Task-Related Reorganization of Functional Connectivity in Early Detection of Consciousness in Patients With Severe Brain Injury
Article Information
Zigmantovich A.S1*, Oknina L.B1, Kopachka M.M2, Masherow E.L2, Alexandrova E.V2
1Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Science, Russian Federation
2Burdenko National Medical Research Center of Neurosurgery, Moscow, Russian Federation
*Corresponding author: Alexandra Zigmantovich, Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Science, Russian Federation
Received: 25 October 2019; Accepted: 07 November 2019; Published: 11 November 2019
Citation: Zigmantovich A.S, Oknina L.B, Kopachka M.M, Masherow E.L, Alexandrova E.V. Task-Related Reorganization of Functional Connectivity in Early Detection of Consciousness in Patients With Severe Brain Injury. Archives of Clinical and Biomedical Research 3 (2019): 374-385.
Share at FacebookAbstract
In recent years the number of patients with early postcomatosis long-term states of unconsciousness is increasing in the world. Accurate assessment of current functional state of patients in unconscious states is very important for correct treatment strategy and rehabilitation activities especially in cases when the difference between those states is not clinically obvious.
To investigate features of resting-state wavelet synchrony in unconscious patients with severe brain injury and how TMS-therapy can affect resting state functional connectivity.
We used stimulus-based EEG paradigm to detect changes of functional connectivity after TMS-therapy. Fifteen patients were recruited. They had the diagnosis of minimally conscious state (MCS) (male = 9, female = 2; n = 11), vegetative state/unresponsive wakefulness syndrome (VS/UWS) (male = 4, n = 4).
We analyzed functional connectivity, values of wavelet synchrony in resting state and for responses on stimuli of patients before and after TMS-therapy. Of the 15 patients, eleven (eight MCS, two VS/UWS) had positive clinical dynamics including improvement of moving, emergence of glance fixation and gaze tracking, speech understanding and attempts of speech productions.
The appearance of interhemispheric and diagonal connections is a favorable prognostic sign. TMStherapy makes it possible to include activate functional networks in resting state.
Keywords
Vegetative state; Minimally conscious state; Unresponsive wakefulness syndrome; transcranial magnetic stimulation; Functional connectivity
Article Details
List of the abbreviations
ERP - event-related potentials
MCS - minimally conscious state
TBI - traumatic brain injury
TMS - transcranial magnetic stimulation
UW - unresponsive wakefulness state
VS - vegetative state
Introduction
Posttraumatic unconscious states are currently classified in three categories as follows: comatose state, unresponsive wakefulness state or vegetative state (UW or VS) and minimally conscious state (MCS) [1, 2, 3]. The main issue related to minimally conscious state lacks any direct evidence of patient’s conscious content or conscious state. It is also worth noting that this posttraumatic state has been divided into MCS+ and MCS−,depending on the complexity of behavioral responses (i.e., presence or absence of understanding conversations, following commands, respectively) [4]. Over the last 20 years, empirical and theoretical studies have demonstrated that conscious state do not rely on a single cortical area or networks, but require, instead, a brain-scale communication that has to be sustained, complex, and differentiate [5].
Transcranial magnetic stimulation (TMS) study clarified the functional involving of cortical regions in mental activity after the stimulation [6]. Recently TMS became used in rehabilitation and treatment patients with severe traumatic brain injury (TBI). TMS is non-invasive method changing cortex activation [7].
In order to reveal changes of task-related brain regions activation we can make use of functional brain imaging tools like EEG and combine with cognitive neuroscience paradigms to record brain activity. According to the recent studies, the brain responses to naturalistic stimuli have sufficient data for assessment of integrity of consciousness and prognosis of cognitive functions. Speech and music are used as naturalistic stimuli that are closely related to social life [8, 9, 10].
Using both TMS and event-related potentials (ERP) for naturalistic stimuli could increase accuracy of forecasting of mental recovery. TMS would change local activation and facilitate perception of stimuli, and ERP for music would help to reveal hidden mental activity. Using functional connectivity for assessment of changing brain activation under the TMS and during listening to music could help estimate connectivity between different brain regions and integration of task-related brain areas [7].
Calculated functional networks reflect an activation of brain regions during different cognitive tasks. Moreover, functional networks include areas of brain activation and functional connectivity between these areas. Functional networks are considered as a measure of integrity of anatomical networks involved in mental activity [11]. Currently, the most studied network is the so-called “default mode network” (DMN). This is a system of regions including the precuneus/posterior cingulate gyrus, the lateral parietal region, the medial prefrontal cortex [12, 13]. It was shown that this network is more actively involved in dormant conditions and is relatively deactivated whenever the subject participates or performs active tasks [14].
Functional connectivity reflects a degree of the activation of brain regions involved in a task and reveals the reorganization of functional connectivity during perception of stimuli or task solving. Revealed activated functional networks could be considered as neurophysiological markers of current state of patients and forecast a potential of mental recovery [15, 16, 17].
Wavelet analysis is one of the methods to calculate functional connectivity [18], using EEG data and phase synchrony [19].
The aim of current study was to research the reorganization of functional connectivity under TMS during resting state and listening to naturalistic stimuli (songs) in unconscious patients with severe brain injury. In order to estimate functional connectivity, the analysis of wavelet synchrony was carried out in patients in unresponsive wakefulness state and minimally conscious state in resting state.
Materials and Methods
Patients
Brain activity of 15 in-patients with severe brain TBI treated in Burdenko National Medical Research Center of Neurosurgery was analyzed in the study. The experimental protocol was approved by the local Ethics Committee (Burdenko Neurosurgery Institute Research Center of Neurosurgery). Taking into account that all patients were in unconscious state the written informed consent was obtained from patients’ relatives. They received a complete information about the methods and goals of research prior to the experiment. Patients’ age varied from 17 to 49 (30,2±9,1 y.o., median = 31). Characteristics of patients, their functional state during the first EEG record after TBI and outcome are presented in Table 1.
TMS course
All these patients had TMS course. Parameters of stimulation, accurate area of stimulation and number of sessions were controlled by neurologists and depended on patient’s state and neurological dynamics. TMS were conducted using MagPro x 100 (MagVenture). Parameters of stimulation were individual. Each TMS session included 1000-4000 impulses with 30% of maximum stimulator output (MSO). Before the treatment classical diagnostic TMS procedure - the diagnostic bilateral stimulation of motor cortex (M1) and neck area (CVII) with registration of responses from contralateral m. abductor pollicis brevis was carried out. MSO caused a minimal motor response (resting motor threshold) in 50% cases was considered as a threshold value. 50% from resting motor threshold was taken as a power of therapeutical stimulation. The frequency of epi-activity on EEG was excluded from stimulation. Area of stimulation was frontal (F3, F4, Fz) and depended on traumatic area and integrity of brain. EEG-control was carried out before the TMS. The frequencies that had provoked epi-activity on EEG were excluded from stimulation [20]. In some cases the changes of area of stimulation were due to the appearance of focal epi-activity on the area of stimulation.
The number of TMS sessions and area of stimulation are presented in Table 1.
For the off-line analysis all patients were divided into two groups. The first group (n=11) included patients with positive clinical dynamics after the stimulation course. And the second one (n=4) included patients with no clinical dynamics after TMS which was considered as a negative clinical dynamic group. Clinical assessment included determination of consciousness recovery stages [21] and functional activity scale developed by authors with focus on the attention and movement.
We have a small control group consisting of 8 healthy persons which have one TMS session of the frontal area [20].
EEG and ERP-data acquisition
Brain activity was recorded using 32-channel Neurobotics (Russia) from 32 sites. Horizontal electrooculograms were recorded from the right supra-orbital margin and outside corner of the eye fissure for monitoring blinking and eye movement and further off-line artifact correction (>50mkV). EEG was recorded with common ear electrodes. Impedance was less than 5kOm and the range from 0.1 to 100Hz, the 16-bit amplifier was used. Discretization was 1024Hz.
ERP-study was carried out before, in the middle of the course and after the stimulation course. Totally, 45 studies were analyzed.
Table 1: Characteristics of patients, their functional state during the first EEG-record after TBI and outcome and the features of synchrony mapping in resting state EEG.
* selects patients with negative clinical dynamics after TMS. UWS – unresponsive unconscious state, MCS- – minimally conscious state, MCS+ - minimally conscious state with speech perception.
** - Clinical assessment of patients’ state before and after the TMS course based on Glasgow Coma Scale (GCS), Full Outline of UnResponsiveness Score (FOUR), Rehabilitation Complexity Scale (RCS), Modified Ashworth Scale for Grading Spasticity, modified Bohannon and Smith (MAS) and developed in Burdenko Neurosurgical Center scale of mental recovery.
Positive clinical dynamics included improvement of moving, emergence of glance fixation and gaze tracking, speech understanding and attempts of speech productions.
The control group consisted of 30 healthy subjects aged from 18 to 59 (mean age 30±13) [22].
Stimulus-based EEG paradigm
We used resting state and music (songs) paradigms. We supposed that changes of functional connectivity during listening to songs in comparison to resting state would concern, at least, regions involved in music and speech perception.
The music (songs) paradigm included 6 melodies appearing pseudorandom. Each melody lasted 4 s and repeated 11 times (total 66 stimuli). One melody was familiar to a patient before trauma. Interstimulus interval varied from 5 to 7 s. Stimuli were presented binaurally. In some cases, presentation was interrupted after 30-35 stimuli due to emerging epi-activity on EEG.
In current study we did not analyze features of functional connectivity in dependence on music. So the average ERP included responses to all melodies.
Moreover, we consider songs as stimuli with double significance. On the one hand, they could be used as a stimulus for ERP recording. On the other hand, the listening to a song could be considered as stimulation of the brain. We did not have a goal to estimate detailed measurements during the listening to different melodies. So, we estimated only how a person had reacted to stimuli (songs). Brain responses to songs (ERPsong) including 100 ms before and 600 ms after stimuli were averaged off-line after the correction of artefacts trials.
Resting state paradigm included EEG without artefacts before and after the listening to music. 3 minute resting state EEG without artifacts was used to analyze wavelet synchrony during resting state. 30 points were randomly sited on EEG. Wavelet synchrony was calculated in intervals including 100 ms before and 600 ms after the point and was averaged (ERPrest).
Data analysis
ERP(rest) and ERP(song) were analyzed. For wavelet synchrony calculation [19] maternal wavelet-Morlet with parameters Fb=1 and Fc=1 was used. The values of synchrony were calculated for all pairs of sites. Calculation of synchrony was carried out in the range of 1-15Hz. The range was determined by the fact that 1Hz filter rejects slow artifacts due to oculogramm and 15Hz border allows excluding from calculation of high-frequency miography oscillations. To calculate a repeated measures effect, the permutation test was used [23].
Statistica 10 software was used for statistical analysis. The analysis of multiple variance (ANOVA) was performed to examine value of wavelet synchrony among current state and dynamics of clinical changes under the TMS. Significance was determined with a 95% confidence interval. Bonferonni corrections to the degree of freedom were used, and correction probability values were reported.
T-criteria for dependent variance were used for estimation of changes of functional connectivity before and after TMS.
Results
First of all, we estimated synchrony in resting-state before the TMS course. Healthy subjects have a maximal synchrony in frontal and central areas. The value of wavelet synchrony in patients was lesser than in comparison to norm. The lower value of synchrony was found in patients with UWS and higher value of synchrony was discovered in patients with MCS, but the difference had no statistical significance.
The lesser value of wavelet synchrony was detected in patients with chronic UWS in comparison to the norm or patients with MCS. The difference had a greater value during listening to music in comparison to resting state.
The short wavelet connectivity between right frontal and temporal sites was revealed in patients with chronic UWS.
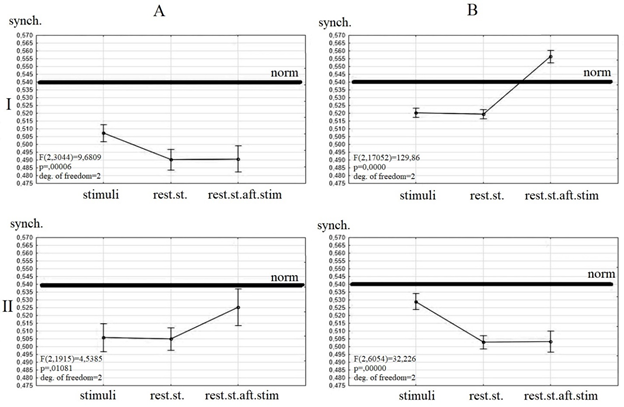
The dependence of wavelet synchrony value and clinical dynamic was revealed (Figure 1).
All patients before the TMS had a value of synchrony lesser then healthy persons. The differences between values of wavelet synchrony during listening to stimuli and resting state after the study were detected between the patients with positive and negative clinical dynamics after the TMS course.
Patients with negative clinical dynamics before TMS have a similar value of synchrony during resting state and listening to songs and an increased synchrony after listening to songs (Figure1 II A). There were no changes of resting state synchrony under the TMS. Only a short-time increase of synchrony during listening to songs was revealed (Figure 1 II B).
Patients with positive clinical dynamics before the TMS have a similar value of synchrony during resting state before and after the listening to stimuli and an increased value of synchrony during listening to stimuli (Figure 1 I A). After TMS these patients had a similar synchrony during resting state and listening to stimuli and an increased synchrony after the listening to stimuli. The normalization of synchrony value was detected only during resting state after listening to stimuli (Figure 1 I B).
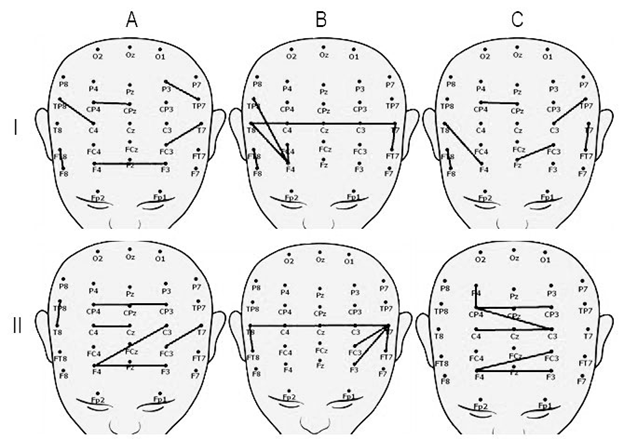
We analyzed wavelet-connectivity in patients with positive clinical dynamics after TMS. We revealed that before the TMS the short connectivity between neighboring sites in frontal, temporal and parietal area were detected during resting state (Figure 2 I A, C). The interhemispheric connectivity between temporal area and interhemispheric connectivity between frontal and temporal areas were revealed (Figure 2 I B).
TMS effects mainly connectivity during resting state. After TMS course, the interhemispheric connectivity between frontal, central and parietal areas during resting state as well as interhemispheric connectivity between temporal areas during listening to stimuli were revealed (Figure 2 II).
Patients with negative clinical dynamics had no interhemispheric connectivity before and after TMS. Functional connectivity mapping for these patients was similar to those during resting state in patients with positive clinical dynamics before TMS. Only a short connectivity between close located sites with no structure was detected.
Discussion
The degree of synchrony value deviation from control level has a significant positive correlation with current state of patients. However, resting state synchrony gives an answer to what the current state is but does not give an answer to what prospects of consciousness recovery the patient has. Although patients in unconscious states showed some changes in wavelet synchrony during listening to songs compared to resting state, the obtained data do not allow us to separate a conscious assessment of the text and/or melody and an involuntary assessment of different melodic series. Thus, in patients in unconscious states, such as unresponsive wakefulness state and minimally conscious state, changes in wavelet synchrony when listening to stimuli relative to the resting state can be used to assess the perception of auditory and speech information.
The difference of wavelet connectivity value before and after listening to naturalistic stimuli before TMS had no statistical significance. Whereas, the way of changing value of connectivity could be considered as a prognostic factor for following reorganization of connectivity mapping.
Determination of the predominant functional connections makes it possible to suggest that the activation of neural networks (such as auditory (including speech) and frontoparietal) and the presence of interhemispheric interactions give the ability to switch and interact between these functional networks, which is characteristic for the control group. Lateralized fronto-parietal networks are presented in both hemispheres. They consist of the inferior and the medial frontal gyri, the precuneus, the inferior parietal gyrus. It is worth noting that these areas in resting state tend to represent other functional networks. This networks are thought to be associated with functions such as memory [24], attention [26], language and speech [25] and visual processes [27]. Auditory network includes the superior temporal gyrus, Geshl's gyrus, postcentral gyrus and Insula. It remains unclear exactly which areas belong to this network and how the interaction with the functions of the language occurs [28, 29, 30].
Patients with positive and negative clinical dynamics had controversial changes of wavelet connectivity under the TMS. Patients with positive clinical dynamics had a higher value of connectivity after the TMS during resting state after functional tasks (listening to naturalistic stimuli). Patients with negative clinical dynamics had the same way of connectivity changing value before TMS. The parameters and area of stimulation for these patients were probably not optimal; this fact did not allow the interhemispheric connectivity to be formed.
For patients with positive clinical dynamics after TMS, high values ??of the resting state after listening to stimuli were detected. Moreover, they were slightly higher than normal. This can be explained by the fact that TMS has changed the level of excitation of the cerebral cortex so much that listening to naturalistic stimuli causes an increase in wavelet synchrony after the end of stimulation therapy. Since the topology of connections coincides with the control group, this can be the evidence that the structures and neural networks that are responsible for clear consciousness were activated and functioning. The obtained data correlate with a study [31], where TMS also caused changes in EEG, which were similar to healthy subjects. It is also worth noting that the values ??of wavelet synchrony in these three states (listening to naturalistic stimuli and resting state before and after listening) were increased after TMS. It can be assumed that a low level of synchronization (before TMS) does not allow stable functional relationships to be established and prevents the effective assessment of incoming information. In this case, TMS therapy contributed to increasing the wavelet synchrony in such a way that this therapy ultimately underlies the establishment of effective functional relationships.
It is also worth noting that a group of patients with positive dynamics after TMS therapy is characterized by a change in state. There was an improvement in motor activity, the appearance of the ability to fix the gaze and tracking moving objects, an understanding of inverted speech, attempts to talk.
Patients with negative dynamics are characterized by the absence of changes in the values ??of wavelet synchrony in a resting state. As mentioned earlier, it is noteworthy that before TMS, listening to melodies caused an increase in the values ??of wavelet synchrony in a resting state after functional tasks (as in the case of patients with positive dynamics), but not significantly. It can be assumed that a low level of synchrony does not allow stable functional relationships to be established and prevents the effective assessment of incoming information. After the TMS course, an increase in the values ??of wavelet synchrony was observed only during stimulation therapy. Compared with the clinical assessment, this effect is most likely of a short-term nature, and therefore only an increase in the wavelet synchrony values ??(and above the control group) can provide the establishment of functional relations for processing information.
It follows from the above, that a positive effect is an increase in values ??in a resting state after listening to stimuli, and it is higher than the values ??of the control group. Thus, the patient’s condition is as close as possible to the control state, and makes it possible to inhibit the processes to the maximum possible compliance with the values ??of the control group.
We consider changes of wavelet connectivity as activation of functional nets of brain. We suppose that revealed connectivity reflects interaction between functional nets involved in detection of stimuli and activation of voluntary processing and speech.
Conclusion
Patients in UWS have a lower value of wavelet synchrony in comparison to norm. Patients with positive clinical dynamics have interhemispheric connectivity between frontal, central and parietal areas. Positive effect of TMS includes increasing wavelet synchrony value with emergence of interhemispheric connectivity during resting state.
Acknowledgements
This study was partially supported by the RFBR grant 18-013-00967 and funds within the state assignment of Ministry of Education and Science of the Russian Federation for 2019-2021 (????-?17-117092040004-0).
Conflict of Interest Statement
The authors declare that they have no conflicts of interests.
References
- Giacino JT, Fins JJ, Laureys S, et al. Disorders of consciousness after acquired brain injury: the state of the science. Nature Reviews Neurology10 (2014): 99.
- Gosseries O, Di H, Laureys S, et al. Measuring consciousness in severely damaged brains. Annual Review of Neuroscience37 (2014): 457-478.
- Vanhoecke J, Hariz M. Deep brain stimulation for disorders of consciousness: systematic review of cases and ethics. Brain stimulation10 (2017): 1013-1023.
- Bruno MA, Majerus S, Boly M, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. Journal of neurology259 (2012): 1087-1098.
- Naccache L. Minimally conscious state or cortically mediated state?. Brain141 (2017): 949-960.
- Oliveira FTP, Diedrichsen J, Verstynen T, et al. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proceedings of the National Academy of Sciences107 (2010): 17751-17756.
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, et al. Noninvasive brain stimulation in traumatic brain injury. The Journal of head trauma rehabilitation 27 (2012): 274.
- Monti M, Schnakers C, Korb A, et al. Non-invasive ultrasonic thalamic stimulation in disorders of consciousness after severe brain injury: a first-in-man report. Brain Stimul9 (2016): 940-941.
- Magee WL, Tillmann B, Perrin F, et al. Music and disorders of consciousness: Emerging research, practice and theory. Frontiers in psychology7 (2016): 1273.
- Owen AM, Coleman MR, Boly M, et al. Detecting awareness in the vegetative state. science313 (2006): 1402-1402.
- Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurological sciences32 (2011): 773-785.
- Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proceedings of the National Academy of Sciences98 (2001): 676-682.
- Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences100 (2003): 253-258.
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of cognitive neuroscience9 (1997): 648-663
- Boly M, Tshibanda L, Vanhaudenhuyse A, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Human brain mapping30 (2009): 2393-2400.
- Varotto G, Fazio P, Sebastiano DR, et al. Altered resting state effective connectivity in long-standing vegetative state patients: an EEG study. Clinical Neurophysiology125 (2014): 63-68.
- Chennu S, Annen J, Wannez S, et al. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain140 (2017): 2120-2132.
- Quiroga RQ, Garcia H. Single-trial event-related potentials with wavelet denoising. clinical neurophysiology114 (2003): 376-390.
- Romanov A, Sharova E, Kuznetsova O, et al. Possibility of waveletsynchronization methods for estimation of spatial distribution of components of auditory evoked potentials in healthy subjects. Neuroscience and Behavioral Physiology 61 (2011): 112-118.
- Kopachka MM, Sharova EV, Troshina EM, et al. Asymmetries of long-latent components of auditory evoked potential on the background of rTMS in healthy subjects and patients with post-traumatic depression of consciousness. Asymmetry 9 (2015): 18-29.
- Dobrokhotova TA, Bragina NN, Zaitsev OS, et al. , Unilateral Spatial Agnosia, Book Ltd., Moscow (1996).
- Oknina LB, Kuptsova SV, Romanov AS, et al. Comparative analysis of changes in short EEG segments during music perception based on event-related synchronization/desynchronization and wavelet synchronicity. Human Physiology 38 (2012): 348-353
- Blair RC, Troendle JF, Beck RW. ?ontrol of familywise errors in multiple endpoint assessments via stepwise permutation tests. Statistics in medicine15 (1996): 1107-1121.
- Damoiseaux JS, Rombouts SARB, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the national academy of sciences103 (2006): 13848-13853.
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences106 (2009): 13040-13045.
- Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences102 (2005): 9673-9678.
- De Luca M, Beckmann CF, De Stefano N, et al. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage29 (2006): 1359-1367.
- Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology21 (2000): 1636-1644.
- Koyama MS, Kelly C, Shehzad Z, et al. Reading networks at rest. Cerebral cortex 20 (2010): 2549-2559.
- Turken U, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience5 (2011).
- Sarasso S, Rosanova M, Casali AG, et al. Quantifying cortical EEG responses to TMS in (un) consciousness. Clinical EEG and neuroscience45 (2014): 40-49.