximum values.
Mixed-effects regression models with robust standard errors were performed, considering both repeated measurements within the two separate studies and differences between the two studies. Intra-subject correlations also were incorporated into the mixed-effects regression models on grounds of the cross-over study design,. All the regression equations were controlled for the studied patients age and gender.
A level of P < 0.05 was considered statistically significant. All the statistical computations were carried out by means of Stata/Special Edition, release 14.2 (StataCorp LP, College Station, Texas, USA).
Results
The present cross-over study comprised the total of 23 patients, the baseline demographics of the study groups were included in table 1. Detailed descriptive measures are displayed in Table 2.
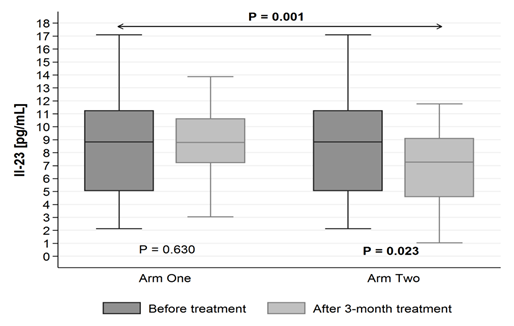
The level of IL-23 found in EBC decreases after three months of supplementation with 1000 IU of cholecalciferol with 1000 mg of omega-3 (in vitamin D+omega-3 group): from 9,39 pg/mL (± SD = 6,40) to 7,33 pg/mL (± SD = 3,88) (p = 0.023). Whereas it did not statistically significantly change in vitamin D group (p = 0.630) (Figure 2.)
The assessment of 25 OH D serum level seems to be the best to assess the effectiveness of cholecalciferol supplementation. The blood concentration of 25OHD significantly augmented, observing both study arms. The difference between the two study arms was not statistically significant (p = 0.320), which indicates that both investigated “regimes” were efficacious to a similar extent (Figure 3).
The level of PTH increased due to the supplementation in vitamin D group (p = 0.314), whereas it decreased when the study participants were being supplemented with omega-3 fatty acid 1000 mg + cholecalciferol 1000 IU (p = 0.150). Albeit the discussed changes were not statistically meaningful. The difference between the two study arms was statistically significant (p = 0.011).
After 3 months of treatment we found no changes in IL-17 level in EBC in both study arms as well as in FEV1, ALT, and lipid balance values in our patients (Table 2).
|
Analyzed trait |
Study arm* |
Phase of the study |
Statistical parameter |
Level of statistical significance (p-value) |
||||
|
M** |
SD*** |
95% CI**** |
Min. – max. |
|||||
|
Age |
18,04 |
4,18 |
16,42-19,66 |
12-25 |
a |
b |
||
|
Total cholesterol (mg/dL) |
1 |
Before treatment |
121,45 |
25,54 |
109,50-133,40 |
89-183 |
= 0.275 |
= 0.061 |
|
After treatment |
127,20 |
30,41 |
112,97-141,43 |
84-199 |
||||
|
2 |
Before treatment |
121,45 |
25,54 |
109,50-133,40 |
89-183 |
= 0.969 |
||
|
After treatment |
120,55 |
31,17 |
105,96-135,14 |
75-186 |
||||
|
HDL-cholesterol (mg/dL) |
1 |
Before treatment |
44,10 |
11,04 |
38,93-49,26 |
22,00-72,00 |
= 0.101 |
= 0.017 |
|
After treatment |
47,56 |
12,15 |
41,87-53,24 |
27,00-72,00 |
||||
|
2 |
Before treatment |
44,10 |
11,04 |
38,93-49,26 |
22,00-72,00 |
= 0.906 |
||
|
After treatment |
44,12 |
11,13 |
38,90-49,32 |
17,70-66,00 |
||||
|
LDL-cholesterol (mg/dL) |
1 |
Before treatment |
56,06 |
22,00 |
45,77-66,36 |
26,80-98,30 |
= 0.247 |
= 0.096 |
|
After treatment |
59,84 |
24,42 |
48,41-71,27 |
26,00-124,00 |
||||
|
2 |
Before treatment |
56,06 |
22,00 |
45,77-66,36 |
26,80-98,30 |
= 0.971 |
||
|
After treatment |
54,66 |
22,07 |
44,33-64,99 |
26,00-104,80 |
||||
|
Triglycerides |
1 |
Before treatment |
122,30 |
69,34 |
89,85-154,75 |
46-270 |
= 0.149 |
= 0.435 |
|
After treatment |
99,00 |
52,82 |
74,28-123,72 |
43-241 |
||||
|
2 |
Before treatment |
122,30 |
69,34 |
89,85-154,75 |
46-270 |
= 0.420 |
||
|
After treatment |
108,30 |
54,52 |
82,78-133,82 |
32-226 |
||||
|
Alat (U/L) |
1 |
Before treatment |
20,90 |
9,03 |
16,68-25,12 |
8-42 |
= 0.954 |
= 0.546 |
|
After treatment |
20,50 |
8,60 |
16,47-24,52 |
9-38 |
||||
|
2 |
Before treatment |
20,90 |
9,03 |
16,68-25,12 |
8-42 |
= 0.689 |
||
|
After treatment |
21,55 |
9,69 |
17,02-26,08 |
10-45 |
||||
|
Phosphor |
1 |
Before treatment |
1,48 |
0,27 |
1,35-1,61 |
1,03-1,93 |
= 0.314 |
= 0.104 |
|
After treatment |
1,53 |
0,23 |
1,42-1,64 |
1,05-1,87 |
||||
|
2 |
Before treatment |
1,48 |
0,27 |
1,35-1,61 |
1,03-1,93 |
= 0.574 |
||
|
After treatment |
1,44 |
0,18 |
1,35-1,54 |
1,21-1,82 |
||||
|
Calcium |
1 |
Before treatment |
3,50 |
2,60 |
2,28-4,72 |
2,22-9,80 |
= 0.386 |
= 0.088 |
|
After treatment |
2,81 |
1,62 |
2,03-3,59 |
2,22-9,48 |
||||
|
2 |
Before treatment |
3,50 |
2,60 |
2,28-4,72 |
2,22-9,80 |
= 0.790 |
||
|
After treatment |
3,62 |
2,58 |
2,41-4,83 |
2,15-9,80 |
||||
|
Parathormone |
1 |
Before treatment |
35,64 |
17,61 |
27,40-43,88 |
16,99-89,12 |
= 0.314 |
= 0.011 |
|
After treatment |
38,30 |
13,62 |
31,92-44,67 |
17,56-72,73 |
||||
|
2 |
Before treatment |
35,64 |
17,61 |
27,40-43,88 |
16,99-89,12 |
= 0.150 |
||
|
After treatment |
32,21 |
15,56 |
25,31-39,10 |
15,30-89,12 |
||||
|
Urinary |
1 |
Before treatment |
0,26 |
0,19 |
0,17-0,36 |
0,03-0,80 |
= 0.892 |
= 0.005 |
|
After treatment |
0,25 |
0,14 |
0,19-0,32 |
0,02-0,50 |
||||
|
2 |
Before treatment |
0,26 |
0,19 |
0,17-0,34 |
0,03-0,80 |
= 0.027 |
||
|
After treatment |
0,18 |
0,13 |
0,12-0,23 |
0,02-0,50 |
||||
|
25-OH D |
1 |
Before treatment |
24,64 |
8,31 |
21,04-28,23 |
5,20-40,00 |
< 0.001 |
= 0.645 |
|
After treatment |
29,41 |
7,78 |
26,04-32,77 |
15,60-49,00 |
||||
|
2 |
Before treatment |
24,31 |
8,28 |
20,72-27,89 |
5,20-40,00 |
< 0.001 |
||
|
After treatment |
30,67 |
8,01 |
27,20-34,13 |
16,60-49,00 |
||||
|
FEV1 |
1 |
Before treatment |
89,42 |
21,02 |
79,29-99,55 |
52-123 |
= 0.083 |
= 0.067 |
|
After treatment |
92,75 |
21,96 |
82,47-103,03 |
44-125 |
||||
|
2 |
Before treatment |
89,42 |
21,02 |
79,29-99,55 |
52-123 |
= 0.407 |
||
|
After treatment |
91,57 |
23,12 |
80,75-102,39 |
53-125 |
||||
|
IL-17A |
1 |
Before treatment |
0,48 |
0,51 |
0,23-0,72 |
0,05-1,91 |
= 0.682 |
= 0.001 |
|
After treatment |
0,45 |
0,39 |
0,26-0,63 |
0,05-1,91 |
||||
|
2 |
Before treatment |
0,48 |
0,51 |
0,23-0,72 |
0,05-1,91 |
= 0.066 |
||
|
After treatment |
0,29 |
0,19 |
0,20-0,38 |
0,07-0,71 |
||||
|
IL-23 |
1 |
Before treatment |
9,39 |
6,40 |
6,39-12,38 |
2,12-30,72 |
= 0.630 |
=0.001 |
|
After treatment |
8,90 |
4,07 |
7,00-10,81 |
3,04-20,72 |
||||
|
2 |
Before treatment |
9,39 |
6,40 |
6,39-12,38 |
2,12-30,72 |
= 0.023 |
||
|
After treatment |
7,33 |
3,88 |
5,51-9,15 |
1,03-18,20 |
||||
Table 2: Descriptive statistics for analyzed measurable traits in the studied patients by study arm and phase of the study
(* Study Arm No. 1: 25OHD, 1000 IU, administered during the three-month treatment period; Study Arm No. 2: Omega-3 fatty acid 1000 mg + 25OHD, 1000 IU, administered during the three-month treatment period. ** M – mean; *** SD – standard deviation; **** CI – confidence interval.
Mixed-effects regression models with robust standard errors, due to the small sample size, were performed: a – considering repeated measurements in the two separate studies;
b – considering the above-mentioned repeated measurements, along with differences between the two studies and intra-subject correlation, on grounds of the cross-over study design.
All statistical calculations were controlled for the studied patients’ age and sex.)
Discussion
The persistent inflammatory response in the airways connected with T helper type 2 and Th17 T cells activation, which stimulates IL-17A/IL-23 axis to IL-23 production is believed to play a central role in the progression of lung damage in CF patient infected with P. aeruginosa [16,18-20, 23]. In this pilot study, we observed that administering 1000 mg of omega-3 per day together with 1000 IU of cholecalciferol per day significantly reduced IL-23 in EBC in CF patients with chronic P. aeruginosa infection. Furthermore, the level of IL-23 in the exhaled air did not statistically significantly change due to the supplementation with cholecalciferol alone (1000 IU per day). Additionally, the blood concentration of 25OHD significantly increased in both study groups. In vitamin D + omega-3 group the beneficial changes in PTH level were noticed. Both treatments had no other effect on calcium-phosphorus metabolism as well as serum lipid balance.
The dominant neutrophilic type of inflammation in the pathophysiology of CF lung inflammation has been established [24,25]. Other study have previously shown that airway IL-17 and IL-23 are connected with infection status and antigen presenting cells (such as dendritic cells) are activated by bacterial antigens in the bronchial mucus layer to produce IL-23 [3,5]. Available studies reported elevated IL-23 protein levels in bronchoalveolar lavage of CF patients during exacerbation and its decreased after antibiotic therapy [26]. Others have shown that elevated levels of IL-23 in sputum are observed also in clinically stable CF patients with chronic infection with P. aeruginosa CF [1].
Long-chain PUFA - linoleic acid (omega-6) and alpha-linolenic (omega-3) are essential for normal growth and function in humans. Essential fatty acids deficiency was reported in more than 80% of patients suffered from CF due to defects in fatty acid metabolism and fat malabsorption [9-11]. This imbalance of n-3 and n-6 in cell membranes causes overall suppression of the anti-inflammatory pathway mediated by omega-3 metabolites and exacerbation of the inflammatory pathway mediated by metabolites of arachidonic acid, which is the most common n-6 PUFA [27]. PUFA disorders in patients with CF may contribute to the inflammatory process and correlated with disease severity. It may lead to progressive tissue damage. Clinical trials performed on CF patients have revealed that oral supplementation with omega-3 modifies fatty acid profiles in both plasma and cell membrane levels [11] and down-regulate the production of inflammatory mediators and thus improve clinical outcomes. The results of present study also confirm the findings of previous reports. We observed decreased of IL-23 level in EBC in patients supplemented with omega-3, what is more the therapy was well tolerated by CF patients, we did not noticed modification in the liver enzyme, total cholesterol and LDL cholesterol. The anti-inflammatory effect of omega-3 on the airways has been demonstrated previously [7-9,11,12,15] and PUFA supplementation in CF patients, often for short time, was shown to reduce inflammatory markers [28,29].
The other date demonstrated that omega-3 display a positive relationship as wall as with lower inflammatory status and better lung function [13,30]. Whereas we did not observe modification in the values of FEV1.
In this pilot study, selective inhibition of IL-23 level in EBC, an inflammatory marker in CF airway, might represent a high-priority target for CF therapy. The major limitation to our pilot study is a small sample size. Larger CF population and longer study will be required to confirm improvement of pulmonary inflammation and to determine the influence of disease severity, dosage and duration of treatment.
It has been known that vitamin D has anti-inflammatory effect. It inhibits proliferation, maturation and differentiation of dendritic cells, which leads to decrease activation and proliferation of T cells [16-18]. T cells, in turn, produce interleukin IL-17 which stimulate IL-23 production [31]. Surprisingly, we did not observe statistically significant changes of IL-23 and IL-17 due to the supplementation with cholecalciferol alone (1000 IU per day). This is probably related to low dose of administered cholecalciferol what is the limitation of our study, as we were using earlier valid guidelines [21]. In this pilot trial, despite administering only low dose of vitamin D the blood concentration of 25OHD significantly augmented observing both study arms. What is more in vitamin D + omega-3 group this increasing was bigger and it was reflected in favorable changes in parathyroid glands and lowering of the PTH concentration in serum. Our results generate a hypothesis that taken together (with omega-3) cholecalciferol exerts some immunomodulatory effect and is more efficient at improving vitamin D status, which would speak in favor of using a combination supplement. Larger studies are needed to test this hypothesis. This study has generated novel observations that need to be follow- up in larger long-term placebo-controlled studies.
In summary, this trial found that regular omega-3 supplementation with cholecalciferol may provide some benefits for people with CF and plays an anti-inflammatory effect. We demonstrated the ability of such supplementation to modulate the immunity and inflammatory response in airway CF patients with chronic P. aeruginosa infection reflected as reduction of the level of IL-23 in EBC.
Financial information
The study was performed in the Department of Pediatrics and Allergy, Medical University of Lodz, Copernicus Memorial Hospital in Lodz, Poland under the project supported by the grant No. 503/2-056-01/503-21-001-18 from Medical University of Lodz.
Conflict of interest
All authors state no conflict of interests
References
- Decraene A, Willems-Widyastuti A, Kasran A, De Boeck K, Bullens DM, Dupont LJ. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable Cystic Fibrosis patients. Respir Res 11 (2010): 177.
- Bullens DM, Decraene A, Seys S, Dupont LJ. IL-17A in human respiratory diseases: innate or adaptive immunity? Clinical implications. Clin Dev Immunol 2013 (2013): 840315.
- Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK. Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection. Infect Immun 80 (2012): 398-409.
- Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK. Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection. Infect Immun 80 (2012): 398-409.
- Dubin PJ, McAllister F, Kolls JK. Is cystic fibrosis a TH17 disease? Inflamm Res 56 (2007): 221–227.
- McAllister F. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony- stimulating factor in bronchial epithelium: implications for airway in- flammation in cystic fibrosis. J Immunol 175 (2005): 404–412.
- Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: Cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 57 (2002): 596–601.
- Portal C, Gouyer V, LeÂonard R, Husson M-O, Gottrand F, Desseyn J-L. Long-termdietary (n-3) polyunsaturated fatty acids show benefits to the lungs of Cftr F508del mice. PLoS ONE 13 (2018): 0197808.
- AldaÂmiz-EchevarrõÂa L, Prieto JA, Andrade F, Elorz J, Sojo A, Lage S, et al. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatr Res 66 (2009): 585-9.
- Wouthuyzen-Bakker M, Bodewes FA, Verkade HJ. Persistent fat malabsorption in cystic fibrosis; lessons from patients and mice. J Cyst Fibros 10 (2011): 150-158.
- Hanssens L, Thiébaut I, Lefèvre N, Malfroot, A, Knoop C et all. The clinical benefits of long-term supplementation with omega-3 fatty acids in cystic fibrosis patients—A pilot study. Prostaglandins Leukot. Essent. Fatty Acids 108 (2016): 45–50.
- Leggieri E, De Biase RV, Savi D, Zullo S, Halili I, et all. Clinical effects of diet supplementation with DHA in pediatric patients suffering from cystic fibrosis. Minerva Pediatr 65 (2013): 389–398.
- Olveira, G, Olveira C, Acosta E, Espíldora F, Garrido-Sánchez L, et all. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch Bronconeumol 46 (2010): 70–77.
- Cantin AM, Bilodeau G, Larivée P, Richter, M.V. Plasma biomarkers and cystic fibrosis lung disease. Clin Invest Med 35 (2012): E173-E181.
- Oliver C, Watson H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst Rev 5 (2016): CD002201.
- McNally P, Coughlan C, Bergsson G, Doyle M, Taggart C, Adorini L, Uskokovic MR, et al. Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis. J Cyst Fibros 10 (2011): 428-434.
- Grzelak T, Miko?ajczyk K. Pleiotropic effect of vitamin D in cystic fibrosis. Adv Respir Med 2018.
- Wani WA, Nazir M, Bhat JI, Malik EU, Ahmad QI, Charoo BA, Ali SW. Vitamin D status correlates with the markers of cystic fibrosis-related pulmonary disease. Pediatr Neonatol 60 (2019): 210-215.
- Moustaki M, Loukou I, Priftis KN, Douros K. Role of vitamin D in cystic fibrosis and non-cystic fibrosis bronchiectasis. Pediatr Neonatol 60 (2019): 210-215.
- Pincikova T, Paquin-Proulx D, Sandberg JK, Flodström-Tullberg M, Hjelte L. Clinical impact of vitamin D treatment in cystic fibrosis: a pilot randomized, controlled trial. Eur J Clin Nutr 71 (2017): 203-205.
- Walkowiak J, Pogorzelski A, Sands D, Skorupa W, Milanowski W, Nowakowska A, et Rules for the diagnosis and treatment of cystic fibrosis / Recommendations of the Polish Society of Cystic Fuibrosis 2009. Standardy Medyczne Pediatria 6 (2009): 352-378.
- Miller MR, Hankinson J, Brusasco V. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26 (2005): 319–338.
- De Rose V. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur Respir J 19 (2002): 333–340.
- Margaroli C, Garratt LW, Horati H, Dittrich AS, Rosenow T, Montgomery ST, Frey DL, et al. Elastase Exocytosis by Airway Neutrophils Associates with Early Lung Damage in Cystic Fibrosis Children. Am J Respir Crit Care Med 199 (2019): 873-881.
- Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: An update. Pediatr Pulmonol 53 (2018): S30-S50.
- McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175 (2005): 404-412.
- Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat 99 (2012): 57-67.
- Coste TC, Armand M, Lebacq J, Lebecque, P, Wallemacq P. et all. An overview of monitoring and supplementation of omega 3 fatty acids in cystic fibrosis. Clin Biochem 40 (2007): 511–520.
- De Vizia B, Raia V, Spano C, Pavlidis C, Coruzzo A, et. all. Effect of an 8-month treatment with omega-3 fatty acids (eicosapentaenoic and docosahexaenoic) in patients with cystic fibrosis. J Parenter Enteral Nutr 27 (2003): 52-57.
- Morin C, Cantin AM, Vezina FA, Fortin S. The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients Mar Drugs 16 (2018): pii: E184.
- Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 7 (2015): 3011–3021.