Soil-Transmitted Protozoans and Helminths from Market Gardening Sites of Yaounde, Cameroon
Article Information
Herman Parfait Awono-Ambene1,2, Laurelle Djieukap Njieyap1,3, Patrick Akono Ntonga2, Josiane Désirée Etang1,4, Christophe Antonio-Nkondjio1,5, Cyrille Ndo1,4, Jacques Etame6, Flobert Njiokou3, Serge Hubert Zebaze Togouet7,*
1Research Institute of Yaounde, OCEAC, Cameroon
2Laboratory of Animal Organism Biology and Physiology, Faculty of Sciences, University of Douala, Cameroon 3Laboratory of Parasitology and Ecology, Faculty of Sciences, University of Yaounde I, Cameroon
4Faculty of Medicine and Pharmacy, University of Douala, Cameroon
5LSTM Wellcome Trust Senior Fellowship, Cameroon
6Department of Earth Sciences, Faculty of Sciences, University of Douala, Cameroon
7Laboratory of Hydrobiology and Environment, Faculty of Sciences, University of Yaounde I, Cameroon
*Corresponding Author: Pr Serge Hubert Zebaze Togouet , University of Yaounde I, Campus of Ngoa-Ekele, Yaounde 3, Center region, Cameroon, Africa
Received: 03 March 2020; Accepted: 17 March 2020; Published: 8 May 2020
Citation:
Herman Parfait Awono-Ambene, Laurelle Djieukap Njieyap, Patrick Akono Ntonga, Josiane Désirée Etang, Christophe Antonio-Nkondjio, Cyrille Ndo1, Jacques Etame, Flobert Njiokou, Serge Hubert Zebaze Togouet. Soil-Transmitted Protozoans and Helminths from Market Gardening Sites of Yaounde, Cameroon. Journal of Environmental Science and Public Health 4 (2020): 61-.70.
Share at FacebookAbstract
Background: In Cameroon, the practice of market gardening exposes both the farmers and consumers to a certain number of health-related hazards. Environmental health risks associated to this activity showed that it was responsible for the increased of transmission risk of the human and animal intestinal worms.
Methods: The epidemiology of soil borne diseases associated with urban agriculture was furthermore considered in three market garden sites of the city of Yaounde (Tsinga, Nkolondom, Nkolbisson). From October to December 2016, water and soils from rivers, wells and furrows were analysed for physicochemical characteristics and parasite identification using Kato-Katz and Formol-Ether methods.
Results: Means of water parameters and parasite composition varied per locations and environments in the 82 samples analyzed. Ranges of mean temperature, pH, electrical conductivity, turbidity and color of water samples were 25.2-25.5°C (), 6.53-7.01 UC, 308-502 µS.cm-1, 637-1,300 FTU and 2,451-6,422 Co-Pt, respectively. Soil parasites consisted of 3 protozoans (Entamoeba histolytica, Sarcocystis sp. and Giardia intestinalis) and 11 helminths (Fasciola hepatica, Diphyllibotrium latum, Ascaris lumbricoides, Trichuirus trichiura, Ancylostoma sp., Trichostrongylus sp., Schistosoma mansoni, Schistosoma sp., Paragonimus westermanii, Strongyloides stercoralis and Hymenolepis nana).
Conclusion: The presence of viable helminths suggests that harmful soil pathogens such as protozoans and helminths can spread through market gardening. This reminds on the need to find better ways to alleviate the control of intestinal parasitic infections which, nevertheless, are classified as neglected tropical diseases.
Keywords
Distribution; Soil parasites; Market gardening; Yaounde
Distribution articles, Soil parasites articles, Market gardening articles, Yaounde articles
Distribution articles Distribution Research articles Distribution review articles Distribution PubMed articles Distribution PubMed Central articles Distribution 2023 articles Distribution 2024 articles Distribution Scopus articles Distribution impact factor journals Distribution Scopus journals Distribution PubMed journals Distribution medical journals Distribution free journals Distribution best journals Distribution top journals Distribution free medical journals Distribution famous journals Distribution Google Scholar indexed journals Soil parasites article Soil parasites Research articles Soil parasites review articles Soil parasites PubMed Central articles Soil parasites 2023 articles Soil parasites 2024 articles Soil parasites Scopus articles Soil parasites impact factor journals Soil parasites Scopus journals Soil parasites PubMed journals Soil parasites medical journals Soil parasites free journals Soil parasites best journals Soil parasites top journals Soil parasites free medical journals Soil parasites famous journals Soil parasites Google Scholar indexed journals Market gardening articles Market gardening Research articles Market gardening review articles Market gardening PubMed Central articles Market gardening 2023 articles Market gardening 2024 articles Market gardening Scopus articles Market gardening impact factor journals Market gardening Scopus journals Market gardening PubMed journals Market gardening medical journals Market gardening free journals Market gardening best journals Market gardening top journals Market gardening free medical journals Market gardening famous journals Market gardening Google Scholar indexed journals environmental health articles environmental health Research articles environmental health review articles environmental health PubMed articles environmental health PubMed Central articles environmental health 2023 articles environmental health 2024 articles environmental health Scopus articles environmental health impact factor journals environmental health Scopus journals environmental health PubMed journals environmental health medical journals environmental health free journals environmental health best journals environmental health top journals environmental health free medical journals environmental health famous journals environmental health Google Scholar indexed journals bacteria articles bacteria Research articles bacteria review articles bacteria PubMed Central articles bacteria 2023 articles bacteria 2024 articles bacteria Scopus articles bacteria impact factor journals bacteria Scopus journals bacteria PubMed journals bacteria medical journals bacteria free journals bacteria best journals bacteria top journals bacteria free medical journals bacteria famous journals bacteria Google Scholar indexed journals urban farming articles urban farming Research articles urban farming review articles urban farming PubMed Central articles urban farming 2023 articles urban farming 2024 articles urban farming Scopus articles urban farming impact factor journals urban farming Scopus journals urban farming PubMed journals urban farming medical journals urban farming free journals urban farming best journals urban farming top journals urban farming free medical journals urban farming famous journals urban farming Google Scholar indexed journals groundwater articles groundwater Research articles groundwater review articles groundwater PubMed Central articles groundwater 2023 articles groundwater 2024 articles groundwater Scopus articles groundwater impact factor journals groundwater Scopus journals groundwater PubMed journals groundwater medical journals groundwater free journals groundwater best journals groundwater top journals groundwater free medical journals groundwater famous journals groundwater Google Scholar indexed journals plant watering articles plant watering Research articles plant watering review articles plant watering PubMed Central articles plant watering 2023 articles plant watering 2024 articles plant watering Scopus articles plant watering impact factor journals plant watering Scopus journals plant watering PubMed journals plant watering medical journals plant watering free journals plant watering best journals plant watering top journals plant watering free medical journals plant watering famous journals plant watering Google Scholar indexed journals pathogens article pathogens Research articles pathogens review articles pathogens PubMed Central articles pathogens 2023 articles pathogens 2024 articles pathogens Scopus articles pathogens impact factor journals pathogens Scopus journals pathogens PubMed journals pathogens medical journals pathogens free journals pathogens best journals pathogens top journals pathogens free medical journals pathogens famous journals pathogens Google Scholar indexed journals Soil articles Soil Research articles Soil review articles Soil PubMed articles Soil PubMed Central articles Soil 2023 articles Soil 2024 articles Soil Scopus articles Soil impact factor journals Soil Scopus journals Soil PubMed journals Soil medical journals Soil free journals Soil best journals Soil top journals Soil free medical journals Soil famous journals Soil Google Scholar indexed journals
Article Details
1. Introduction
Market gardening is expanding with little regulation in African cities as alternative responses to poverty and food insecurity [1]. Landscapes used for its practice, are usually valleys and swampy areas with easy access to rivers and groundwater for plant watering. Wells and furrows are created between crops to facilitate plant’s irrigation. People living in these areas rely on market gardening for living despite the potential impact on health. Health risks in urban farming may be contamination with pathogens and chemical residues from polluted water sources, human disease transmitted by some vectors and zoonosis transmitted by domestic animals [2]. This situation calls for good management of risks through holistic approaches presently driven by the “One health” concept, which is promoting integrated actions for prevention of zoonotic and infectious diseases [3]. In Cameroon, market gardening contributes to food supply at the national level and neighboring country markets [4, 5]. Despite his positive economic impact as source of income and wellbeing for local communities, the practice of market gardening exposed both the farmers and consumers to a certain number of health-related hazards, such as organic pollution and spreading of communicable diseases [6, 7]. Almost 32 000 households were involved in market gardening in Yaounde [1] and the number has certainly increased over years. Previous studies on environmental health risks associated with urban farming and livestock activities showed that the combination of the two activities was responsible for increased transmission risk of the human and animal intestinal worms [8, 9]. Soil pathogens are usually coliform bacteria, protozoans and helminths, but there are knowledge gaps on their potential dissemination routes. This paper provides a comprehensive mapping of soil transmitted protozoans and helminths in vegetable farming environments of the capital city Yaounde.
2. Materials and Methods
2.1 Study period and sitesThe study was conducted from October to December 2016 in 3 selected market gardening locations of the capital city Yaounde, Cameroon. Figure 1 shows the 3 different studied areas. These sites were Nkolondom (N 3° 57’ 11.89”; E 11° 29' 41.01'') crossed by the river Ntsas, Nkolbisson (N 3° 52' 8.48''; E 11° 27' 14.39'') crossed by Afeme and Mefou rivers, and Tsinga (N 3° 52' 57.16''; E 11° 30' 40.66'') watered by West Abiergue stream. The study area belongs to the equatorial domain and experienced a bimodal precipitation regime. The landscape is dominated by plains and valleys. The local hydrographic network is mainly composed of the river Mfoundi and its tributaries [10, 11]. Market gardening activities peak with an increase access to farming ground and water sources during dry seasons.
Figure 1: Map of administrative subdivisions of Yaounde showing selected study sites.
2.2 Field sampling procedures
Muddy water samples were selected from aquatic environments used for crop’s irrigation, like furrows, rivers/streams, wells. Environments were prospectively chosen based on their availability in the study area. After the measurement of temperature in the field, each sample consisted of a minimum of 250 ml per collection in different location, stored in plastic bottles and transported immediately to the laboratory for subsequent analysis. 2.3 Physicochemical measurementsPhysicochemical parameters were measured in water samples collected from various aquatic environment of the studied areas. In water samples stored properly in laboratory, electrical conductivity and pH were measured by a multimeter and a portable spectrophotometer was used to quantify color and turbidity [12].
2.4 Parasite identification in soil samples
Muddy water samples were left for 24 h at room temperature before proceeding for parasite identification. Kato-Katz and Formalin-ether techniques currently used for stools were adapted for parasite identification in soil samples. Sample preparations were performed according standards WHO protocols [13]. About 150 µl of sample were used for slide preparation, prior the detection of protozoan and helminths. All stages were identified following their morphology using microscope (magnification ×100 or ×400) [14]. Parasite viability was determined using morphological criteria that combined egg integrity (size, color, shape) and the presence of visible and/or motile larvae under microscope.
2.5 Data analysis
Parasites were counted for each 150 µl samples and the density (D) was estimated per gram of pellet according to WHO formula: D , with n as the number of parasites. The species richness and the multiple site similarity were used to analyze diversity and distribution [15]. The following formula was used to estimate the three site similarity index: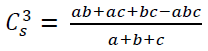 [16]; with a, b, c as numbers of species found in location A, B, C; ab, ac, bc, abc as the number of species shared by locations A+B, A+C, B+C and A+B+C, respectively. There is no similarity when C3stends to 0 and complete species similarity when C3stends to 1.
[16]; with a, b, c as numbers of species found in location A, B, C; ab, ac, bc, abc as the number of species shared by locations A+B, A+C, B+C and A+B+C, respectively. There is no similarity when C3stends to 0 and complete species similarity when C3stends to 1.
3. Results
About 82 samples were checked for physicochemical and parasitological analysis, including 50 (61%) in Nkolondom (14 in wells, 13 in rivers and 23 in furrows), 20 (24%) in Tsinga (6 in wells, 6 in rivers and 8 in furrows) and 12 (15%) in Nkolbisson (3 in wells, 6 in rivers and 3 in furrows).
|
Physicochemical parameters |
Furrows (n=33) |
Wells (n=22) |
Rivers (n=25) |
|
Temperature ± CI 95% (°C) |
25.5 ± 0.7 |
25.5 ± 0.8 |
25.2 ± 0.7 |
|
pH ± CI 95% (U.C) |
6.99 ± 0.12 |
6.53 ± 0.28 |
7.01 ± 0.29 |
|
Conductivity ± CI 95% (µS/cm) |
502.2 ± 104.4 |
315.0 ± 116.7 |
308.8 ± 115.3 |
|
Turbidity ± CI 95% (FTU) |
1,299.5 ± 725.8 |
637.0 ± 185.3 |
715.0 ± 387.1 |
|
Color ± CI 95% (Pt-Co) |
6,422.4 ± 3,118.5 |
2,451.53 ± 1,126.6 |
4,961.9 ± 4,420.9 |
Table 1: Overall mean values of physicochemical parameters recorded from October to December 2016 in sample sites of the three selected study locations.
3.1 Physicochemical characterization
(Table 1) shows an overview of the means variations of some Physicochemical parameters of furrows (N=33), rivers (N=26) and wells (N=23). We observed a range of 25.2-25.5 °C for temperature, 6.53-7.01 UC for pH, 308.8-502.2 µS.cm-1 for electrical conductivity, 637-1,300 FTU for turbidity and 2,451-6,422 Co-Pt for color. High values of electrical conductivity of studied environments indicated mineralization in Tsinga, whereas pH and turbidity values in such environments in Nkolbisson suggested abundance of organic matters (data not shown).
3.2 Parasite density and diversity
Five hundred and one parasite stages were identified including 207 protozoan cysts (three species) and 294 helminths eggs/larvae (eleven species). The overall parasite load was 7,056/L and 4,968/L for helminths and protozoans, respectively, with significant variations in locations and environments. The mean parasite load was 151.2/L in Nkolondom (102.2/L helminths+ 49.0/L protozoans), 224.0/L in Nkolbisson (72.0/L helminths+152.0/L protozoans) and 88.8/L in Tsinga (54.0/L helminths+34.8/L protozoans). In (Figure 2) variation in parasites density is observed respectively in wells, rivers and furrows 171.1/L (68.9/L helminths; 102.3/L protozoans), 116.1/L (78.7/L helminths, 37.4/L protozoans), 152.5/L (103.1/L helminths, 49.3/L protozoans). Strongyloides stercoralis (2,280 larvae/L) was the most abundant helminth, followed by Fasciola hepatica (1,536 eggs/L), Ascaris lumbricoïdes (1,008 eggs/L), Diphyllobotrium latum (1,008 eggs/L) and Trichuris trichiura (408 eggs/L). In protozoans, Entamoeba histolytica represented 84% (4,152 cysts/L) of samples, Giardia intestinalis (672 cysts/L) and Sarcocystis sp. (144 cysts/L) 13% and 3%, respectively.
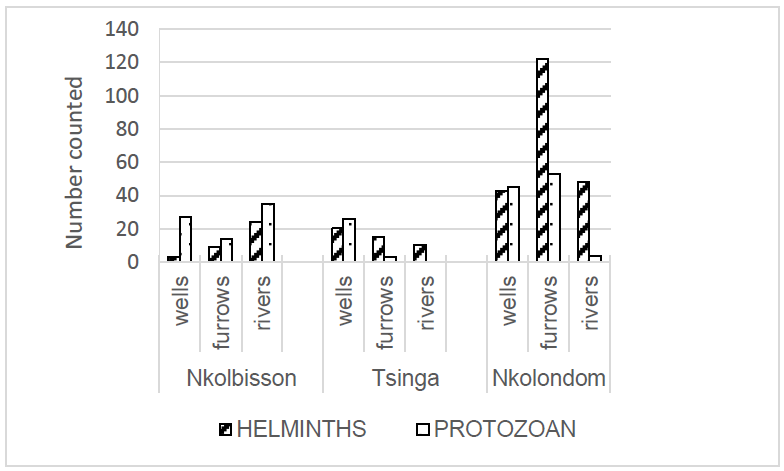
Figure 2: Number of helminths stages (egg/larvae) and protozoans (cysts) distributed in soil samples from three aquatic environments.
3.3 Parasite distribution and site species similarity
(Figure 2) indicates a significant variation of the parasite distribution by locations (X2=43.05, df=2, P<10-4) and environments (X2=34.19, df=2, P<10-4). Nkolondom (315 parasites, 68% helminths vs 32% protozoans) showed high parasite density compared to Nkolbisson (112 parasites, 32% helminths vs 68% protozoans) and Tsinga (74 parasites, 61% helminths vs 39% protozoans). In environments, furrows recorded 216 parasites (68% helminths vs 32% protozoans), wells 164 parasites (40% helminths vs 60% protozoans) and rivers 121 parasites (68% helminths vs 32% protozoans). Some disparities were observed between helminths and protozoans within study locations, in Nkolondom (helminths ? protozoans; X2=28.964, df=2, P<10-4), in Tsinga (helminths ? protozoans; X2=13.463, df=1, P=0.0002) and in Nkolbisson (protozoans ~ helminths; X2=3.378, df=1, P=0.066). The species richness reached 14, 8 and 7 in Nkolondom, Tsinga and Nkolbisson respectively.
*: sample less than 10
Table 2: Frequency, density and viability of parasites per studied locations and environments using combined Kato-Katz and Formol-Ether methods.
From (Table 2), we can clearly see the species richness in different areas. This observation was consistent with the species similarity between locations (Cs=0.52) and environments (Cs=0.56), indicating that almost 52% and 56% of species are shared by all the locations and environments respectively. Ascaris lumbricoïdes, Trichuris trichuira, Fasciola hepatica, Strongyloides stercoralis and Entamoeba histolytica were found in all locations while Hymenolepis nana, Paragonimus westermanii, Sarcocystis sp. and Giardia intestinalis were identified in Nkolondom. Three species were apparently confined in furrows.
3.4 Parasite viability
The overall parasite viability rate is clearly indicated in Table 2. Effectively, 5.1% and only seven helminths showed viable stages (larvae or eggs). The rate was 7.1% in wells, 2.2% in rivers and 1.6% in furrows. Viable helminths were found in Nkolondom (3.8%) and Tsinga (15.6%) while all parasites identified in Nkolbisson were unviable.
4. Discussion
The study was designed to gather updated information on the distribution of protozoans and helminths in three market gardening sites of the city of Yaounde. It came out from the results that helminths and protozoans were present in studied locations. This finding was consistent with previous works carried out in the same area and suggests the endemicity of these groups of pathogens involved in diarrheal diseases and zoonosis in these environments [7]. Entamoeba histolytica (~84%) as a protozoan and helminths i.e. roundworms (S. stercolaris and A. lumbricoïdes), sheep fluke F. hepatica and fish tapeworm D. latum represented almost 80% of total soil parasite species. These pathogens together with fecal coliform bacteria are distributed in environments through rivers and traditional wells used by farmers for plant watering and irrigation. They can cause diarrheal diseases and/or zoonosis in farmers or consumers of farming products. Entamoeba histolytica distributed in all locations, and the similar trend observed in marshy sites in Douala [17] and Ndjamena [18] suggests his predilection for urban areas. These parasites are those causing intestinal disorders like roundworms (S. stercolaris and A. lumbricoïdes), the whipworm T. trichuira and the tapeworm H. nana species. Other zoonotic helminths i.e. D. latum and F. hepatica, and Trichostrongylus sp. seems to be related to slaughter activities nearby selected locations. The presence of Schistosoma sp. (probably S. guineensis or S. intercalatum) and P. westermanii considered as non-endemic in our study locations is indicative for possible importation from other sources of contamination.
Few observations in study location had already identified: i) inappropriate agricultural practices, ii) various health constraints (feces excreted by human/animals, hospital’s wastewater and iii) health status of local populations as factors contributing to environmental contaminations [19-21]. Poor hygiene and environmental conditions in lowlands and agricultural settings were related to the abundance of helminths, protozoans and faecal coliform bacteria [10, 22, 23]. Species distribution showed modest diversity per location and environment, and discrepancies observed in our study could be due to soil composition and water physicochemical properties. In fact, the highest parasite density and species richness in clay soils in Nkolondom contrasted with the reduction in species abundance in sandy environments from Tsinga. The predominance of organic matter in water (furrows) was advantageous for parasite conservation than high electrical conductivity conditions (rivers and wells) resulting from the water mineralization [12].
Only helminths showed viable stages in Tsinga and Nkolondom, and especially in wells. Therefore, the persistence of viable parasites although in low frequencies constitutes a potential health risk associated with agriculture. Several factors may contribute to this parasite viability. Firstly, low temperature, high humidity, poor exposure to sun and clay soils may enhance the survival of some helminth eggs and protozoan cysts in the environment [24]. Secondly, external membrane of helminth eggs may exhibit more resistance compared with that of protozoan cysts [25, 26] and then pesticide utilization can reduce parasite survival [27]. Our findings are certainly informative in assessing the potential environment health risk of urban agriculture practices but could be limited the concentration methods used to assess the composition and the viability of parasite stages. These concentration microscopy-based techniques usually performed for the detection parasite in stools may display low performances compared with mini-FLOTAC and molecular techniques [28].
5. Conclusion
The potential environmental health risk due to the presence of harmful protozoans and helminths in market gardening areas of the capital city of Cameroon reminds us on the need to find better ways to alleviate the control of intestinal parasitic infections which, nevertheless, are classified as neglected tropical diseases. Adopting an integrated strategy based on the environmental management of health risks and the development of capacities of farmers and other actors of the sector should be preliminary steps towards the reduction of negative health effects of urban agriculture in Africa.
Acknowledgements
We thank Mr Bayibeki Ngano Albert and Mr Mvondo Narcisse for their technical assistance in the field. This work forms part of a ‘‘PADY-2 project funded by BAD-AFD-GEF through a service provision of OCEAC to Société Services Camerounais d’Assainissement (SECA).
Conflict of Interest
The authors declare that they have no conflict of interests relevant to the subject of this manuscript.
References
- Growing greener cities in Africa. First status report on urban and peri-urban horticulture in Africa. Rome, Food and Agriculture Organization of the United Nations (2012).
- Birley M, Lock K. Health and periurban natural resource production. Environment and Urbanisation 10 (1999): 89-106.
- Destoumieux GD, Mavingui P, Boetsch G, et al. The one health concept: 10 years old and a long road ahead. Frontiers in Veterinary Sciences (2018):
- Asongwe GA, Yerima BP, Tening AS. Vegetable Production and the Livelihood of Farmers in Bamenda Municipality, Cameroon. International Journal of Current Microbiology and Applied Sciences 3 (2014): 682-700.
- Ball A. The Future of Agriculture in Cameroon in the Age of Agricultural Biotechnology Independent Study Project 2287 (2016): 53.
- Antonio-Nkondjio C, Fossog BT, Ndo C, et al. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaounde (Cameroon): influence of urban agriculture and pollution. Malaria Journal 10 (2011): 154.
- Djouaka R, Zeukeng F, Soglo ME, et al. Heavy metal contamination and faecal coliforms in peri-urban market gardening sites in Benin and Cameroon. International Journal of Agriculture and Environmental Research 5 (2016): 1013-1044.
- Bopda A, Brummet R, Dury S, et al. Urban farming systems in Yaoundé-Building a Mosaic, G. Prain et al. (Ed), African Urban Harvest (2010): 39-59.
- Ajeagah GA, Moussima D. Study of the influence of environmental factors on the occurrence of Balantidium coli cysts in an urban aquatic system in Cameroon. Journal of Ecology and the Natural Environment 6 (2014): 190-199.
- Ajeagah GA, Chumtchoua AL, Mbouombouo M, et al. Evaluation de l’abondance des kystes des protozoaires flagellés dans les eaux usées exploitées en agriculture maraîchère en zone urbaine de Yaoundé (Cameroun). Journal of Applied Biosciences 107 (2016): 10450-10459.
- Nguendo YH. Human Settlement, Land Management and Health in Sub Saharan Cities. International Journal of Social Sciences 4 (2009): 10-17.
- Suchel J. Les climats du Cameroun. Thèse de doctorat d’état, Université Saint-Etienne, France (1988): 1177.
- Rodier J, Legube B, Merlet N. L’analyse de l’eau. In Dunod (9e Ed), Paris (2009): 1526.
- Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organization (1994).
- Tanowitz HB, Weiss LM, Diagnostic and treatment of intestinal helminths, Common intestinal cestodes. The Gastroenterologist 1 (1993): 265-273.
- Diserud OH, Odegaard F. A multiple-site similarity measure. Biology Letter 3 (2007): 20-22.
- Magurran A. Measuring biological diversity. Oxford, UK: Blackwell Publishing (2004).
- Lehman L, Kouodjip NL, Bilong C. Diagnostic des parasitoses intestinales à l’aide de la microscopie à fluorescence. Médecine d’Afrique Noire 59 (2012): 377-385.
- Hamit A, Tidjiani M, Bilong C. Prevalence of intestinal parasites in N’Djamena, Chad Republic. African Journal of Environmental Science and Technology 2 (2008): 407-411.
- Kenfack Essougong UP. Urban and peri-urban agriculture in Cameroon: Status and perspectives for development. International Journal of Agronomy and Agricultural Research 11 (2017): 116-127.
- Hinson AV, Mbaduet Y, Djouaka R, et al. Health hazards linked to the quality of the irrigation water and to the consumption of the vegetables grown in the market gardening site of Nkolondom, (Yaounde-Cameroon) Journal of Agriculture and Research 4 (2018): 35-52.
- Bouhoum K, Mahmid O, Habba K et al. Devenir des œufs d’helminthes et des kystes de protozoaires dans un canal à ciel ouvert alimenté par les eaux usées de Marrakech. Revue des Sciences de l'Eau 2 (1997): 217- 232.
- Etewa S, Adel-Rahman S, Naglaa F, et al. Geohelminths distribution as affected by soil properties, chemical factors and climate change in Sharkyia governorate Egypt. Journal of Parasitic Diseases 40 (2014): 496-504.
- Keffala C, Harerimana C, Vasel J. Œufs d’helminthes dans les eaux usées et les boues de station d’épuration: enjeux sanitaires et intérêt du traitement par lagunage. Environnement Risque Santé 11 (2012): 511-520.
- Kpoda N, Ouela A, Yélézouomin S, et al. Physicochemical and parasitological quality of vegetables irrigation water in Ouagadougou city, Burkina-Faso. African Journal of Microbiology Research 9 (2015): 308-317.
- Maya C, Ortiz M, Jiménez, B. Viability of Ascaris and other helminth genera non larval eggs in different conditions of temperature, lime (pH) and humidity. Water Science. Technology 62 (2010): 2616-2624.
- Monte T, Junior A. What do we know about the effects of pesticides on helminths? Journal of Science and Medical Biology 2 (2017): 1-6.
- Khurana S, Sethi S. Laboratory diagnosis of soil transmitted helminthiasis. Tropical parasitology 7 (2017): 86-91.
