Structured Jaw Exercise in Head and Neck Cancer Survivors with Trismus Greatly Increases Chances of Being Pain Free 3-Years After Oncological Treatment
Article Information
Paulin Andréll MD, PhD1,2, Therese Karlsson MD, PhD3,4*, Ove Karlsson MD, PhD1,5, Susan Aghajanzadeh, MD3,4, Caterina Finizia MD, PhD, Prof3,4
1Department of Anesthesiology and Intensive Care Medicine, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
2Region Västra Götaland, Sahlgrenska University Hospital Department of Anaesthesiology and Intensive Care Medicine/Paincenter, Sahlgrenska University Hospital, Gothenburg, Sweden
3Department of Otorhinolaryngology, Head and Neck Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
4Region Västra Götaland, Sahlgrenska University Hospital, Department of Otorhinolaryngology, Head and Neck Surgery, Gothenburg, Sweden
5Region Västra Götaland, NU-hospital, Trollhättan, Sweden
*Corresponding Author: Dr. Therese Karlsson, Department of Otorhinolaryngology, Head and Neck Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Citation: Paulin Andréll, Therese Karlsson, Ove Karlsson, Susan Aghajanzadeh, Caterina Finizia. Structured Jaw Exercise in Head and Neck Cancer Survivors with Trismus Greatly Increases Chances of Being Pain Free 3-Years After Oncological Treatment. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 557-573.
Share at FacebookAbstract
Background: The aim of the study was to investigate the prevalence of facial pain in patients with Head and Neck Cancer (HNC) and trismus three years after cancer treatment. Furthermore, the study aimed to assess the effect of a structured jaw exercise program on pain.
Methods: One hundred patients with HNC and trismus (i.e. Maximum Interincisal Opening (MIO) ≤35 mm) post-radiotherapy were prospectively followed up to three years with regard to prevalence of facial pain. Fifty of the patients participated in a structured 10-week intervention program with jaw exercise post-cancer treatment and 50 of the patients comprised a matched control group. Facial pain during last month was reported by the patient on a 7-point Likert scale.
Results: Facial pain was common in HNC patients with trismus before undergoing cancer treatment (n=59, 59%) and more than half of the patients (n=46, 51%) reported facial pain at the three-year follow-up. Patients with facial pain at the three-year follow-up had lower MIO (34.8 vs. 39.6 mm, p=0.0005) compared to pain free patients. Fewer patients in the exercise intervention group (n=13, 28%) reported facial pain compared to the control group (n=33, 77%) (p<0.0001). The odds ratio for being pain free three years post-cancer treatment was 8.63 times higher (p<0.0001) if the patients had undergone structured jaw exercise.
Conclusion: There is a high prevalence of facial pain in HNC and trismus. Structured jaw exercise seems to be effective for improving facial pain in trismus patients up to three years after HNC treatment.
Keywords
Exercise therapy; Head and neck cancer survivors; Health-related quality of life; Mouth opening ability; Oncology; Pain; Prevalence; Radiotherapy; Trismus
Exercise therapy articles; Head and neck cancer survivors articles; Health-related quality of life articles; Mouth opening ability articles; Oncology articles; Pain articles; Prevalence articles; Radiotherapy articles; Trismus articles
Article Details
1. Introduction
Head and neck cancer (HNC) accounts for 5% of malignancies worldwide [1], which is often treated by surgery, chemotherapy, radiotherapy or a combination thereof. More patients survive the initial cancer disease resulting in an increased numbers of patients suffering from pain post treatment [2]. In a recent systematic review and meta-analysis, pain prevalence after curative cancer treatment was 39.3% in cancer survivors [3]. However, the prevalence of pain in HNC survivors is even higher, where 45.1% of the patients report pain and 11.5% of the patients report severe pain [4], which may increase up to 100% if the patients have advanced stages tumours [5]. This is not unexpected due to the intense innervation of the head and neck area as well as the proximity of multiple anatomical structures in a small confined space [6]. Pain post-cancer treatment in HNC patients can be multifactorial, i.e. radiation-induced neural damage and osteoradionecrosis, chemotherapy-induced peripheral neuropathy and may develop after surgery (persistent post-surgical pain), thus involving both nociceptive and neuropathic pain mechanisms [2, 4, 5]. Pain affects the cancer survivors’ physical and mental health negatively and is associated with impaired health related quality of life (HRQL) [2, 5, 7]. Hence, a biopsychosocial approach is recommended when treating cancer-related pain. Opioid treatment is often the
main component in cancer pain management [1] albeit non-pharmacological alternatives such as mindfulness-training and physical exercise potentially also play a role [8, 9].
The pain experienced post-radiotherapy in HNC patients is often caused by inflammation of the mucosa (oral mucositis) yet can also result from radiation-induced fibrosis of the masticatory muscles and temporomandibular joint [10], thereby reducing mouth opening ability - a condition known as trismus. Trismus is defined as a maximum interincisal opening (MIO) of ≤ 35 mm [11] and develops in up to 40% of patients post-radiotherapy [12]. It complicates nutritional intake, dental hygiene, wound healing and negatively impacts patients’ HRQL [13]. Rehabilitation using jaw exercise training has been shown to increase MIO in HNC patients [14].
However, its effect on facial pain in trismus patients over time is unclear. Hence, the aim of the study was to investigate the prevalence of facial pain in HNC patients with trismus three years post-cancer treatment. Furthermore, the study aimed to assess the effect of a structured jaw exercise program on facial pain. The primary hypothesis of the study was that pain is frequent in HNC patients with trismus three years after completed cancer treatment. Our secondary hypothesis was that HNC patients with trismus undergoing structured jaw exercise would report less pain, greater MIO and better HRQL three years post cancer treatment compared to patients who were followed-up according to clinical routine (control group).
2. Material and Methods
2.1 Study participants
All patients with primary HNC tumours diagnosed at five
tertiary centres in Western Sweden were asked for study participation between 2007-2012. Inclusion criteria were curative treatment by radiotherapy ± chemotherapy and the development of trismus. Patients were excluded if they had difficulties filling out questionnaires, were edentulous, had recurrent disease, undergoing primary surgical treatment or had trismus pre-radiotherapy. Patients with tumour locations not expected to develop trismus (hypopharyngeal, laryngeal oesophageal and skin malignancies) were also excluded. Patients residing in Gothenburg were followed clinically post-radiotherapy by an oral surgeon and if trismus developed, patients were invited to enrol in a structured jaw exercise program. A control group was selected from patients outside of Gothenburg city but within its catchment area and were matched according to gender, age, tumour location and stage, comorbidity and radiotherapy dose.
2.2 Study design
Patients were followed pre-radiotherapy, post-radiotherapy (pre-exercise intervention), post-intervention and 36 months post-radiotherapy completion. The intervention group and matched control group were followed at above time-points and at each visit MIO was registered and patients filled in symptom-specific and HRQL-questionnaires.
2.3 Intervention
All patients developed trismus within nine months of completing radiotherapy, where the majority fulfilled the trismus criterion at six months. The intervention group partook in a 10-week structured exercise program where exercises were performed five times daily [14]. Firstly, warm up exercises were performed. Thereafter, passive stretching of the jaw using a jaw mobilising device and finally active moments, i.e. biting down against resistance concluded the exercise. Following completion of the 10-week program, patients were instructed to continue exercising a minimum of three times weekly.
2.4 Oncological treatment
All patients received radiotherapy, of which 77% (n=77) were treated using intensity modulated radiotherapy (IMRT). Between 2007-2009, radiotherapy was administered as accelerated hyperfractionated therapy yielding 1.7 Gy per fraction, twice daily, five times per week totalling 64.6 Gy. During 2010-2012, it was administered as accelerated fractionated therapy of 2 Gy per fraction, once or twice daily, five days per week, totalling 68 Gy. Chemotherapy was given either as induction chemotherapy (cisplatin-fluorouracil) or as concurrent chemotherapy (cisplatin). Radical neck dissection was performed in addition to radiotherapy for 8 patients.
2.5 Outcome measures
2.5.1 Maximum interincisal opening (MIO): MIO was measured using a ruler and encompassed the maximum distance in millimetres between the incisors of the mandible and the maxilla.
2.5.2 Gothenburg Trismus Questionnaire (GTQ) and facial pain: The GTQ is a trismus-specific questionnaire totalling 21 items. It is composed of three domains containing 13 items: jaw related problems (six items), eating limitations (four items) and muscular tension (three items). The remaining eight items are single items addressing facial pain, pain associated with trismus and if trismus is affecting work, leisure or social life. Domains and single items range from 0 to 100, where 100 equals a high symptom burden and 0 represents no symptoms [15]. Facial pain during last month was reported in the GTQ by the patient on a 7-point Likert scale (“no facial pain” to “unbearable pain”). Patients were considered to be pain free if the patient reported “no facial pain” during last month (“no pain” group). Patients who reported any facial pain during last month according to the GTQ, i.e. “very mild pain”, “mild pain”, “moderate pain”, “severe pain”, “very severe pain” or “unbearable pain” were considered to have pain (“pain” group).
2.5.3 European Organisation for Research and Treatment of Cancer (EORTC) Quality of life Core 30 (C30) and Head and Neck 35 (HN35): The EORTC QLQ-C30 is a cancer-specific questionnaire that measures HRQL in patients with cancer [16]. It consists of 30 items, divided into five functional domains; one Global quality of life domain, three symptom scales and six single items. For functional domains and the Global quality of life domain, scores range from 0 to 100, where a high score is equal to a high level of functioning or a high level of global quality of life. For single items, the scores also range from 0 to 100, but a higher score is indicative of a higher symptom burden. A complementary 35-item module is also used, namely the EORTC QLQ-H&N35 [17], which consists of seven symptom scales and 11 single items. As described before in symptom scales and single items, a score of 100 indicates the worst possible symptoms and 0 indicates no symptoms.
2.6 Statistical analysis
The primary variable “facial pain intensity during last month” according to the GTQ was dichotomised in order to distinguish patients that were completely pain free during last month from patients reporting facial pain. Descriptive statistics were calculated according to standard procedures. For comparison between groups, the Mann-Whitney U-test was used for continuous variables, the Mantel-Haenszel Chi2 exact test was used for ordered categorical variables, Chi2 exact test was used for non-ordered categorical variables and Fisher’s exact test was used for dichotomous variables. Univariate and multivariable logistic regression was used for prediction analyses of HNC patients being pain free at three years post-cancer treatment. Continuous variables are reported using mean and confidence intervals. All tests are two-tailed and conducted at a 5% significance level.
2.7 Ethical considerations
The Regional Ethical Review Board at Gothenburg University approved the study. The study was performed in accordance with the Declaration of Helsinki and all participants gave their informed consent prior to study participation.
3. Results
3.1 Patient characteristics
Clinical characteristics for all patients are presented in Table 1. There was no statistically significant difference between the intervention and control group in any of the measured characteristics before start of oncologic treatment. All patients developed trismus within nine months of completing radiotherapy, of which the majority presented with trismus within 3–6 months. At the three-year follow-up, 10 patients were lost to follow-up of which three were in the intervention group (unspecified n=1, deceased n=2) and seven in the control group (unspecified n=1, deceased n=6). At the three-year follow-up, 41 of the 47 (87%) intervention patients no longer had trismus and 32 patients (68%) still exercised. In the control group 14 of 43 (33%) patients were exercising, and 23 of 43 patients (53%) no longer had trismus.
|
Intervention group (n=50) |
Control group (n=50) |
|
|
Mean (range) |
Mean (range) |
|
|
Age mean (range) |
57.9 (30-75) |
58.0 (29-80) |
|
n (%) |
n (%) |
|
|
Gender |
||
|
Female |
19 (38) |
19 (38) |
|
Male |
31 (62) |
31 (62) |
|
ACE-27 |
||
|
No comorbidity |
29 (58) |
20 (40) |
|
Mild comorbidity |
13 (26) |
18 (36) |
|
Moderate comorbidity |
7 (14) |
10 (20) |
|
Severe comorbidity |
1 (2) |
2 (4) |
|
Tumour location |
||
|
Orofarynx |
38 (76) |
38 (76) |
|
Tumor colli |
6 (12) |
6 (12) |
|
Oral cavity |
1 (2) |
1 (2) |
|
Nasofarynx |
5 (10) |
5 (10) |
|
Stadium* |
||
|
I |
1 (2) |
0 (0) |
|
II |
8 (18) |
4 (9) |
|
III |
8 (18) |
12 (27) |
|
IV |
27 (61) |
28 (64) |
|
Treatment regimen |
||
|
Radiotherapy only |
7 (14) |
8 (16) |
|
Radiochemotherapy |
39 (78) |
38 (76) |
|
Radiotherapy + surgery |
4 (8) |
4 (8) |
|
*missing data n=6 in each group since tumor colli/unknown primary is not classified. There were no statistically significant differences between the intervention group and control in any of the measured characteristics at baseline i.e. directly following radiotherapy completion (prior to starting exercise intervention). Abbreviations: ACE-27, Adult Comorbidity Evaluation. |
||
Table 1: Patient characteristics in the intervention group and the control group.
3.2 Prevalence of facial pain over time
A total of 59 patients (59%) reported facial pain prior to commencing radiotherapy. At baseline, i.e. directly following radiotherapy completion (prior to starting exercise intervention), 86 patients (86%) reported facial pain, whilst at the post-intervention and 3-year follow-up corresponding figures were 72 (72%) and 46 (51%) respectively. There was no statistically significant difference between the intervention group and control group in terms of having “no pain” or “pain” at baseline or post-intervention. However, at three years post-radiotherapy completion, facial pain was reported by 33 patients (77%) in the control group compared to 13 patients (28%) in the intervention group (p<0.0001).
3.3 Predictors for being pain free at three years post-radiotherapy
Being in the intervention group increased the chance of being pain free at the three-month follow-up by an odds ratio of 8.63 (p <0.0001) on univariate analysis. This variable remained significant on multivariate analysis and increased to an odds ratio of 9.37 (p <0.0001). No other of the analysed factors were significant in order to predict being pain free at the study end-point.
3.4 Trismus over time
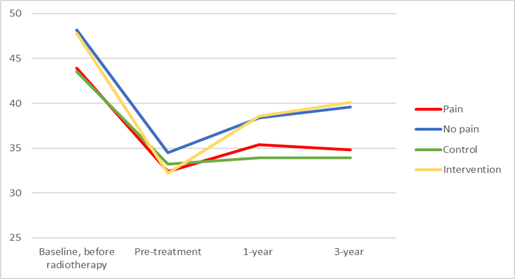
Pre-radiotherapy, patients reporting any presence of facial pain had a mean MIO of 43.9 mm compared to a MIO of 48.2 mm in those reporting no facial pain (p <0.01). Pre-intervention, post-intervention and at the three-year follow-up, the corresponding figures were 32.4 mm vs 34.5 mm (p=0.04), 35.4 mm vs 38.4 mm (p <0.01) and 34.8 mm vs 39.6 mm (p<0.01) for the pain group vs no pain group respectively (Figure 1).
3.4.1 Gothenburg Trismus Questionnaire (GTQ): Results from the GTQ are shown in Table 2. Patients reporting presence of facial pain last month (ranging from mild to unbearable pain) scored significantly worse compared to patients who were pain free in all domains of the GTQ at pre-intervention, post-intervention and at the three-year follow-up. This was also seen at the pre-radiotherapy time-point (data not shown).
3.4.2 EORTC QLQ-C30 and EORTC QLQ-HN35: Pre-radiotherapy, patients with pain reported significantly worse scores on EORTC QLQ-C30 domains Role function, Global quality of life and Pain as well as on EORTC QLQ-HN35 domains Pain, Swallowing, Speech, Social Eating, Sexuality, Opening mouth, Sticky saliva and higher use of Pain killers (data not shown).
Results from EORTC HRQL questionnaires for pre-intervention, post-intervention and at three-year follow-up are presented in Tables 3 and 4. At pre-intervention, patients reporting pain had significantly worse scores in all but three domains on the EORTC QLQ C30 and in 8/14 items on EORTC QLQ-HN35. At post-intervention, patients suffering from facial pain had lower (worse) scores in 5/6 functioning domains on EORTC QLQ C30 and in 7/14 items on the EORTC QLQ HN35. At the three-year follow-up, patients with pain reported significantly worse scores in all EORTC QLQ C30 domains and items (except Constipation and Diarrhoea) and in 12/14 domains/items on the EORTC QLQ HN35. A significantly higher use of pain killers was reported pre-intervention by those patients suffering from pain, whilst no difference in use of pain killers between the “pain” and “no pain” group was observed at post-intervention or the three-year follow-up.
Table 2: GTQ-scores for HNC patients with trismus reporting pain or no pain, pre-intervention, post-intervention and at three-year follow-up.
Table 3: EORTC QLQ C30-score for HNC patients with trismus reporting pain or no pain, pre-intervention, post-intervention and at three-year follow-up, compared to reference data.
Table 4: EORTC QLQ H&N35-score for HNC patients with trismus reporting pain or no pain, pre-intervention, post-intervention and at three-year follow-up, compared to reference data.
4. Discussion
This study showed that the majority of HNC patients (59%) suffered from facial pain prior to cancer treatment and more than half (51%) of the HNC survivors with trismus report facial pain after three years of follow-up. However, by participating in a 10-week structured jaw exercise program, the chance of being pain-free three years after cancer treatment is greatly improved for this patient group. The study provides increased knowledge of the long-term prevalence of facial pain as well as the long-term benefits of structured jaw exercise on facial pain in this patient group.
At three years post treatment completion, the prevalence of facial pain remains nearly unchanged (51%), which is much in line with systematic reviews, reporting pre-treatment pain in up to 51% of patients [7]. Cramer et al found that 45% of 175 HNC patients reported pain at a median of 6.6 years following diagnosis, of which 11.5% had severe pain [4]. Similarly, Rogers et al reported the presence of pain in 50% of patients three to 14 years following oncological treatment for oral and oropharyngeal tumours [18]. Whilst the chronic pain experienced by HNC survivors is multifactorial, trismus is a major potential aetiological factor being a late radiotherapeutic side-effect caused by fibrosis of the masticatory muscles and temporomandibular joint [10]. At all study time-points, patients suffering from pain consistently had significantly lower MIO as well as reduced HRQL compared to those reporting no pain. There is a clear relationship between pain, trismus and HRQL, which was also highlighted by Cramer et al, where 46% of patients with pain had low overall quality of life (QOL) versus only 12% of those without pain. Having pain increased the odds ratio of a low QOL by 2.2 [4]. Hence, this study emphasises that pain is reported by the majority of HNC patients and they exhibit both reduced MIO and HRQL.
Post-intervention and at the three-year follow-up, there was surprisingly no significant difference in the use of pain killers between patients reporting pain and those having no pain, suggesting that patients with pain may be under-treated. It is estimated in the literature that cancer pain is insufficiently treated in up to 50% of patients, mainly due to fear of creating opioid dependency [1, 3]. However, pain at one-year post-treatment in HNC patients has been shown to be the strongest predictor of 5-year HRQL outcomes [5]. Even more importantly, Scharpf et al found that the survival rate was 81.8% for patients with low post-treatment pain compared to 65.1% for those with high pain, concluding that pain level is an independent predictor of 5-years survival [19]. Therefore, it is incredibly important to identify HNC patients suffering from pain and treat them as effectively as possible.
With pain being a predominant symptom in post-cancer care, studies have tried to pinpoint predictors of pain among HNC populations. Trimodality treatment (surgery and chemoradiotherapy) has been associated with an increased risk of pain (odds ratio 3.55) along with pre-treatment pain, depressive symptoms, feeding tube at one year, less physical activity, neck dissection and poor sleep quality [20]. Chronic pain, however, is more difficult to record due to the choice of outcome measure, alterations of patient perceptions and the time-definition of chronic. Its prevalence varies between 8-60% and risk is associated with radiation technique, tumour subsite and psychological issues [5]. Despite the focus on pain predictors, there are no studies investigating predictors of being pain free. We found that if patients with trismus perform a 10-week structured jaw exercise program, they have a 9 times higher chance of being pain-free three years following treatment. This pain may otherwise be treated by opioid analgesics, which is often necessary in HNC [7]. Thus, by performing jaw exercises the use of opioid analgesics could potentially be avoided long-term or at least minimized, thereby limiting risk of known long-term side effects such as opioid-induced hyperalgesia and endocrine dysfunction as well as negative effects on cognitive function [1]. In addition, jaw exercise is a safe non-pharmacological treatment option compared to for instance Pregabalin, which has been proposed to improve HRQL in patients with trismus following treatment for nasopharyngeal carcinoma, albeit with no significant effect on MIO (21).
5. Clinical Implications
In light of the high pain prevalence, potential under-treatment and having been shown to negatively influence both survival and HRQL, it is undoubtedly extremely important to address and treat the symptom. A simple, time-efficient, safe, non-pharmacological and cost-effective 10-week jaw training exercise programme has been shown to greatly increase chance of being completely pain-free up to three years after treatment and should be offered to all patients with pain and trismus following radiotherapy. The patients were recommended to continue to perform jaw exercises after the 10-week exercise program. According to clinical experience, many patients continued to perform jaw exercises and increased exercise frequency if they experienced reduced mouth opening ability. Hence, continued jaw exercise, especially when experiencing deterioration, should be recommended.
Rehabilitative measures for the surviving HNC population was also recently highlighted in an extensive review by Rodriguez et al. [22] and as focus is shifting to preventing symptoms rather than treating them once they surface, the aspect of prophylactic jaw exercise training needs to be investigated. In order to improve pain management in this patient group, careful follow-up by a multidisciplinary team is warranted, including otorhinolaryngologists, oncologists and medical pain experts.
6. Strengths and Limitations
A strength in this study lies in its prospective design as well as the dichotomisation of the main variable pain, as it assures the freedom of pain. The prevalence of pain at each time-point is a point-prevalence and consists of different patients at each measurement. A limitation here is that pain has not been assessed over time for each individual patient. Thus, the prevalence of chronic pain (i.e. pain lasting >3 months) has not been assessed. The cause of pain and the underlying pain mechanism were not assessed in the study. However, regardless of type of pain and underlying cause, structured jaw exercise was effective for reducing pain in HNC survivors with trismus, making the results generalizable. Since the study was not randomized, selection bias was minimized by the inclusion of a carefully matched control group. Nevertheless, further randomized controlled studies are required to confirm this finding.
7. Conclusions
Facial pain is reported by more than half of HNC survivors with trismus prior to cancer treatment as well as at three years post-treatment completion. Facial pain severely impairs jaw opening and is associated with a reduced HRQL. Structured jaw exercise greatly improves the chance of being pain free three years after radiotherapy.
Funding
This study was funded by the Swedish Cancer Society, the Research and Development Council (FoU), Västra Götaland County, Sweden, The Assar Gabrielsson Foundation Göteborg and the Medical Faculty of Gothenburg University Sweden.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We would like to thank oral surgeon Bodil Fagerberg-Mohlin at the Department of Oral and Maxillofacial Surgery, Institute of Odontology and Public Dental Service, Gothenburg, Sweden for performing all patient interventions, data collection, and expert advice.
References
- Bossi P, Ghiani M, Argenone A, Depenni R. Is pain part of a systemic syndrome in head and neck cancer? Support Care Cancer. 28 (2020): 451-459.
- Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain 8 (2014): 139-153.
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 51 (2016): 1070-90.e9.
- Cramer JD, Johnson JT, Nilsen ML. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol Head Neck Surg 159 (2018): 853-858.
- Bossi P, Giusti R, Tarsitano A, Airoldi M, De Sanctis V, Caspiani O, et al. The point of pain in head and neck cancer. Crit Rev Oncol Hematol 138 (2019): 51-59.
- Chua KS, Reddy SK, Lee MC, Patt RB. Pain and loss of function in head and neck cancer survivors. J Pain Symptom Manage 18 (1999): 193-202.
- Mirabile A, Airoldi M, Ripamonti C, Bolner A, Murphy B, Russi E, et al. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit Rev Oncol Hematol 99 (2016): 100-106.
- van Nieuwenhuizen AJ, Buffart LM, van Uden-Kraan CF, van der Velden LA, Lacko M, Brug J, et al. Patient-reported physical activity and the association with health-related quality of life in head and neck cancer survivors. Support Care Cancer 26 (2018): 1087-1095.
- Kvillemo P, Bränström R. Experiences of a mindfulness-based stress-reduction intervention among patients with cancer. Cancer Nurs 34 (2011): 24-31.
- Blanchard D, Bollet M, Dreyer C, Binczak M, Calmels P, Couturaud C, et al. Management of somatic pain induced by head and neck cancer treatment: pain following radiation therapy and chemotherapy. Guidelines of the French Otorhinolaryngology Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis 131 (2014): 253-256.
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg 35 (2006): 337-342.
- Pauli N, Johnson J, Finizia C, Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol 52 (2013): 1137-1145.
- Johnson J, Johansson M, Rydén A, Houltz E, Finizia C. Impact of trismus on health-related quality of life and mental health. Head Neck 37 (2015): 1672-1679.
- Pauli N, Fagerberg-Mohlin B, Andréll P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol 53 (2014): 502-509.
- Johnson J, Carlsson S, Johansson M, Pauli N, Rydén A, Fagerberg-Mohlin B, et al. Development and validation of the Gothenburg Trismus Questionnaire (GTQ). Oral Oncol 48 (2012): 730-736.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85 (1993): 365-376.
- Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 36 (2000): 1796-1807.
- Rogers SN, Miller RD, Ali K, Minhas AB, et al. Patients' perceived health status following primary surgery for oral and oropharyngeal cancer. Int J Oral Maxillofac Surg 35 (2006): 913-919.
- Scharpf J, Karnell LH, Christensen AJ, et al. The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Head Neck Surg 135 (2009): 789-794.
- Shuman AG, Terrell JE, Light E, et al. Predictors of pain among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 138 (2012): 1147-1154.
- Li H, Yao Q, Huang X, et al. Therapeutic effect of pregabalin on radiotherapy-induced trismus in nasopharyngeal carcinoma patients. Eur Ann Otorhinolaryngol Head Neck Dis 136 (2019): 251-255.
- Rodriguez AM, Komar A, Ringash J, et al. A scoping review of rehabilitation interventions for survivors of head and neck cancer. Disabil Rehabil 41 (2019): 2093-2107.