Tacrolimus Reverses the Pemphigus Vulgaris Serum-Enhanced Expression of Desmoglein in HaCaT Cells
Article Information
Xie Zhimin1,2, Dai Xiangnong2,3, Pan Qiaolin2, Li Qingqing2,3, Ye Xingdong2,3
1Department of Dermatology, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, The China
2Institute of Dermatology, Guangzhou medical University, Guangzhou, The China
3Department of Dermatology, Guangzhou Institute of Dermatology, Guangzhou, The China
Xie Zhimin and Dai Xiangnong are co-first authors.
*Corresponding author: Xingdong Ye, Guangzhou Institute of Dermatology, NO. 56, Heng Fu Street, Guangzhou, Guangdong province, China.
Received: 11 September 2022; Accepted: 21 September 2022; Published: 19 October 2022
Citation: Xie Zhimin, Dai Xiangnong, Pan Qiaolin, Li Qingqing, Ye Xingdong. Tacrolimus Reverses the Pemphigus Vulgaris Serum-Enhanced Expression of Desmoglein in HaCaT Cells. Archives of Clinical and Biomedical Research 6 (2022): 873-879.
Share at FacebookAbstract
Background: Pemphigus vulgaris (PV) is associated with autoantibodies against desmoglein (Dsg), including Dsg1 and Dsg3. However, the precise mechanism by which acantholysis occurs in response to PV-IgG and the effect of tacrolimus for PV remains unclear.
Method: To co-culture human HaCaT keratinocytes with DMEM medium containing 5%PV-sera to establish a cell model of pemphigus that can determine the effect of PV-sera and tacrolimus on Dsg mRNA transcription and protein expression in HaCaT cells. Dsg protein expression in HaCaT cells was evaluated by Western blotting and Dsg mRNA transcription by real-time PCR (RT-PCR). The distribution of Dsg1 and Dsg3 in HaCaT cells was determined by indirect immunofluorescence (IIF).
Results: The application of 5% PV serum resulted in an increase in the transcription and expression levels of Dsg1 and Dsg3, whereas tacrolimus suppressed Dsg1 and Dsg3 expression. Tacrolimus inhibited PV seruminduced disruption of cell-cell contacts. Tacrolimus also downregulated the expression of Dsg1and Dsg3 compared with the PV group. IIF revealed that the linear deposits of Dsg1 on the surface of HaCaT cells in the PVsera group disappeared and were replaced by granular and agglomerated fluorescent particles on the cell surface, whereas the Dsg3 linear deposits still existed, but this effect could be reversed by tacrolimus.
Conclusion: The Dsg3 antibody disrupts desmosome junctions by inducing endocytosis, resulting in desmosomal dissociation. Tacrolimus could reverse PV serum-induced enhancement Dsg expression in HaCaT cells.
Keywords
Acantholysis; Desmogleins; Pemphigus Vulgaris; Tacrolimus
Acantholysis articles; Desmogleins articles; Pemphigus Vulgaris articles; Tacrolimus articles
Article Details
Abbreviations:
PV- Pemphigus Vulgaris; Dsg- Desmoglein; AZA- Azathioprine; MMF - Mycophenolate Mofetil; CP- Cyclophosphamide; NH- Normal Healthy; PBS- Phosphate-buffered Saline; DAPI- 4′,6-diamidine-2′-Phenylindole Dihydrochloride
1. Introduction
Pemphigus is a rare, chronic, potentially life-threatening, autoimmune blistering disease characterized by stained skin mucous crusting, erosion, and blisters in mucous membranes and skin. The aetiopathogenesis of pemphigus is characterized by acantholysis and intraepidermal blister formation, resulting from IgG autoantibodies directed against transmembrane desmosomal glycoproteins, including Dsg3 and/or Dsg1 [1]. a recent study from China identified HLADRB1*14:04 and also rs7454108 at the TAP2 gene (implicated in the presentation of intracellular proteins on major histocompatibility complex I HLAs) as associated with PV in a Han Chinese Population [2, 3], Based on clinical and pathological findings, pemphigus can be classified into four major forms [4]: (i) pemphigus vulgaris (PV), (ii) pemphigus erythematosus, (iii) pemphigus foliaceus (PF), and (iv) proliferative pemphigus. However, in Europe and North America, pemphigus is divided into two subtypes, that is, PV and PF with contribution of 75% and 20% patients, respectively [5]. Li et al reported an increase in Dsg3 mRNA abundance in HaCaT cells after culturing with the addition of sera of patients with PV and PF, whereas the fluorescence intensity of Dsg3 on the surface of HaCaT cells decreased [6]. However, their study neither detected Dsg3 expression in HaCaT cells nor employed a positive control, and further study on the effect of pemphigus serum on Dsg expression in HaCaT cells is needed. Tacrolimus (FK506) is calcineurin inhibitor that reduces T-cell activation, and topical application of tacrolimus has been studied in the treatment for mucosal and skin lesions in PV and PF, with beneficial results observed [7-9]. There are also two case reports on the successful use of systemic tacrolimus for recalcitrant PV unresponsive to prednisolone, azathioprine (AZA), mycophenolate mofetil (MMF), dapsone, and cyclophosphamide (CP) [10]. A randomized controlled trial demonstrated that tacrolimus effects are comparable to AZA as PV adjuvant treatment, although with less severe side effects [11]. Takae et al used a pemphigus mouse model to evaluate various immunosuppressive therapies and reported the suppressive effect of tacrolimus on the production of anti-Dsg3 IgG [12]. However, tacrolimus has not been studied at the cellular level, to date. This study aimed to explore the effect of PV-sera on mRNA and protein expression of Dsg1 and Dsg3 in HaCaT cells, and the capacity of tacrolimus to reverse the effect of PV-induced Dsg upregulation in HaCaT cells was also assessed.
2. Materials and Methods
2.1 Participants and Specimens
Serum samples were collected from seven patients with PV (Table 1) and three healthy volunteers. All the patients were in the active stage of the disease without treatment and met PV diagnostic criteria as follows: (i) multiple flaccid, easily ruptured bullae on the basic of mucocutaneous erythema, (ii) progressive refractory erosions covered crust secondary to blisters, (iii) positive Nikolsky’s sign, (iv) histopathological finding of intraepidermal blister formation, and (v) immunological feature of reticular bright green fluorescence IgG deposition on keratinocyte cells. We used the sera of healthy volunteers as control and inclusion criteria of PV as follows: (i) consistent with the diagnostic criteria for PV; (ii) neither immunosuppressant nor glucocorticosteroid was used in the last 30 days; (iii) without other autoimmune diseases, except for PV; and (iv) no significant organ dysfunction, as well as informed consent. Exclusion criteria are as follows: (i) PV patient is pregnant, (ii) pemphigus induced by drugs, (iii) patients with malignant tumor, and (iv) administration of tetracycline and macrolide antibiotics in the last month.
|
Case |
Gender |
Age |
Brief description of case history |
|
1 |
F |
44 |
Progressive erythema, erosion with filthy crust in trunk for 2 weeks |
|
2 |
M |
43 |
Erythema, blister on extremities and trunk for 3 months |
|
3 |
F |
32 |
Recurrent painful erythema and blisters all over the body for half a year |
|
4 |
F |
40 |
Erythema, blisters, erosion with pain all over the body for more than 2 months |
|
5 |
M |
63 |
Oral ulcers for 3 months, recurrent blisters on extremities and trunk for more than 1 month |
|
6 |
M |
72 |
Trunk and oral blisters, erosions with pain for 2 months |
|
7 |
F |
56 |
Erythema, blisters, erosions with pain all over the body for more than 3 months |
Table 1: Description of seven PV patients in the study.
2.2 Cell Culture and Study Design
The human keratinocyte cell line HaCaT (MssBio Co., Ltd. Guangzhou, China) was used to establish an in vitro model of PV according to a previous study [13]. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Cat. No. SH30022.01B) supplemented with penicillin (50 U/ mL, Hyclone, Cat. No. SH30010) with 10% fetal calf serum (Hyclone, Cat. No.SH30087.0), then seeded at a density of 2 ×105 cells/cm2 on 25-mm2 cell culture flasks at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed every 24 h until the cultures reached 80% confluency. The HaCaT cells were subcultured in 12-well plates up to 80% confluency. The 3rd passages of HaCaT cells were cultured continuously for 24 h in DMEM high glucose medium containing 5% PV-sera at 37°C in a humid, 5% CO2 incubator. For tacrolimus studies, cells were incubated in 5% PV-sera with 100 nM tacrolimus or NH sera with 100 nM tacrolimus (Absin Bioscience, China). The cells were harvested after 24 h, and total mRNA was extracted for analysis. Six experimental groups were designed in the study: control group (cells were incubated with medium without treatment), normal healthy (NH) sera group (cells were incubated with 5% sera from healthy donors), PV-sera group (cells exposed to 5% PV-sera), positive group (cells were incubated with Dsg3 monoclonal antibody containing culture medium), PV-sera + FK506 group (cells were incubated with tacrolimus-treated and 5% PV-sera), and healthy-sera + FK506 group (cells were incubated with tacrolimus-treated and NH-sera).
2.3 Transcription Assay for Dsg mRNA
Total RNA was extracted from the HaCaT cells using TRIzol reagent. First-strand cDNA synthesis was performed using a kit and following the manufacturer’s instructions. Using NCBI for RT- PCR primer design, the primer sequences for amplification of the Dsg1 (NCBI reference sequence: NM_001942), Dsg3 (NM_001944), and GAPDH (NM_001289746) gene fragments are shown in Table 2. The reaction volume was 20 μL and prepared as follows: 5 μL of cDNA template (1:10 dilution, ViiA7 software), 0.5 μL of the upstream primer, 0.5 μL of the downstream primer, 10 μL of SYBR® Premix Ex Taq™ (Tli RNase H Plus) (2×), and 4.0 μL of ddH2O. PCR cycling conditions were as follows: incubation 95°C for 30s min, followed by 40 cycles of 95°C for 3 s and 60°C for 34 s, with the addition of 60°C 1 min for elongation.
|
Gene |
Sequence |
Locus at target gene |
PCR product size |
|
Dsg1 |
F 5'-GGCATTCAATCCGAAGGCAG-3' |
263-282 |
112 bp |
|
R 5'-AGTGAATTTTGGCGATTGGGTT-3' |
364-343 |
||
|
Dsg3 |
F 5'-TTGAGCTGCTTGGAAAAAGGGA-3' |
3,889-3,910 |
73 bp |
|
R 5'-TATATGGCTTCCCAGCACCAAG-3' |
3,961-3,940 |
||
|
GAPDH |
F 5'-TGTTGCCATCAATGACCCCTT-3' |
406-426 |
202 bp |
|
R 5'-CTCCACGACGTACTCAGCG-3' |
607-589 |
Table 2: Description of primer sequences included in the study.
2.4 Western Blot Analysis of Dsg Expression
The HaCaT cells were grown in 12-well plates for 18 h, the medium was replaced with DMEM containing 5% PV-sera or NH sera, and incubated for another 18 h, then 1×108 to 1×109 cells were collected for western blot analysis. The primary antibodies used were mouse anti-Dsg3 antibody (1:160 dilution; Abcam, Cambridge, UK) and mouse anti-Dsg1 antibody (1:500 dilution; Abcam). Goat anti-mouse antibody (1:1,000 dilution, Alexa Fluor-488, Thermo Fisher Scientific, Dreieich, Germany) served as secondary antibody.
2.5 Indirect Immunofluorescence (IIF) Detection of Dsg in HaCaT cells
The cells were grown directly (1× 105 cells/ml) on glass coverslips for 4 h in a 12-well plate and then continuously for 24 h after replacing the medium with or without supplements as earlier described. After washing with phosphate-buffered saline (PBS), the cells were fixed in 4% paraformaldehyde solution for 30 min. The samples were then washed thrice with PBS for 5 min each time. The cells were then permeabilized with 0.2% Triton X-100 for 5 min. After three rinses with PBS, the samples were blocked using 10% normal goat serum for 30 min. Then, the samples were incubated with the primary antibody (Dsg1 at 1:500 dilution; Dsg3 at 1:160 dilution) at 4°C overnight. As secondary antibody, fluorescently labeled goat anti-mouse antibodies were used at a dilution of 1:1,000 for 1 h at room temperature. The anti-Dsg1 and anti-Dsg3 antibodies were used against cell surface proteins Dsg1 and Dsg3. The nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI). Images of the cells morphology were captured by standard light microscopy. An inverted fluorescence microscope (DMI6000B, Leica, Japan) was used for image acquisition at 40× magnification. Average OD value was assayed by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
2.6 Statistical Analysis
Western blot analysis was visualized using ImageJ (NIH, USA). Statistical significance was assessed using one-way ANOVA followed by Bonferroni correction using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) for comparison of multiple groups. Differences were deemed significant when the calculated p value was <0.05. The data were expressed as the mean ± SD.
3. Results
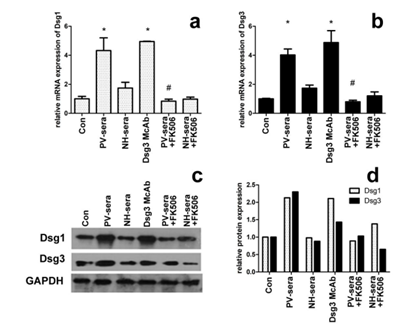
3.1 5% PV-sera Enhances Dsg1 and Dsg3 mRNA Abundance in HaCaT cells and inhibited by Tacrolimus
The mRNA transcription level of Dsg1 and Dsg3 in HaCaT cells incubated with 5% PV-sera is indirectly reflected by the amount of cDNA template detected by RT-PCR (Figure 1a and b). Significantly higher Dsg1 and Dsg3 mRNA transcription levels were observed in the HaCaT cells in the presence of 5% PV-sera, whereas this effect decreased in the presence of 100 nM tarcrolimus (Figure 1a for Dsg1 and b for Dsg3, P< 0.05).
3.2 The Addition of PV-sera Increases Dsg1 and Dsg3 Protein Expression in HaCaT Cells and is inhibited by Tacrolimus
The expression of Dsg1 and Dsg3 in the PV-sera group significantly increased after the addition of PV-sera. However, when the cells were treated with PV-sera and tarcrolimus, the expression of Dsg1 and Dsg3 dramatically decreased (Figure 1, c and d).
Figure 1: Dsg1/Dsg3 transcription and expression levels in HaCaT cells under different medium conditions. The effect of PV-sera on Dsg1and Dsg3 mRNA abundance of in HaCaT cells measured by RT-PCR (a, b), mRNA levels were normalized to that of GAPDH (=1). Each bar represents the mean ± SD. (*P <0.05 vs. control group, #P <0.05 vs. PV-sera group). One-way ANOVA was used to evaluate differences in the average Ct values among the four groups. The level of Dsg1 and Dsg3 expression was analyzed by western blotting (c), normalized using GAPDH, and plotted using GraphPad Prism (d). GAPDH was used as a control for SDS/PAGE loading.
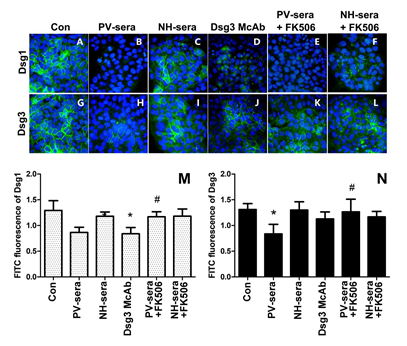
3.3 IIF Analysis of Dsg1 and Dsg3 in HaCaT Cells
Figure 2: Immunofluorescence staining of anti-Dsg1/Dsg3 and histogram of optical density (OD) values at the surface of HaCaT cells as detected by IIF. Nuclear staining of HaCaT cells using DAPI. The cells were treated for 18 h by medium with supplements (A, G), 5% PV-sera (B, H), sera for normal healthy (NH) donors (C, I), Dsg monoclonal antibody (D, J), 5% PV-sera and tacrolimus (E, K), and NH-sera and tacrolimus (F, L). Dsg1 and Dsg3 levels were assessed by immunofluorescence, the graphics shows the median of FITC (green) fluorescence intensity (M, N). Each bar represents the mean ± SD (*P < 0.05 vs. control group, #P<0.05 vs. PV-sera group).
4. Discussion
This study has shown that incubation with PV-sera can induce HaCaT cell colony contraction and formation of intercellular gaps, and gene transcription and protein translation levels of Dsg1 and Dsg3 in HaCaT cells were significantly higher than that of the control. In addition, both PV-serum and anti-Dsg3 autoantibodies induced a disruption of the linear distribution of Dsg1, resulting in a dispersed distribution of smaller dots throughout the cytoplasm. In addition, our results confirmed that Dsg3 antibodies can result in Dsg1 antigen internalization on HaCaT cells. However, the linear distribution of Dsg3 was remarkably reduced in the HaCaT cells cultured with PV-sera compared to those of the control group and NH serum. The calcineurin inhibitor tacrolimus reversed the PV serum-induced up-regulation transcription and expression of Dsg. The IIF results suggested that incubation of HaCaT cells with PV-sera and tacrolimus did not reverse Dsg1 internalization. The decrease in Dsg1 fluorescence may be related to protein endocytosis and its possible tendency of triggering a cascade reaction involving cytokines, and the expression of MMPs may lead to Dsg1/Dsg3 decomposition. Studies demonstrating that the serum from pemphigus can affect the amount of Dsg in HaCaT cells are limited. Li Hui and her colleagues described that the serum containing anti-Dsg1 antibody decreased the expression of Dsg3 mRNA, and that containing anti-Dsg3 antibody increased the expression of Dsg3 mRNA, but Dgs3 protein expression decreased because of endocytosis of keratinocyte and the presence of anti-Dsg3 antibody in the serum [6] which was confirmed in this study using IIF. Lanza et al. reported that PV serum and PV IgG can induce acantholysis and reduce the total amount of Dsg3 in cultured keratinocytes, whereas linear epitopes of Dsg3 (anti-Dsg3-L IgG) fail to do so when administered at concentrations comparable to those present in pathogenic PV-sera [14]. However, the Dsg3-depleting activity of such polyclonal anti-Dsg3 IgG was acquired when the Dsg3 antibody was used at a concentration of 1 mg/mL, and this may be because of increasing of Dsg3 autoantibodies that bind to the epitopes at the surface of KC, which, in turn, hinders the binding of foreign antibodies, although autoantibodies preferentially bind to mature Dsg3-antigens and causes Dsg3 depletion from desmosomes. Dsg3 depletion may be due to the following: activation of p38MAPK, the binding of the autoantibodies leads to internalization of Dsg3, a collapse of the keratin cytoskeleton, and Dsg dissociation through MAPK pathway. Jolly et al. confirmed that PV IgG causes the internalization of cell-surface Dsg3 into endosomes (as early as 4 h), which are then depleted from both detergent-soluble and -insoluble pools, and this could be blocked by the p38MAPK inhibitor SB202190 [15]. The depletion of Dsg3 induced by autoantibodies from PV patients is dependent on PKC signaling [16]. Anti-Dsg3 antibodies have been shown to induce KC cell apoptosis, which may contribute to the depletion of Dsg3. Anti-Dsg3 antibodies increased the expression of mRNAs for proinflammatory cytokines (IL-1β, TNF-α, and IL-6), Bax, and uPAR, whereas decreased the levels of procaspase-3 and Bcl-2 [17]. Dsg1-positive sera from patients can reduce the recognition of Dsg3 peptides. Anti-Dsg1 antibodies can recognize the epitopes of Dsg3 and thus anti-Dsg1 antibodies would bind to the peptides of Dsg3 and thus activated the p38MAPK/PKC pathway as well, which may result in the depletion of Dsg3 [18,19]. Serum containing anti-Dsg1 antibodies can decrease the expression of Dsg3 mRNAs, but serum containing anti-Dsg3 antibodies can increase the expression of Dsg3 mRNAs. The effect of Dsg1 on Dgs3 may be a negative feedback of KC, and anti-Dsg1 antibodies trigger the PKC pathway, which, in turn, causes a downregulation of Dgs3 mRNAs. Anti-Dsg1 antibodies can decrease the expression of Dsg3, while anti-Dsg3 antibodies can increase the expression of Dsg3. However, the increased expression of Dsg3 will be consumed or offset through Dsg3 internalization as mediated by anti-Dsg3 antibodies or KC. Differences in the effects of anti-Dsg1and Dsg3 autoantibodies indicate that both Dsg1 and Dsg3 are not supposed to fully compensate for each other, and thus, are suggestive of the complexity of its pathogenesis. Tacrolimus has been shown to potentiate the action of glucocorticoids by preventing their degradation, resulting in a steroid-sparing effect [20]. Interaction of desmoglein-3-reactive T cells with naïve B cells is required for induction of synthesis of pathogenic IgG [21], and thus, tacrolimus may be effective in controlling B-cell-mediated auto-immune in PV. In this study, an enhancement of the expression of Dsg1and Dsg3 by PV-sera was observed and reversed with the addition of 100 nM of tarcrolimus. Previous studies have been demonstrated that tarcrolimus inhibited the activation of p38MAPK pathway in atopic dermatitis, human colonic myofibroblasts and rheumatoid arthritis [22-24]. Thus, we assumed that tarcrolimus reserves the Dsg depletion by blocking the activation of p38MAPK pathway, which requires more in-depth research.
There is growing evidence suggesting that anti-Dsg autoantibodies cannot account for the loss of cell-cell adhesion as seen in PV, indicating that multiple combinations of pathogenic or subpathogenic autoantibodies may function together to contribute to acantholysis. “Desmoglein compensation hypothesis” and “steric hindrance theory” are the widely recognized pathogenic mechanisms of pemphigus [25-28]. The formation of blisters in PV is due to the synergistic effect of autoantibodies targeting multiple keratinocytes antigens [29,30]. For example, in addition to Dsg, a variety of other autoantibodies have been described in PV, including cholinergic receptors [31], anti-mitochondrial Proteins [32], non-Dsg adhesion proteins (desmocollin and plakophillin) [33,34], and additional targets concerning γδ-T lymphocytes, ATP2C1 and IL-36 [35-37]. Fujimura et al. demonstrated that CD163+ tissue-associated macrophages (TAMs) accumulate in the skin lesions of PV. The expression of periostin (POSTN), IL-36γ, and MMP-12 is prominent in the skin lesions of PV patients. In addition, serum levels of CXCL5 and sCD163 are significantly higher in PV patients compared to healthy donors [38]. These experiments provide key insights into the mechanism underlying multiple autoantibodies specificities that may be involved in blister formation in PV. To our knowledge, this study is the first to investigate the effect of tacrolimus on Dsg expression in a HaCaT cell model of pemphigus, but there are some limitation: first, the lack of inactivation of serum complement may affect Dsg expression; second, when studying the effect of tacrolimus on Dsg expression, no positive control was used. This study also provides a basis for further elucidating the mechanism of tacrolimus in the treatment of pemphigus.
5. Conclusions
Taken together, our study has shown that PV serum can promote the transcription and expression of Dsg, and tacrolimus can reverse this effect of PV. Our findings may be utilized in elucidating the mechanism of action of tacrolimus in the treatment of pemphigus.
Ethics Approval and Consent to Participate
This study and all relevant experiments were reviewed and approved by Guangzhou Institute of Dermatology Research Ethics Committee (NO.201802). The purpose of the study, type and amount of specimen needed were explained to the participants and written informed consent was obtained from all study participants recruited in this study. The methods were conducted in accordance with approved guidelines and regulations.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
This study was financially supported in part by the “Scientific research plan (2019)” (No. 201904010352) and “the School & Institute Joint Fund Project in basic and applied research areas” Guangzhou Science and Technology Bureau 2023.
Author’s Contributions
Xie ZM drafted the manuscript. Ye XD conceptualized the research design and revised the draft, Pan QL conducted the experiment, and Dai XN and Li QQ reviewed and analyzed the data. All authors were involved in revising the manuscript and approving the final version.
References
- Pollmann, R, Schmidt, T, Eming, R, et al. Pemphigus: a Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches[J]. Clinical reviews in allergy & immunology 54 (2018): 1-25.
- Hammers CM, Stanley JR. Recent Advances in Understanding Pemphigus and Bullous Pemphigoid[J]. The Journal of investigative dermatology 140 (2020): 733-741.
- Gao J, Zhu C, Zhang Y, et al. Association Study and Fine-Mapping Major Histocompatibility Complex Analysis of Pemphigus Vulgaris in a Han Chinese Population[J]. The Journal of investigative dermatology 138 (2018): 2307-2314.
- Xucheng Shen, Zhang Yi, Ye Xd. Advances in the treatment of pemphigus[J]. J Diagn Ther Dermato-Venereol 25 (2018): 373-376.
- Beyzaee AM, Rahmatpour RG, Patil A, et al. Rituximab as the treatment of pemphigus vulgaris in the COVID-19 pandemic era: A narrative review[J]. Dermatologic therapy 34 (2021): e14405.
- Li H, Lin L, Zhang Jc. Effects of serum from different subtypes of pemphigus on the expression of desmoglein-3 in HaCaT cells. [J]. China journal of Leprosy and Skin Diseases 31 (2015): 131-135.
- Hodgson TA, Malik F, Hegarty AM, et al. Topical tacrolimus: a novel therapeutic intervention for recalcitrant labial pemphigus vulgaris[J]. European journal of dermatology: EJD 13 (2003): 142-144.
- Gach JE, Ilchyshyn A. Beneficial effects of topical tacrolimus on recalcitrant erosions of pemphigus vulgaris[J]. Clinical and experimental dermatology 29 (2004): 271-272.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (protopic) for the treatment of a localized pemphigus foliaceus[J]. Journal of the European Academy of Dermatology and Venereology: JEADV 18 (2004): 636-637.
- Busing V, Kern JS, Bruckner-Tuderman L, et al. Recalcitrant pemphigus vulgaris responding to systemic tacrolimus[J]. Dermatology (Basel, Switzerland) 221 (2010): 122-126.
- Dastgheib L, Sadati MS, Baghernejhad M. Assessment of the adjuvant effect of tacrolimus in the management of pemphigus vulgaris: A randomized controlled trial[J]. The Journal of dermatological treatment 26 (2015): 90-93.
- Takae Y, Nishikawa T, Amagai M. Pemphigus mouse model as a tool to evaluate various immunosuppressive therapies[J]. Experimental dermatology 18 (2009): 252-260.
- Cirillo N, Campisi G, Gombos F, et al. Cleavage of desmoglein 3 can explain its depletion from keratinocytes in pemphigus vulgaris[J]. Experimental dermatology 17 (2008): 858-863.
- Lanza A, Perillo L, Landi C, et al. Controversial role of antibodies against linear epitopes of desmoglein 3 in pemphigus vulgaris, as revealed by semiquantitative living cell immunofluorescence microscopy and in-cell ELISA[J]. International journal of immunopathology and pharmacology 23 (2010): 1047-1055.
- Mao X, Li H, Sano Y, et al. MAPKAP kinase 2 (MK2)-dependent and -independent models of blister formation in pemphigus vulgaris[J]. The Journal of investigative dermatology 134 (2014): 68-76.
- Dehner C, Rotzer V, Waschke J, et al. A desmoplakin point mutation with enhanced keratin association ameliorates pemphigus vulgaris autoantibody-mediated loss of cell cohesion[J]. The American journal of pathology 184 (2014): 2528-2536.
- Narbutt J, Boncela J, Smolarczyk K, et al. Pathogenic activity of circulating anti-desmoglein-3 autoantibodies isolated from pemphigus vulgaris patients[J]. Archives of medical science: AMS 8 (2012): 347-356.
- Szabados H, Bosze S, Sillo P, et al. The mapping of linear B-cell epitope regions in the extracellular parts of the desmoglein 1 and 3 proteins: recognition of immobilized peptides by pemphigus patients' serum autoantibodies[J]. Journal of peptide science: an official publication of the European Peptide Society 19 (2013): 84-94.
- Berkowitz P, Chua M, Liu Z, et al. Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signaling in the skin[J]. The American journal of pathology 173 (2008): 1628-1636.
- Madan V, Griffiths CE. Systemic ciclosporin and tacrolimus in dermatology[J]. Dermatologic therapy 20 (2007): 239-250.
- Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease[J]. Nature reviews. Immunology 1 (2001): 147-153.
- Lan CC, Fang AH, Wu PH, et al. Tacrolimus abrogates TGF-beta1-induced type I collagen production in normal human fibroblasts through suppressing p38MAPK signalling pathway: implications on treatment of chronic atopic dermatitis lesions[J]. Journal of the European Academy of Dermatology and Venereology: JEADV 28 (2014): 204-215.
- Aomatsu T, Imaeda H, Takahashi K, et al. Tacrolimus (FK506) suppresses TNF-alpha-induced CCL2 (MCP-1) and CXCL10 (IP-10) expression via the inhibition of p38 MAP kinase activation in human colonic myofibroblasts[J]. International journal of molecular medicine 30 (2012): 1152-1158.
- Choe JY, Lee SJ, Park SH, et al. Tacrolimus (FK506) inhibits interleukin-1beta-induced angiopoietin-1, Tie-2 receptor, and vascular endothelial growth factor through down-regulation of JNK and p38 pathway in human rheumatoid fibroblast-like synoviocytes[J]. Joint bone spine 79 (2012): 137-143.
- Waschke J, Spindler V. Desmosomes and extradesmosomal adhesive signaling contacts in pemphigus[J]. Medicinal research reviews 34 (2014): 1127-1145.
- Amagai M, Stanley JR. Desmoglein as a target in skin disease and beyond[J]. The Journal of investigative dermatology 132 (2012): 776-784.
- Tsunoda K, Ota T, Aoki M, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3[J]. Journal of immunology (Baltimore, Md.: 1950) 170 (2003): 2170-2178.
- Mahoney MG, Wang Z, Rothenberger K, et al. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris[J]. The Journal of clinical investigation 103 (1999): 461-468.
- Sinha AA, Sajda T. The Evolving Story of Autoantibodies in Pemphigus Vulgaris: Development of the "Super Compensation Hypothesis"[J]. Frontiers in medicine 5 (2018): 218.
- Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus[J]. Journal of dermatological science 48 (2007): 1-14.
- Chernyavsky AI, Arredondo J, Piser T, et al. Differential coupling of M1 muscarinic and alpha7 nicotinic receptors to inhibition of pemphigus acantholysis[J]. The Journal of biological chemistry 283 (2008): 3401-3408.
- Kalantari-Dehaghi M, Chen Y, Deng W, et al. Mechanisms of mitochondrial damage in keratinocytes by pemphigus vulgaris antibodies[J]. The Journal of biological chemistry 288 (2013): 16916-16925.
- Fuchs M, Foresti M, Radeva MY, et al. Plakophilin 1 but not plakophilin 3 regulates desmoglein clustering[J]. Cellular and molecular life sciences: CMLS 76 (2019): 3465-3476.
- Rafei D, Muller R, Ishii N, et al. IgG autoantibodies against desmocollin 3 in pemphigus sera induce loss of keratinocyte adhesion[J]. The American journal of pathology 178 (2011): 718-723.
- Ding L, Wang X, Hong X, et al. IL-36 cytokines in autoimmunity and inflammatory disease[J]. Oncotarget 9 (2018): 2895-2901.
- Das D, Anand V, Khandpur S, et al. T helper type 1 polarizing gammadelta T cells and Scavenger receptors contribute to the pathogenesis of Pemphigus vulgaris[J]. Immunology 153 (2018): 97-104.
- Kalantari-Dehaghi M, Anhalt GJ, Camilleri MJ, et al. Pemphigus vulgaris autoantibody profiling by proteomic technique[J]. PloS one 8 (2013): e57587.
- Fujimura,T, Kakizaki A, Furudate S, et al. A possible interaction between periostin and CD163(+) skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid[J]. Experimental dermatology 26 (2017): 1193-1198.