The Correlation Between the Serum Serglycin Level and the Burden of Coronary Artery Disease in Subjects with Non-St-Elevation Myocardial Infarction
Article Information
Zeynep ULUTAS1*, Hasan Ata BOLAYIR2
1Elazig City Hospital Department of Cardiology, Elazig, Turkey
2Sivas Numune State Hospital, Department of Cardiology, Sivas, Turkey
*Corresponding author: Zeynep ULUTAS, Elazig City Hospital Department of Cardiology, Elazig, Turkey
Received: 25 February 2020; Accepted: 10 March 2020; Published: 22 April 2020
Citation: Zeynep ULUTAS, Hasan Ata BOLAYIR. The Correlation Between the Serum Serglycin Level and the Burden of Coronary Artery Disease in Subjects with Non-St-Elevation Myocardial Infarction. Cardiology and Cardiovascular Medicine 4 (2020): 118-129.
Share at FacebookAbstract
Background: Despite the fact that serglycin takes a crucial part in the inflammatory status, the correlation between the coronary artery disease (CAD) severity in subjects having non-ST segment elevation myocardial infarction (NSTEMI) and serglycin is still unknown.
Methods: A total of 129 participants, including 90 NSTEMI subjects and 39 healthy controls, were included in the present research prospectively. The patient group was separated into two groups as subjects with a high SYNTAX score, which was equal to or higher than 32 (40 subjects), and subjects with a low SYNTAX score, which was lower than 32 (50 subjects). The enzyme-linked immunosorbent assay test was utilized to measure serglycin level from the collected blood serum samples.
Results: A considerably higher serum serglycin level (17.2±3.4 ng/mL) was determined in NSTEMI subjects having the high SYNTAX score in comparison with NSTEMI subjects having the low SYNTAX score (11.4±2.1 ng/mL) and the control group (7.9±2.7 ng/mL). The serglycin cut-off value to predict the high SYNTAX score as a result of receiver-operating characteristic curve analysis was identified as 14.8 ng/mL, with a sensitivity of 67% and a specificity of 58%. Serglycin independently predicted the high SYNTAX score with an odds ratio of 0.999, a confidence interval of 95% (0.998–1.000), and p=0.007.
Conclusion: Serglycin may represent a possible blood sample value in order to predict the CAD severity in NSTEMI subjects.
Keywords
Acute coronary syndrome; Serglycin; SYNTAX score
Acute coronary syndrome articles, Serglycin articles, SYNTAX score articles
Article Details
Introduction
Studies conducted recently have demonstrated the vital role of inflammation in coronary artery disease (CAD) and other atherosclerosis manifestations. Early atherosclerotic lesions are dominated by immune cells, the progression of the lesions is accelerated by their effector molecules, and the activated inflammation may cause acute coronary syndrome. Atherosclerosis, which is the primary reason for CAD, represents an inflammatory disease with an interaction between immune mechanisms and metabolic risk factors for the purpose of initiating, propagating, and activating lesions in the arterial tree [1]. The SYNTAX score represents an angiographic scoring system, defining the CAD complexity and grade [2,3]. Various studies have demonstrated that poor outcomes are observed in patients having a comparatively high SYNTAX score and that the score independently predicts major advanced cardiovascular outcomes (MACE) in order to perform a percutaneous coronary intervention (PCI) [4,5].
Serglycin is correlated with intracellular proteoglycan and hematopoietic cells. Previous research has shown that serglycin is also synthesized by a number of non-hematopoietic cell types. After the synthesis process by inflammatory cells, serglycin is stored in granules to enter into reaction with mediators, including chemokines, cytokines, proteases, and growth factors [6,7]. Furthermore, serglycin may take part in the occurrence of atheromatous lesions and atherosclerosis. Lipopolysaccharides in macrophages, interleukin 1-b in smooth muscle cells, and tumor necrosis factor (TNF) in endothelial cells have been demonstrated to up-regulate serglycin [8].
The present research aimed to examine the correlation between serglycin levels and the CAD severity in subjects having non-ST segment elevation myocardial infarction (NSTEMI). To the best of our knowledge, this research represents the first one in the literature on NSTEMI subjects.
Methods
Study population
Ninety subjects, who had undergone coronary angiography (CA) due to NSTEMI at XXX Hospital or XXX Hospital, and 39 subjects having normal coronary artery (NCA) were included in the research between November 2017 and May 2018. Subjects, who had undergone coronary artery bypass grafting (CABG) before, were not included in the research due to the appropriateness of the SYNTAX score only for subjects having native coronary artery lesions. Having active infection, severe renal or hepatic dysfunction, chronic inflammatory diseases, and malignancy represents the other exclusion criterion. NSTEMI was defined as presenting with acute chest pain or overwhelming shortness of breath, without ST-elevation but with classical increase and decrease in minimum 1 cardiac enzyme, such as troponin or creatine kinase-myocardial band. During the outpatient clinic visit, subjects having NCA were sent to coronary angiography because of a positive result of stress test (myocardial perfusion scintigraphy test or exercise stress test), or highly clinically suspected CAD (such as subjects having the strong family history of CAD or early death having or not having the related risk factors, and subjects having the unexplained chest pain following the scrupulous laboratory and clinical investigation in case of the suspicion of ischemic heart disease). NCA is described as the absence of observable disease or luminal irregularity (below 50%), as can be evaluated visually on CA. Approval was acquired from the Local Ethics Committee for the research, and written and informed consent was received from all the subjects.
Coronary angiography
Coronary angiography was carried out by employing the Judkins technique (Siemens Axiom Artis Zee 2011; Siemens Healthcare, Erlangen, Germany) via the femoral or the radial artery. Minimum two various plane images displayed every coronary artery. Standard techniques were employed to perform PCI procedures. In accordance with baseline CA, two competent interventional cardiologists, not aware of the laboratory or clinical outcomes of the subjects, calculated the SYNTAX score for all the subjects. Calculation of the SYNTAX score was performed for every coronary lesion having diameter stenosis higher than 50% in a vessel larger than 1.5 mm on the basis of the SYNTAX score calculator 2.1 (www. syntaxscore.com). Subjects with NSTEMI were separated into two groups: subjects having the high SYNTAX score (≥32) (40 subjects) and subjects having the low SYNTAX score (<32) (50 subjects).
Reproducibility
For the purpose of defining intra-observer variability, fifteen subjects were selected from the study group in a random manner. Measurements were taken in three repetitions under identical basal conditions. The coefficient of variation between the measurements was utilized with the aim of assessing the reproducibility of the SYNTAX score by CA. As for the SYNTAX score, intra-observer variability was determined to be 5.5%.
Laboratory measurements
Peripheral venous blood specimens were collected from the antecubital vein at admission. Measurements of the baseline creatinine concentration, platelet count, hemoglobin level, and white blood cell (WBC) count were taken. Measurements of high-sensitivity C-reactive protein (hs-CRP), lipid profile, and some biochemical parameters were taken on the first morning following admission by employing standard methods. Furthermore, records of the peak and baseline levels of creatinine kinase-myocardial band and troponin level were taken. An enzyme-linked immunosorbent assay that had been previously described was used to determine serglycin and TNF-alpha levels in plasma [9].
Statistical analysis
SPSS version 20.0 statistics package (SPSS Inc., Chicago, II, USA) was utilized to perform data analysis. Continuous variables were expressed as mean±SD, while categorical variables were presented as percentage and count. Variables that exhibited a normal distribution were compared by Student’s t-test, whereas variables not normally distributed in case of the presence of two groups were compared by the Mann-Whitney U test. Variables that exhibited a normal distribution between three groups were compared by the one-way analysis of variance. Tukey’s test was utilized in order to conduct a posthoc analysis. The comparison of the categorical variables was performed as a result of conducting the χ2 test or Fisher’s exact test appropriately. The power of the correlation between continuous variables was evaluated by Pearson’s correlation coefficients, while Spearman’s correlation analysis was conducted for categorical and non-continuous variables. The receiver-operating characteristic (ROC) curve analysis was utilized for the purpose of determining the optimal cut-off value of serglycin to predict the high SYNTAX score. The SYNTAX score equal to or higher than 32 and variables, such as hs-CRP, serglycin, TNF-alpha, left ventricle ejection fraction, WBC, dyslipidemia, high-density lipoprotein [HDL] cholesterol, constituted univariate and multiple models. While conducting all the analyses, the p-value below 0.05 was considered to be statistically significant.
Results
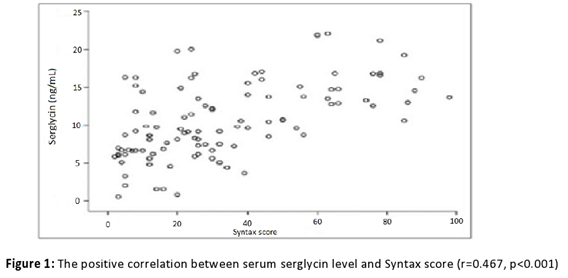
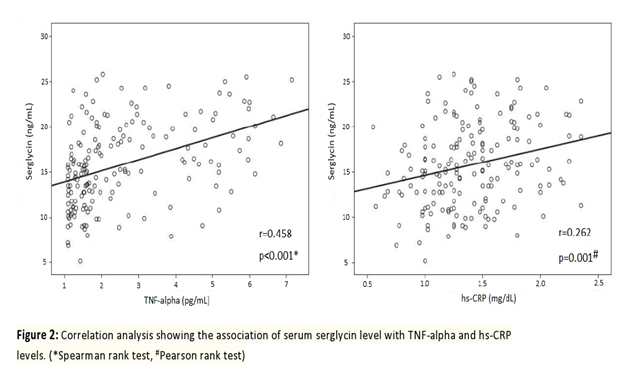
Table 1 contains information on the baseline clinical and angiographic features of the research population. Information on age, sex, body mass index, diabetes mellitus (DM), systolic and diastolic blood pressure, and smoking status were found to be statistically insignificant between the groups. Compared to the low SYNTAX group subjects (with the SYNTAX score below 32), the high SYNTAX group subjects (with the SYNTAX score equal to or higher than 32) had considerably more previous myocardial infarction (MI), chronic total occlusion, multi-vessel coronary involvement, collateral vessels, and CABG procedure, and fewer stent implantations (p=0.015, p<0.001, p<0.001, p<0.001, p=0.018, and p<0.001, respectively). Table 2 contains information on the biochemical, hematological, and serum serglycin, hs-CRP, and TNF-alpha measurements taken from the study population. Except for the WBC count and HDL cholesterol, the groups did not differ statistically significantly between each other (p=0.003, p=0.001). In the current study, significantly higher serum serglycin, hs-CRP, and TNF-alpha levels (17.2±3.4 ng/mL, 2.0±0.5 mg/dL, 6.02±0.7 pg/mL, respectively) were determined in subjects with NSTEMI having a high SYNTAX score (score ≥32) in comparison with NSTEMI subjects having a low SYNTAX score (score <32) (11.4±2.1 ng/mL, 1.2±0.3 mg/dL, 4.98±0.4 pg/mL, respectively), and the control group (7.9±2.7 ng/mL, 0.8±0.3 mg/dL, 3.27±0.6 pg/mL, respectively). With regard to serglycin, hs-CRP, and TNF-alpha levels, all the groups differed statistically significantly between each other (p<0.001, p=0.02, p<0.001). Moreover, a statistically significant difference was revealed between subjects having the high SYNTAX score and subjects having the low SYNTAX score (p=0.003, p=0.03, p=0.004), the high score group and the control group (p=0.001, p=0.01, p=0.002), and the low score group and the control group (p=0.009, p=0.04, p=0.008) with regard to serglycin, hs-CRP, and TNF-alpha levels. As seen from Figure 1, serum serglycin level and SYNTAX score were positively associated with each other (r=-0.467, p<0.001). On the contrary, a positive relationship was determined between plasma serglycin level and TNF-alpha level, while a positive correlation was found between plasma serglycin level and hs-CRP level in the current study population (Figure 2). As can be observed from Table 3, univariate and multiple linear regression analyses were carried out with the aim of predicting the SYNTAX score ≥32. In the univariate regression analysis, there was a correlation between hs-CRP (odds ratio [OR]=1.874; 95% confidence interval [CI]: 1.179–2.979; p=0.008), TNF-alpha (OR=0.999; 95% CI: 0.998–1.000; p=0.002), serglycin (OR=0.999; 95% CI: 0.998–1.000; p=0.001), and the high SYNTAX score. Following the multiple linear regression analysis, higher serum levels of serglycin and TNF-alpha independently predicted the high SYNTAX score in subjects having NSTEMI (OR=0.999; 95% CI: 0.998–1.000; p=0.007 and OR=0.999; 95% CI: 0.998–1.000; p=0.009).
|
Variable |
NSTEMI SYNTAX score ≥32 (n=40) |
NSTEMI SYNTAX score<32 (n=50) |
NCA (n=39) |
P* |
P§ |
Pβ |
Pα |
|
Age, years |
63.74±9.49 |
61.29±6.88 |
56.90±8.64 |
0.287 |
|||
|
BMI, kg/m2 |
31.03±5.67 |
30.66±4.99 |
29.80±6.13 |
0.888 |
|||
|
Female, n (%) |
12 (30.0%) |
13 (26%) |
10 (25.6%) |
0.782 |
|||
|
Systolicbloodpressure, mm Hg |
136.48±7.35 |
135.66±10.87 |
136.79±5.75 |
0.843 |
|||
|
Diastolicbloodpressure, mm Hg |
84.65±6.36 |
84.60±9.53 |
85.00±4.51 |
0.973 |
|||
|
Diabetesmellitus, n (%) |
13 (32.5%) |
14 (28%) |
9 (23.1%) |
0.218 |
|||
|
Currentsmoking, n (%) |
20 (50%) |
29 (58%) |
18 (46.1%) |
0.09 |
|||
|
Dyslipidemia, n (%) |
26 (65%) |
27 (54%) |
8 (20.5%) |
0.041 |
|||
|
Previous MI, n (%) |
14 (35%) |
11 (22%) |
0 (0%) |
0.015 |
|||
|
Multi-vesseldisease, n (%) |
34 (85%) |
30 (60%) |
0 (0%) |
<0.001 |
|||
|
LVEF, % |
53.92±10.86 |
51.82±8.89 |
58.04±6.85 |
0.035 |
0.619 |
0.24 |
0.026 |
|
Chronic total occlusion, n (%) |
15 (37.5%) |
8 (16%) |
0 (0%) |
<0.001 |
|||
|
Stentimplantation, n (%) |
15 (37.5%) |
30 (60%) |
0 (0%) |
<0.001 |
|||
|
Decisionfor CABG, n (%) |
15 (37.5%) |
5 (10%) |
0 (0%) |
<0.001 |
<0.001 |
<0.001 |
<0.001 |
|
Collateralvessel, n (%) |
10 (25%) |
5 (10%) |
0 (0%) |
0.018 |
|||
|
SYNTAX score |
38.77±4.86 |
16.18±7.13 |
0±0 |
<0.001 |
BMI - body mass index; CABG – coronary artery bypass grafting; LVEF – left ventricular ejection fraction; MI – myocardial infarction; NCA - normal coronary artery; NSTEMI - non-ST segment elevation myocardial infarction.
P*- P value between all groups;
P§- P value between SYNTAX score<32 and SYNTAX score ≥32;
Pβ- P value between SYNTAX score ≥32 and controls;
Pα- P value between SYNTAX score<32 and controls
Table 1: Baseline clinical and angiographic characteristics of the study population
|
Variable |
NSTEMI SYNTAX score ≥32 (n=40) |
NSTEMI SYNTAX score<32 (n=50) |
NCA (n=39) |
P* |
P§ |
Pβ |
Pα |
|
WBC count, x109/L |
9.20±2.25 |
8.81±2.32 |
7.52±1.80 |
0.003 |
0.668 |
0.003 |
0.064 |
|
Plateletcount, x109/L |
258.91±70.66 |
268.83±74.45 |
251.46±66.39 |
0.787 |
|||
|
Hemoglobin, g/dL |
13.79±1.63 |
13.82±1.76 |
13.37±1.28 |
0.494 |
|||
|
Serum glucose, mg/dL |
151.92±72.33 |
131.08±47.76 |
133.75±45.02 |
0.234 |
|||
|
Creatinine, mg/dL |
0.99±0.26 |
1.12±0.78 |
0.91±0.113 |
0.234 |
|||
|
Peak CK-MB, U/L |
46.50 (15–229) |
38.86 (0–131) |
– |
0.588 |
|||
|
Peaktroponin-T, ng/mL |
4.51 (0–34.7) |
5.27 (0.09–31.00) |
– |
0.884 |
|||
|
Total cholesterol, mg/dL |
199.71±49.03 |
188.44±55.27 |
176.20±45.28 |
0.237 |
|||
|
HDL-cholesterol, mg/dL |
47.65±12.56 |
46.18±10.11 |
58.54±16.91 |
0.001 |
0.876 |
0.006 |
0.001 |
|
LDL-cholesterol, mg/dL |
118.96±42.78 |
112.18±45.09 |
98.75±37.77 |
0.215 |
|||
|
Triglyceride, mg/dL |
174.84±113.24 |
150.76±91.30 |
147.13±79.68 |
0.479 |
|||
|
hs-CRP, mg/dL |
2.0±0.5 |
1.2±0.3 |
0.8±0.3 |
0.02 |
0.03 |
0.01 |
0.04 |
|
TNF-alpha, pg/mL |
6.02±0.7 |
4.98±0.4 |
3.27±0.6 |
<0.001 |
0.004 |
0.002 |
0.008 |
|
Serglycin, ng/mL |
17.2±3.4 |
11.4±2.1 |
7.9±2.7 |
<0.001 |
0.003 |
0.001 |
0.009 |
CK-MB – creatine kinase-myocardial band; HDL – high density lypoprotein; hs-CRP – high sensitivity C-reactive protein; LDL – low density lypoprotein; NCA - normal coronary artery; NSTEMI - Non ST-segment elevation myocardial infarction; WBC – white blood cell, TNF – tumour necrosis factor.
P*- P value between all groups;
P§- P value between SYNTAX score<32 and SYNTAX score ≥32;
Pβ- P value between SYNTAX score ≥32 and controls;
Pα- P value between SYNTAX score<32 and controls
Table 2: Biochemical and hematological measurements of the study patients
|
Univariable |
Multivariable |
|||
|
Variables |
OR (95% CI) |
P |
OR (95% CI) |
P |
|
White bloodcell |
1.874 (1.179–2.979) |
0.008 |
1.652 (0.810–3.371) |
0.67 |
|
Serglycin |
0.999 (0.998–1.000) |
0.001 |
0.999 (0.998–1.000) |
0.007 |
|
Leftventricle EF |
1.023 (0.972–1.077) |
0.386 |
– |
– |
|
Dyslipidemia |
0.926 (0.383–2.244) |
0.866 |
– |
– |
|
hs-CRP |
1.874 (1.179–2.979) |
0.008 |
1.652 (0.810-3.371) |
0.67 |
|
TNF-alpha |
0.999 (0.998–1.000) |
0.002 |
0.999 (0.998–1.000) |
0.009 |
|
HDL cholesterol |
1.012 (0.971–1.055) |
0.571 |
– |
– |
CI – confidence interval; OR – odds ratio; EF – ejection fraction; hs-CRP – high sensitive C-reactive protein; HDL – high density lipoprotein; TNF – tumour necrosis factor.
Table 3: Univariate and multiple linear regression analysis showing the predictors for SYNTAX ≥32 score
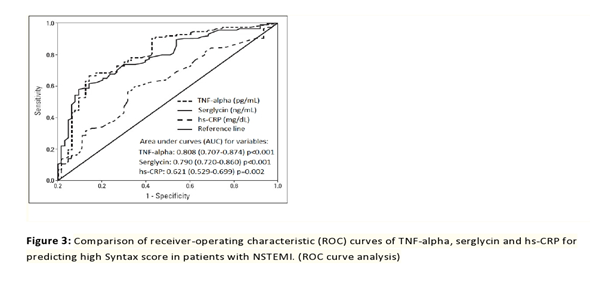
The serglycin cut-off value at admission in order to predict the high SYNTAX score in all the subjects included in the research population on the basis of the receiver operating characteristic curve analysis was 14.8 ng/mL, with a sensitivity of 67% and a specificity of 58% (with an area under the curve of 0.790; 95% CI: 0.720–0.860; p<0.001). Furthermore, serglycin, TNF-alpha, and hs-CRP have comparable diagnostic values for predicting the high SYNTAX score in subjects with NSTEMI (Figure 3).
Discussion
This study showed that NSTEMI patients had a considerably higher serum serglycin level compared to NCA patients. Furthermore, patients having a high SYNTAX score had a higher serum serglycin level in comparison with patients having a low SYNTAX score. In addition, the increased serum serglycin level and SYNTAX score were positively associated with each other. The high serum serglycin level independently predicted the high SYNTAX score in NSTEMI subjects.
NSTEMI commonly presents in subjects having acute coronary syndromes. Despite a lower in-hospital mortality rate in subjects with NSTEMI compared to subjects having ST-segment elevation, there is a similarity between them in terms of a 6-month mortality rate. Furthermore, NSTEMI patients have a two-fold higher 4-year mortality rate compared to patients with ST-segment MI [10-12]. Success has been achieved with invasive procedures and intensive medical treatment in reducing the mortality and morbidity in NSTEMI [11]. Nevertheless, the CAD severity in coronary angiography represents the most significant determinant in finding the most appropriate treatment strategy. The SYNTAX score represents a visual angiographic score, which shows the complexity of CAD by considering the lesion number and their functional and anatomic components, such as location, the existence of total occlusions, bifurcations, tortuosity, collaterals, calcification, and thrombus. The SYNTAX score has been shown to play a significant role in making decisions on the most appropriate revascularization strategy, in other words, CABG or PCI, in subjects having CAD. High SYNTAX scores point at a more complex disease and are associated with a therapeutic challenge. An increase in the major adverse cardiac or cerebrovascular events rates is determined in subjects having a high SYNTAX score [13-15]. In this study, subjects with a high SYNTAX score, which is equal to or higher than 32, have more chronic total occlusion, the decision on performing CABG, and collateral vessels in comparison with subjects having a low to moderate SYNTAX score, which is lower than 32.
Serglycin represents a dominant intracellular proteoglycan expressed by immune cells, in which its interaction with many inflammatory mediators, including chemokines, proteases, growth factors, and cytokines, occurs [6,7]. In a study conducted recently, serglycin has been determined to be one of the proteins most plentifully expressed in the adiposities of epicardial adipose tissue in subjects having CAD. Furthermore, the above-mentioned study demonstrated that TNF-alpha induces serglycin secretion in adipocytes, indicating that TNF-alpha-serglycin interaction can lead to the onset and progress of CAD through adipocyte/macrophage cross-talk [16]. Moreover, circulating serglycin may contribute to the formation of atheromatous lesions [17,18]. Lipopolysaccharides in macrophages [17,18], interleukin-1b in smooth muscle cells [8], and TNF in endothelial cells up-regulate the biosynthesis of serglycin. It is still not clear what cell type and tissue are responsible for circulating serglycin; circulating serglycin, in combination with pro-inflammatory proteins that are secreted from adipose tissue-derived macrophages, may probably play a systemic role in inflammation in adipose tissues. TNF-alpha is an important mediator of inflammation, and it can ensure a potential association between systemic inflammation and visceral fat [19]. It is a known fact that both of them stimulate the secretion of free fatty acids and lipolysis, by taking part in the increased output of hepatic glucose and insulin resistance, impaired adipocyte differentiation, and stimulate inflammation. The release of the above-mentioned factors occurs from the vessel wall in the course of an inflammatory reaction, which consequently promotes the release of acute-phase reactant, hs-CRP [20]. Furthermore, serum levels of TNF-alpha and hs-CRP increase in CAD patients [21]. Kundi et al. [22] determined a considerably higher serglycin level in patients having coronary artery ectasia. On the other hand, Bolayır et al. [23] demonstrated a considerable correlation between the burden of atherosclerosis and serum serglycin level in subjects having stable angina pectoris. In the other study by Bolayır HA. [24], it was demonstrated that subjects having a slow coronary flow, which represents an atherosclerotic condition mediated by inflammation, have considerably increased serglycin values in comparison with subjects having a normal coronary flow.
Coronary artery disease represents an atherosclerotic disease mediated by inflammation. It has been indicated that multiple inflammatory cells and mediators assist in sustaining and amplifying pro-inflammatory signals, which causes the onset and progress of atherosclerosis. Thus, the increased plasma serglycin levels in NSTEMI subjects were thought to originate from the inflammatory status. The positive association between plasma serglycin and TNF-alpha - hsCRP levels determined in this study population supported the above-mentioned situation. Moreover, analyzing the ROC curve in a comprehensive manner showed that the levels of serglycin are capable of estimating a high SYNTAX score in NSTEMI subjects in an independent way. Furthermore, hsCRP, TNF-alpha, and serglycin were shown to have comparable diagnostic values for a high SYNTAX score.
As a result, a considerably higher serum serglycin level was determined in NSTEMI patients. Furthermore, patients having a high SYNTAX score had a higher serum serglycin level in comparison with patients having a low SYNTAX score in the NSTEMI group. Serglycin may take part in the pathogenesis of the atherosclerotic burden in NSTEMI patients.
Study limitations
The first limitation of the present research is that it represents a cross-sectional study having a comparatively small sample size. The second limitation is that serglycin level was not measured following discharge and MACE data were not followed up. Thus, it is necessary to verify the findings of this study in multi-center prospective longitudinal studies having a larger sample size. It is required to take into account the limitations of the current study during the interpretation of the results.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352 (2005): 1685–1695.
- Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1 (2005): 219-27.
- Yang CH, Hsieh MJ, Chen CC, Chang SH, Wang CY, Lee CH, et al. SYNTAX score: an independent predictor of long-term cardiac mortality in patients with acute ST-elevation myocardial infarction. Coron Artery Dis 23 (2012): 445-9.
- Chakrabarti AK, Gibson CM. The SYNTAX score: usefulness, limitations, and future directions. J Invasive Cardiol 23 (2011): 511-2.
- Kundi H, Gök M, Çetin M, Kızıltunç E, Topçuoğlu C, Neşelioğlu S, et al. Association of thiol disulfide homeostasis with slow coronary flow. Scand Cardiovasc J 50 (2016): 213-7.
- Kolset SO, Pejler G. Serglycin: a structural and functional chameleon with wide impact on immune cells. J Immunol 187 (2011): 4927–4933.
- Niemann CU, Abrink M, Pejler G, et al. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 109 (2007): 4478–4486.
- Korpetinou A, Skandalis SS, Labropoulou VT, et al. Serglycin: at the crossroad of inflammation and malignancy. Front Oncol 3 (2014): 327.
- Reine TM, Vuong TT, Jenssen TG, et al. Serglycin secretion is part of the inflammatory response in activated primary human endothelial cells in vitro. Biochim Biophys Acta 1840 (2014): 2498–2505.
- Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, Filippatos G, et al. The second Euro Heart Survey on acute coronary syndromes: Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J 27 (2006): 2285-93.
- Terkelsen CJ, Lassen JF, Norgaard BL, Gerdes JC, Jensen T, Gotzsche LB, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 26 (2005): 18-26.
- Savonitto S, Ardissino D, Granger CB, Morando G, Prando MD, Mafrici A, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA 281 (1999): 707-13.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360 (2009): 961-72.
- Farooq V, Serruys PW, Bourantas C, Vranckx P, Diletti R, Garcia Garcia HM, et al. Incidence and multivariable correlates of long-term mortality in patients treated with surgical or percutaneous revascularization in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial. Eur Heart J 33 (2012): 3105-13.
- Garg S, Serruys PW, Silber S, Wykrzykowska J, van Geuns RJ, Richardt G, et al. The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimuseluting stents: a substudy of the RESOLUTE All Comers Trial. JACC Cardiovasc Interv 4 (2011): 432-41.
- Imoto-Tsubakimoto H, Takahashi T, Ueyama T, et al. Serglycin is a novel adipocytokine highly expressed in epicardial adipose tissue. Biochem Biophys Res Commun 432 (2013): 105–110.
- Zernichow L, Abrink M, Hallgren J, et al. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide. J Biol Chem 281 (2006): 26792–26801.
- Kolseth IB, Reine TM, Vuong TT, et al. Serglycin is part of the secretory repertoire of LPS-activated monocytes. Immun Inflamm Dis 3 (2015): 23–31.
- Koster A, Stenholm S, Alley DE, et al. Health ABC Study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 18 (2010): 2354-2361.
- Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 42 (2009): 1331-1346.
- Corcoran TB, Engel A, Sakamoto H, et al. The effects of propofol on lipid peroxidation and inflammatory response in elective coronary artery bypass grafting. J Cardiothorac Vasc Anesth 18 (2004): 592-604.
- Kundi H, Gök M, Topçuoglu C, et al. Association of serglycin levels with isolated coronary artery ectasia. Kardiol Pol 75 (2017): 990–996.
- Bolayır HA, Kıvrak T, Gunes H, et al. The association between serum serglycin level and coronary artery disease severity in patients with stable angina pectoris. Kardiol Pol 74 (2018): 783-790.
- Bolayır HA. Patients with normal coronary arteries via angiography: The relationship between slow coronary flow and serglycin. Cardiol Cardiovasc Med 2 (2018):111-122.