Short-Term Intervention with Garlic-Based diets Modulates mRNA Expression of Calmodulin, Inositol-1,4,5-Trisphosphate Receptor-1, and Intracellular Adhesion Molecule in Cyclosporin-Induced Prehypertensive Wistar Rats
Article Information
Ajayi, Olubunmi Bolanle and Ajayi, Oluwadamilare Oluwaseun*
Department of Biochemistry, Ekiti State University, P.M.B. 5363, Ado-Ekiti, Nigeria
*Corresponding author: Oluwadamilare Oluwaseun, Department of Biochemistry, Ekiti State University, P.M.B. 5363, Ado-Ekiti, Nigeria;
Received: 01 October 2020; Accepted: 08 October 2020; Published: 03 August 2021
Citation: Ajayi, O.B., Ajayi, O.O. Short-Term Intervention with Garlic-Based diets Modulates mRNA Expression of Calmodulin, Inositol-1,4,5-Trisphosphate Receptor-1, and Intracellular Adhesion Molecule in Cyclosporin-Induced Prehypertensive Wistar Rats. Archives of Clinical and Biomedical Research 5 (2021): 613-628.
Share at FacebookAbstract
This study assessed the short term effect of Allium sativum-based diets on mRNA expression of Calmodulin (CaM), Inositol Trisphosphate receptor (IP3R) and Intracellular adhesion molecule (ICAM-1) in the heart and aorta of cyclosporin-induced prehypertensive rats. Plasma levels of Ca2+ and Tumor Necrosis Factor-alpha (TNF-alpha) were also determined. The experimental rats used for this study were randomly allocated into 7 groups (n=4): normotensive control rats (fed basal diet); untreated prehypertensive rats (fed basal diet); prehypertensive rats (fed 10% and 20% Allium sativum based diets respectively); normotensive rats (fed 10% and 20% Allium sativum based diets respectively); prehypertensive rats treated with 10mg/kg captopril respectively for 7 days. Induction of hypertension with cyclosporin caused significant increase (p < 0.05) in mRNA expression of CaM, IP3R and ICAM-1, accompanied by increased (p < 0.05) systolic and diastolic blood pressures, plasma Tumor Necrosis Factor-alpha (TNF-alpha) and Ca2+ levels. These effects were considerably reversed by Allium sativum-based diets fed to the prehypertensive rats. These findings provide evidence which suggest that CaM, IP3R, and ICAM-1 are also upregulated in prehypertension, but are modulated by short term dietary intervention with garlic.
Keywords
Allium sativum; Hypertension; Cyclosporin; Garlic; Prehypertension
Allium sativum articles; Hypertension articles; Cyclosporin articles; Garlic articles; Prehypertension articles
Article Details
Introduction
Hypertension still stands as one of the deadliest cardiovascular diseases that affect large populations across different regions of the world due to its high rate of contribution to mortality cases [1,2]. A number of medical complications like kidney dysfunction, inflammation, dementia, stroke, heart failure have are linked with progression of high blood pressure in hypertensive patients especially when poorly managed [3,4]. Prehypertension is a case whereby a patient records blood pressure values which are higher than the normal range but not considered as full hypertension [5]. Prehypertensive patients stand a risk of developing future medical complications especially for the fact that its symptoms are not noticed in time. Thus, prehypertension contributes to hypertension-related morbidity on the long run. It is thought safe to employ safety measures to circumvent progression to chronic hypertension [6].
Cyclosporin is an immunosuppressive agent linked with hypertension and nephrotoxicity in transplant patients. As a result, it is a potent agent used for inducing hypertension in rat models [7]. At considerably high doses cyclosporin induces hypertension in rat models. It functions by causing systemic vasoconstriction, altering cytosolic calcium translocation, activation of Renin-Angiotensin system (RAS) and pro-inflammatory cytokines [8].
Both pharmacological and non-pharmacological interventions are applied in the management of hypertension as a cardiovascular disease. Non-pharmacological interventions have also been recommended as a first means of action before employing pharmacological alternatives [9]. Dietary intervention as a non-pharmacological means to manage hypertension involves the use of majorly plant food sources including herbs and spices which possess health benefits [10]. This method is important to the health of individuals even down to the genetic level. Genes are important in the production of proteins and related derivatives which play important physiological functions. Many nutrients have been studied against their effects on gene expression within living organisms. It is thought that diet supplemented with nutraceuticals may influence expression of genes bearing in mind that genetic activations are responses to extracellular stimulations which are mediated by signaling molecules [11,12].
Garlic, Allium sativum L., is one of such herbs employed as a functional food in the management of hypertension due to its medicinal and culinary benefits. Raw garlic is rich in nutrients including some of the B-complex vitamins like riboflavin and niacin, Vitamin C as well as potassium, magnesium and zinc [13]. Garlic is consumed in different forms such as raw or aged garlic, garlic powder, garlic oil and the likes. Garlic has been used as a potent antihypertensive and anti-inflammatory remedy for hypertensive subjects [14]. These properties have also been linked with the presence of sulphur-rich compounds present in garlic [15]. A number of pathways are targeted by antihypertensive drugs in their pharmacological dispositions [16]. An example is the calcium ion (Ca2+) associated pathway [17]. Ca2+ is an important second messenger responsible for many physiological functions such as muscle contraction and neurotransmitter release. It stimulates muscle contraction by activating various signaling proteins especially calmodulin (CaM), as well as the inositol triphosphate (IP3) pathway [17].
Calmodulin (CaM) as one of the intracellular targets of Ca2+ is a key player in the activation of the IP3 pathway as well as other proteins which play a role in many physiological events including contraction of smooth muscle and arteries in the build up to hypertension [18]. Inositol trisphosphate receptor (IP3R) is a membrane receptor that acts as a Ca2+ channel on activation by IP3. IP3R is important in the signaling which causes the release of Ca2+ from intracellular store sites. Intracellular Adhesion molecule-1 (ICAM-1) is a pro-inflammatory molecule which belongs to the immunoglobulin family, and is expressed in endothelial cells during inflammatory processes [19]. It is usually stimulated by cytokines like Tumor Necrosis Factor-alpha (TNF- alpha) and interleukin-1 (IL-1) which have been thought to have an effect on the Renin-Angiotensin System (RAS) relevant to all categories of hypertension [20]. From previous studies, mRNA expression of CaM, IP3R and ICAM-1 were upregulated in hypertensive rat models [21,22].
Many studies have focused majorly on chronic hypertension only while less attention has been given to prehypertension (borderline hypertension). This study aimed at investigating the possible effect of prehypertension on mRNA expression of CaM, IP3R, and ICAM-1 in order to be able to determine if these genes are affected at borderline stage of hypertension before progression into the chronic stage. Also, this study investigates the effect of garlic-based diets on the mRNA expression of these molecules.
Materials and Methods
Materials
Fresh Allium sativum (garlic) cloves were purchased from a local market in Ado-Ekiti, Ekiti State, Nigeria.
Preparation of Allium sativum powder
The garlic cloves were separated into bulbs, peeled and properly washed. The bulbs were blended and the garlic slurry was freeze-dried at -10oC. The freeze-dried garlic was finally milled into powder and stored in polythene packs at refrigerated temperature for further use.
Chemicals
Cyclosporin capsules and captopril tablets were purchased from Aromokeye Pharmacy and Veterinary services in Ado-Ekiti, Ekiti State, Nigeria. Trizol reagent was sourced from Zymo Research, USA (Cat: R2050-1-50, Lot: ZRC186885) and chloroform was obtained from BDH Analytical Chemicals, Poole, England (Cat: 10076-6B). Isopropanol was also purchased from Burgoyne Urbidges & Co, India (Cat: 67-63-0). Nuclease-free water was obtained from Inqaba Biotec, West Africa (Lot no: 0596C320, code: E476-500ML).
All other chemicals used were of analytical grade.
Management of Experimental Animals
Wistar strain of male albino rats weighing between 130g and 160g used for this study was purchased from the Animal house of the Department of Biochemistry, University of Ilorin, Kwara State. All the animals used for this work were maintained according to the international guidelines on Animal care and use for scientific purposes [23].
Induction of Prehypertension and experimental feeding with Allium sativum powder
The experimental rats were provided with commercial rat pellet diet and water ad libitum. The rats were randomly allocated into seven experimental groups (n=4) shown in Table 1. Cyclosporin was freshly prepared and administered intraperitoneally at a single daily dose of 25mg/kg/day body weight for 7 days. Captopril (an ACE inhibitor) was used as a standard treatment drug (10mg/kg/day) for one of the experimental groups. This was accompanied with concurrent feeding of the rats with the experimental diets for each of the respective groups.
Diet Formulation
Allium sativum-based diets were formulated [24] as shown in Table 1.
Table 1: Diet formulation
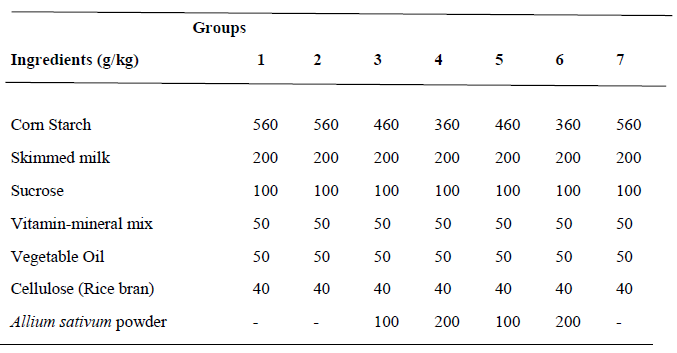
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Total RNA isolation
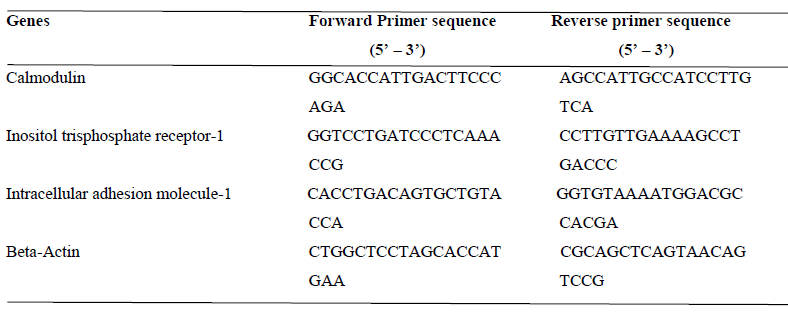
After 7 days of experimental feeding with Allium sativum-based diets, the rats were sacrificed via diethyl ether euthanasia. Total RNA was isolated from freshly excised tissues (heart and aorta) [25]. Briefly, the tissues were homogenized in cold (4°C) TRIZOL reagent (Zymo Research, USA, Cat: R2050-1-50, Lot: ZRC186885). Total RNA was partitioned in chloroform (BDH Analytical Chemicals, Poole, England Cat: 10076-6B) following centrifugation at 15,000 rpm/15 min (Abbott Laboratories, Model: 3531, Lake Bluff, Illinois, United States). RNA from the clear supernatant was precipitated using equal volume of isopropanol (Burgoyne Urbidges & Co, India, Cat: 67-63-0). RNA pellet was rinsed twice in 70% ethanol (70 ml absolute ethanol (BDH Analytical Chemicals, Poole, England Cat: 10107-7Y) in 30 ml of nuclease-free water (Inqaba Biotec, West Africa, Lot no: 0596C320, code: E476-500ML)). The pellets were air-dried for 5 min and dissolved in RNA buffer (1 mM sodium citrate, pH 6.4). Forward and reverse primers for the specific genes of interest (Calmodulin and Inositol-1, 4, 5-trisphosphate receptor) were also obtained (forward and reverse primer sequences are shown in Table 2). Beta-actin was also used as the house-keeping gene.
Table 2: Forward and Reverse primer sequences
Complementary DNA (cDNA) Conversion
Prior to cDNA conversion, total RNA quantity (concentration (µg/ml) = 40 * A260) and quality (≥ 1.8) was assessed using the ratio of A260/A280 (A=absorbance) read using spectrophotometer (Jen-way UV-VIS spectrophotometer model 6305, UK). DNA contamination was removed from RNA was removed following DNAse I treatment (NEB, Cat: M0303S) as specified by the manufacturer. 2 µl solution containing 100 ng DNA-free RNA was converted to cDNA using M-MuLV Reverse transcriptase Kit (NEB, Cat: M0253S) in 20 µl final volume (2 µl, N9 random primer mix; 2 µl, 10X M-MuLV buffer; 1 µl, M-MuLV RT (200 U/µl); 2 µl, 10 mM dNTP; 0.2 µl, RNase Inhibitor (40 U/µl) and 10.8 µl nuclease-free water). The reaction proceeded at room temperature O/N. Inactivation of M-MuLV Reverse transcriptase was performed at 65°C/20 min [25].
PCR amplification and Agarose Gel Electrophoresis
PCR amplification for the expression of genes whose primers (SnapGene software) are listed (see Table 2) was done using the following protocol: PCR amplification was performed in a total of 25 µl volume reaction mixture containing 2 µl cDNA (40 ng), 2 µl primer (100 pmol), 12.5 µl Ready Mix and Taq PCR master mix (One Taq Quick-Load 2x, master mix, NEB, Cat: M0486S) and 8.5 µl nuclease-free water. Initial denaturation at 95°C for 5 minutes was followed by 20 cycles of amplification (denaturation at 95°C for 30 seconds, annealing for 30 seconds and extension at 72°C for 60 seconds) and ending with final extension at 72°C for 10 minutes. In all experiments, negative controls were included where reaction mixture has no cDNA. The amplicons were resolved on 1.5% agarose gel (Cleaver Scientific Limited: Lot: 14170811) in Tris (RGT reagent, china, Lot: 20170605)-Borate (JHD chemicals, China, Lot 20141117)-EDTA buffer (pH 8.4) [25].
Amplicon image processing and semi-quantification
In-gel amplicon bands images captured on camera were processed on Keynote platform. Gel density quantification was done using Image-J software [26]. Each point represent relative expression ((test gene band intensity/ internal control band intensity)*100) plotted using Numbers software (Mac OSX version).
Measurement of Blood pressure
Systolic and diastolic blood pressures were measured in the experimental after 7 days of experimental feeding using tail cuff method. A soft valve extension of the equipment was wrapped round the tail or left leg of the resting experimental rats. The readings were measured in millimeters of mercury (mmHg) [27].
Preparation of Blood Plasma
Blood samples were collected after 7 days of experimental feeding with Allium sativum-based diets into lithium heparin bottles via ocular puncture and centrifuged for plasma preparation prior to analysis. Thereafter, blood plasma were aspirated into plain bottles and stored at -200C until required for assessment of biochemical parameters.
Determination of Plasma Calcium ion (Ca2+) concentration
Determination of calcium ions in plasma was carried out by colorimetric technique [28]. Three clean test tubes were labelled as reagent blank, standard and sample tubes. To the reagent blank tube 25µl of distilled water was added. This was followed by the addition of 1ml of the working reagent. To the standard tube, 25µl of standard solution and 1ml of the working reagent was added. To the sample tube, 25µl of the sample was added and mixed with 1ml of the working reagent. The solutions in each of the test tubes were mixed and the absorbance of the sample (Asample) and standard (Astandard) were read against the reagent blank after 30 minutes at 570nm.
Determination of Plasma TNF-alpha concentration
50µl of incubation buffer was added to wells for the plasma samples, standard and controls. 100µl of standard, controls and plasma samples were added to the appropriate wells. The wells were gently shaken and incubated for 2 hours at room temperature after covering with a plate cover. The solution was thoroughly aspirated and washed four times with wash buffer. 100µl of streptavidin-HRP solution was added to each well except the chromogen blanks. 100μl stabilized chromogen was added to each well. The substrate solution turned blue. The resulting solution was incubated for 30 minutes in a dark place at room temperature. 100μl of stop solution was added to the wells and mixed gently. The solution turned from blue to yellow. The absorbance was read at 450nm [29].
Statistical Analysis
The results of the various biochemical analyses are expressed as Mean ± standard deviation. One-Way Analysis of variance (ANOVA) was used to test for variability using SPSS 20. This was also followed by Duncan Multiple Range Test. The results for the MRNA expression were analyzed using GraphPad Prism 5.01.
Results
Table 3 shows the systolic and diastolic blood pressures (mmHg) of the experimental rats after induction with cyclosporin and concurrent feeding with A. sativum-based diets for 7 days. Systolic blood pressure was found to be significantly increased (p < 0.05) in untreated prehypertensive rats (Group 2) compared to other experimental groups. No significant difference (p < 0.05) was observed between the systolic blood pressures of the normotensive rats fed basal diet (Group 1), prehypertensive rats fed 10% and 20% A.sativum based diets (Groups 3 and 4), normotensive rats fed 20% A.sativum based diets (Group 6) and prehypertensive rats treated with 10mg/kg captopril. However, an insignificant increase (p < 0.05) in systolic blood pressure was observed in the normotensive rats fed 10% A. sativum based diet (Group 5). On the other hand, diastolic blood pressure was found to be significantly increased (p < 0.05) in the untreated prehypertensive rats (Group 2) compared to the other experimental groups.
Table 3: Final systolic and diastolic blood pressures (SBP and DBP) of experimental rats after administration of 25mg/kg/day cyclosporin and concurrent feeding with Allium sativum-based diets
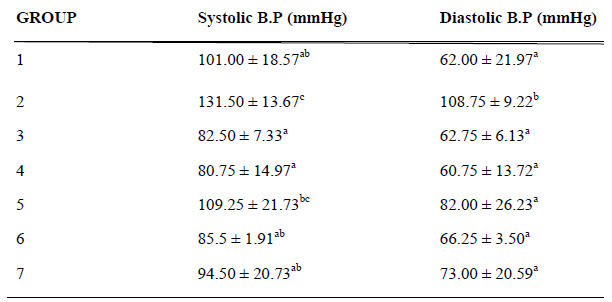
Results are expressed as Mean ± Standard deviation of four (n=4) determinations for each group using Analysis of Variance (ANOVA) followed by Duncan’s (multiple range tests) Post Hoc test; values in the same row for each parameter with superscript different from control are significantly different at p <0.05.
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Figure 1 shows the results of the effect of A.sativum based diets on mRNA expression of Intracellular Adhesion molecule-1 (ICAM-1) in the heart of cyclosporin-induced prehypertensive rats. The expression of ICAM-1 was significantly increased (p < 0.05) in untreated prehypertensive rats (Group 2) compared with other groups. ICAM-1 expression was observed to be lower (significant at p < 0.05) in prehypertensive rats fed 10% and 20% A.sativum based diets (Groups 3 and 4), normotensive rats fed 10% and 20% A.sativum based diets (Groups 5 and 6) and prehypertensive rats treated with 10mg/kg captopril when compared with normotensive rats fed basal diet (Group 1) and untreated prehypertensive rats (Group 2).
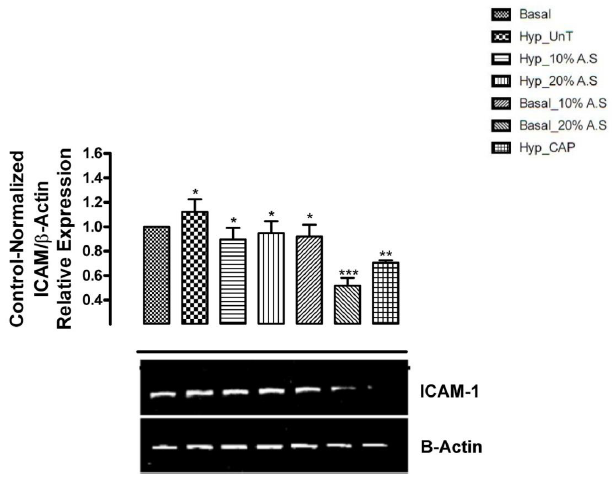
Figure 1: Effect of Allium sativum-based diets on mRNA expression of intracellular adhesion molecule-1 (ICAM-1) in the heart of cyclosporin-induced prehypertensive animals
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Figure 2 shows the results of the effect of A.sativum based diets on mRNA expression of Calmodulin (CaM) in the aorta of cyclosporin-induced prehypertensive rats. Calmodulin gene was observed to show the highest expression (significant at p < 0.05) in untreated prehypertensive rats (Group 2) when compared with normotensive rats fed basal diet (Group 1) and the other experimental groups. Normotensive rats fed 10% and 20% A.sativum based diets (Groups 5 and 6), and prehypertensive rats treated with 10mg/kg captopril (Group 7) also showed low CaM expression compared to the other experimental groups (significant at p < 0.05).
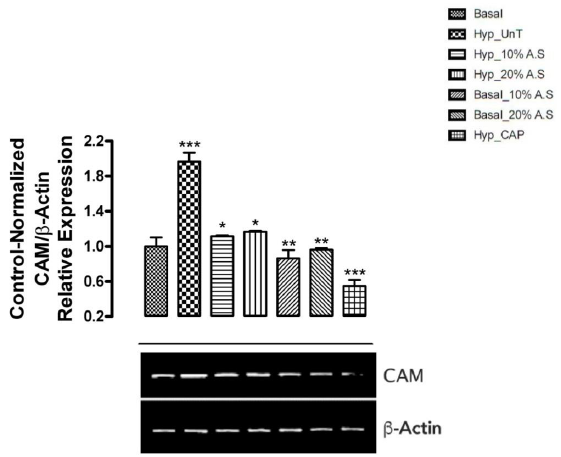
Figure 2: Effect of Allium sativum-based diets on mRNA expression of Calmodulin in the aorta of cyclosporin-induced prehypertensive animals
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Figure 3 shows the results of the effect of A.sativum based diets on mRNA expression of Inositol trisphosphate receptor (IP3R) in the aorta of cyclosporin-induced prehypertensive rats. IP3R expression was observed to be significantly upregulated (p < 0.05) in the untreated prehypertensive rats (Group 2) compared with the normotensive rats fed basal diet (Group 1) and other experimental groups. On the other hand, prehypertensive rats fed 20% A.sativum based diet (Group 4), and normotensive rats fed 10% and 20% A.sativum based diets (Groups 5 and 6) were also observed to show IP3R expression lower (significant at p < 0.05) than that of the normotensive rats fed basal diet (Group 1). Expression of IP3R in prehypertensive rats fed 10% A.sativum based diet (Group 3) is seen to be comparable to the level observed for normotensive rats fed basal diet (Group 1).
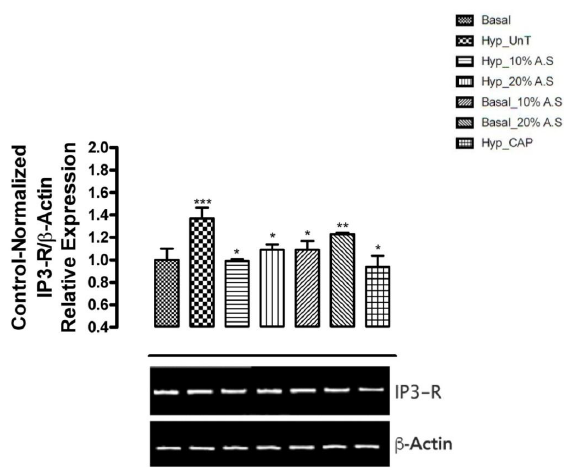
Figure 3: Effect of Allium sativum-based diets on mRNA expression of inositol trisphosphate receptor-1 in the aorta of cyclosporin-induced prehypertensive animals
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Table 4 shows the effect of A. sativum based diets on Calcium ion (Ca2+) and TNF-alpha levels in the plasma of cyclosporin-induced prehypertensive rats. Plasma TNF-alpha and Ca2+ levels were observed to be significantly increased (p < 0.05) in untreated prehypertensive rats (Group 2) compared with the normotensive rats fed basal diet (Group 1) and other experimental groups. The results also show that there is no significant difference (p < 0.05) between plasma TNF-alpha, Ca2+ levels in normotensive rats fed basal diet (Group 1), prehypertensive rats fed 10% and 20% A. sativum based diets (Groups 3 and 4) and prehypertensive rats treated with 10mg/kg captopril. Although, it was observed that TNF-alpha, Ca2+ levels were significantly reduced (p < 0.05) in prehypertensive rats fed 10% and 20% A. sativum based diets (Groups 3 and 4) when compared with untreated prehypertensive rats (Group 2).
Table 4: Effect of Allium sativum-based diets on TNF-alpha and Ca2+ levels in plasma of cyclosporin-induced prehypertensive rats
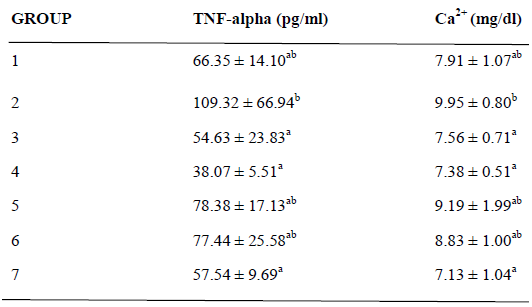
Results are expressed as Mean ± Standard deviation of four determinations(n=4) for each group using Analysis of Variance (ANOVA) followed by Duncan’s (multiple range tests) Post Hoc test; values in the same row for each parameter with superscript different from control are significantly different at p <0.05.
Group 1: Normotensive group; fed basal diet (without Allium sativum powder)
Group 2: Untreated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder).
Group 3: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 10% Allium sativum powder.
Group 4: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet containing 20% Allium sativum powder.
Group 5: Normotensive group; fed basal diet containing 10% Allium sativum powder.
Group 6: Normotensive group; fed basal diet containing 20% Allium sativum powder.
Group 7: Treated prehypertensive group; administered cyclosporin (25mg/kg/day) intraperitoneally + basal diet (without Allium sativum powder) + Captopril (10mg/kg/day)
Discussion
Short-term daily administration of 25mg/kg cyclosporin (for 7 days) caused a significant increase in systolic and diastolic blood pressure readings (p < 0.05) as observed in the untreated prehypertensive rats. This was also accompanied by significantly increased (p < 0.05) mRNA expressions of ICAM-1, CaM and IP3R. These observations show the prehypertension also upregulates ICAM-1, CaM AND IP3R. This is similar to the effect of hypertension on the mRNA expression of ICAM-1, CaM, and IP3R [21,22]. Similarly, plasma levels of TNF-alpha and Ca2+ were also significantly increased (p < 0.05) in untreated prehypertensive rats (p < 0.05). Thus, these results confirm prehypertensive effect on the listed genes as induced by cyclosporin in the experimental rats [30,31].
Intracellular adhesion molecule-1 (ICAM-1), a cell-cell adhesion molecule has been linked with inflammation in hypertensive subjects [20]. ICAM-1 is a proinflammatory protein encoded by ICAM1 gene and its concentration increases with cytokine stimulation. Previous research has shown that inflammation plays a subtle role in the pathogenesis of hypertension [19]. It was also hypothesized that inflammation in hypertension may be due to the activity of the RAS with certain interleukins (1 or 6) standing as mediators [20,32]. From this study, it was observed that untreated prehypertensive rats (Group 2) showed a markedly significant upregulation (p < 0.05) of ICAM-1 mRNA expression compared with the other groups. This shows that inflammation may have set in due to the untreated prehypertensive condition. This was also supported by the significant increase (p < 0.05) in plasma TNF-alpha level observed in the untreated prehypertensive rats. However, prehypertensive rats fed 10% and 20% A. sativum diets (Groups 3 and 4) and normotensive rats fed 10% and 20% A. sativum diets (Groups 5 and 6) showed a downregulation of the ICAM-1 gene expression and plasma TNF-alpha levels which was lower than that observed for normotensive rats fed basal diet (Group 1). It has been previously reported that A. sativum possesses anti-inflammatory activity [33] which forms parts of the reasons for its medicinal uses. Thus, it could be said that one of the ways A. sativum can exhibit its anti-inflammatory ability is by influencing the production of ICAM-1. This downregulation of ICAM-1 expression may be by direct inhibition of TNF-alpha which is also linked with major proinflammatory pathways which can stimulate the production of ICAM-1. A. sativum may also exhibit its anti-inflammatory effect by preventing ICAM-1 ligation i.e. binding of ICAM-1 with other cellular molecules to produce proinflammatory effects [32]. Therefore, this establishes a relationship between the anti-inflammatory properties of A. sativum and its ability to modulate production of ICAM-1 via inhibition of TNF-alpha.
Signal transduction is an important process through which cellular response to internal or external stimuli can be generated. Signaling pathways are well coordinated and they function synergistically to achieve one or more biological functions including cell metabolism, controlling cell growth as well as other processes [34,35]. Signal transduction is mediated via signaling molecules like second messengers and other metabolites, and their genetic expression is linked with the activation of genes i.e. genetic expression of signaling molecules is linked with gene activations [12]. In this work, mRNA expression of calmodulin (CaM) and inositol-1, 4, 5-trisphosphate receptor (IP3R) genes was assessed in the aorta of prehypertensive rats vis-a-vis feeding with A. sativum based diets. CaM, calcium-modulated protein, is a signaling molecule found across many signaling pathways in eukaryotic cells. It serves as a molecular target for Ca2+ ions. When CaM is activated, it has the ability to interact with various proteins such as the kinases or phosphatases which give rise to various cellular responses [36]. There is evidence that CaM activity becomes abnormal in hypertensive rats as a result of calcium abnormalities implicated in the pathogenesis of hypertension [37]. Thus, increased calmodulin levels may be associated with hypertension since it has been already reported to play an indirect role in blood pressure regulation usually through smooth muscle contraction. In this study, mRNA expression of CaM in the heart was significantly upregulated (p < 0.05) in untreated prehypertensive rats (Group 2). Increased levels of CaM cause smooth muscle contraction in blood vessels which ultimately leads to rise in blood pressure. CaM activity is stimulated by the presence of Ca2+ ions which are required for its activation as seen in the untreated prehypertensive rats which showed a significant increase (p < 0.05) in plasma Ca2+ levels. Interestingly, it was seen that mRNA expression of CaM was downregulated in prehypertensive rats fed 10% and 20% A. sativum based diets (Groups 3 and 4). A more pronounced downregulation of CaM gene expression was also observed in normotensive rats fed 10% and 20% A. sativum diets (Groups 5 and 6). These observed effects may be traced with decreased plasma Ca2+ levels observed in the prehypertensive rats fed Allium sativum-based diets. This could be attributed to possible inhibition of the formation of calcium/calmodulin dependent protein kinase II (CaMKII) which contributes to blood pressure in relation with angiotensin II by controlling structural gene expression [38]. This could also be through the activation of cytoplasmic polyadenylation element binding protein (CPEB) which plays a role in angiotensin II mRNA translation. Once again, we see that the significant decrease in systolic and diastolic blood pressure observed in prehypertensive rats fed A. sativum diets can be linked with downregulation of CaM mRNA expression since CaM is a key player which participates in the translation of angiotensin II which has vasoconstrictive properties.
Also linked to CaM signaling is the IP3-DAG signaling pathway which controls the influx of intracellular Ca2+ ions leading to the activation of specific protein kinases (for example protein kinase C) yielding specific biological response. In the IP3 signaling pathway, IP3 (inositol-1,4,5-trisphosphate) is one of the key players. It is produced and activated by the action of the hydrolytic enzyme phospholipase C on phosphatidylinositol-4,5,-bisphosphate. IP3 binds to IP3 receptors (IP3R) on the membrane of the endoplasmic reticulum. The IP3R gene encodes for the production of IP3 receptors on the surface of the endoplasmic reticulum [39]. This results in an influx in intracellular Ca2+. The results from this present study shows significant upregulation (p < 0.05) in the mRNA expression of IP3R in untreated prehypertensive rats (Group 2) when compared with the other groups. This implies an increased in the activity of phospholipase C which is responsible for the production of IP3. Thus, IP3 can bind to IP3 receptors to open calcium channels. Ca2+ ions can then bind calmodulin to activate CAMKII and subsequently CPEB to stimulate translation of angiotensin II to ultimately cause an increase in blood pressure as also seen in this study. Feeding prehypertensive rats with 10% and 20% A. sativum diets was able to cause a considerable downregulation (p < 0.05) of the mRNA expression of IP3R. This was also observed in normotensive rats fed 10% and 20% A. sativum diets. This downregulation of IP3R expression would cause a decline in the influx of Ca2+ ions which can further activate calmodulin and subsequently lead to the production of angiotensin II to cause vasoconstriction. This feature was observed in the prehypertensive rats fed A. sativum based diets. This further consolidates the reduced systolic and diastolic blood pressure values observed in the prehypertensive rats fed A. sativum diets. From these results and observations, it could be depicted that A. sativum plays a multi-dimensional role in exerting its hypotensive effect. This cuts across inducing a decline in IP3 and calmodulin activities. Thus, A. sativum may be able to modulate the activities or functions of key players like calmodulin, IP3 and TNF-alpha at the gene level.
In conclusion, the findings from this study forwards molecular evidence which suggest that CaM, IP3R, and ICAM-1 are also upregulated in prehypertension, but are modulated by short term dietary intervention with garlic. However, further studies should be carried out to determine the possible effects of prehypertension. Attention should also be directed towards taking preventive measures so as to prevent progression to chronic hypertension.
Acknowledgement
Authors acknowledge the contribution of Dr. O.I. Omotuyi of Department of Biochemistry, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria.
Conflict of Interests:
No competing financial interests exist.
References
- Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovascular Therapeutics 28 (2010): e20-e32.
- World Health Organization. CardiovascularDiseases(CVDs). Geneva: World Health Organization. Factsheet No 317 (2013).
- August P. Overview: mechanisms of hypertension: cells, hormones, and the kidney. Journal of the American Society of Nephrology 15 (2004): 1971-1973.
- Freedman BI, Cohen AH. Hypertension-attributed nephropathy: what's in a name?. Nature Reviews Nephrology 12 (2016): 27.
- Chobanian AV, Bakris GL, Black HR, et al. National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42 (2003): 1206-1252.
- Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Archives of Internal Medicine 164 (2004): 2126-2134.
- European Society of Hypertension. Scientific Newsletter: Update on hypertension management 2:8 (2001).
- Niehof M, Borlak J. HNF4alpha dysfunction as a molecular rational for cyclosporine induced hypertension. PloS One 6 (2011): e16319.
- Svetkey LP. Management of prehypertension. Hypertension 45 (2005): 1056-1061.
- Singab AN, Eldahshan O. Medicinal importance of herbs and spices. Medicine and Aromatic Plants 4 (2015): 4.
- Neeha VS, Kinth P. Nutrigenomics research: a review. Journal of Food Science and Technology 50 (2013): 415-428.
- Wilson CH, Ali ES, Scrimgeour N, et al. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+ through a protein kinase C-dependent mechanism. Biochemical Journal 466 (2015): 379-390.
- USDA National Nutrient Database. Nutrition facts for raw garlic. Retrieved November 2, 2014.
- Shouk R, Abdou A, Shetty K, et al. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutrition Research 34 (2014): 106-115.
- Choudhary R. Benificial effect of Allium sativum and Allium tuberosum on experimental hyperlipidemia and atherosclerosis. Pakistan Journal of Physiology 4 (2008): 7-10.
- Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine 18 (2011): 251-258.
- Delbridge LM, O’Riordan MX. Innate recognition of intracellular bacteria. Current Opinion in Immunology 19 (2007): 10-16.
- Martinsen A, Dessy C, Morel N. Regulation of calcium channels in smooth muscle: new insights into the role of myosin light chain kinase. Channels 8 (2014): 402-413.
- Lindmark E, Diderholm E, Wallentin L, et al. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA 286 (2001): 2107-2113.
- Chamarthi B, Williams GH, Ricchiuti V, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin–angiotensin system in humans. American Journal of Hypertension 24 (2011): 1143-1148.
- Rysa J, Leskinen H, Ilves M, et al. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 45 (2005): 927-933.
- Qin X, Hou X, Zhang K, et al. Farrerol modulates aorta gene expression profile in spontaneously hypertensive rats. Planta Medica 84 (2018): 296-303.
- Mohr BJ, Fakoya FA, Hau J, et al. The governance of animal care and use for scientific purposes in Africa and the Middle East. ILAR Journal 57 (2016): 333-346.
- Ajiboye IO, Ajayi OB, Akomolafe SF, et al. Assessment of Physicochemical Properties, Fatty Acid Composition of Afzelia africana Seed Oil and the Effect of Its Oil-Based Diet on Body Weight and Plasma Lipid Profile of Albino Rats. Food and Nutrition Sciences 9 (2018): 983-996.
- Omotuyi OI, Nash O, Inyang OK, et al. Flavonoid-rich extract of Chromolaena odorata modulate circulating GLP-1 in Wistar rats: computational evaluation of TGR5 involvement. Biotech 8 (2018): 124.
- Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18 (2017): 529.
- Wilde E, Aubdool AA, Thakore P, et al. Tail-cuff technique and its influence on central blood pressure in the mouse. Journal of the American Heart Association 6 (2017): e005204.
- Barnett RN. Calcium. J. Clin. Path. 59 (1973): 836.
- Kopf JC, Suhr MJ, Clarke J, et al. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: a randomized controlled trial. Nutrition Journal 17 (2018): 72.
- Biaggioni I, Robertson D. Chapter 9. Adrenoceptor Agonists & Sympathomimetic Drugs. In: B.G. Katzung SB. Masters AJ. Trevor (Eds), Basic & Clinical Pharmacology, 11e (2011).
- Mannu GS. The non-cardiac use and significance of cardiac troponins. Scottish Medical Journal 59 (2014): 172-178.
- Etienne-Manneville S, Chaverot N, Strosberg AD, et al. ICAM-1-coupled signaling pathways in astrocytes converge to cyclic AMP response element-binding protein phosphorylation and TNF-α secretion. The Journal of Immunology 163 (1999): 668-674.
- Mousa AS, Mousa SA. Cellular effects of garlic supplements and antioxidant vitamins in lowering marginally high blood pressure in humans: pilot study. Nutrition Research 27 (2007): 119-123.
- Papin JA, Hunter T, Palsson BO, et al. Reconstruction of cellular signalling networks and analysis of their properties. Nature reviews Molecular Cell Biology 6 (2005): 99-111.
- Krauss G. Biochemistry of Signal Transduction and Regulation. Wiley-VCH (2008): p.15.
- Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. Massachusetts: Sinauer Associates, Inc. Sunderland. 2012.
- Huang SL, Wen YI, Kupranycz DB, et al. Abnormality of calmodulin activity in hypertension. Evidence of the presence of an activator. The Journal of Clinical Investigation 82 (1988): 276-281.
- Prasad AM, Morgan DA, Nuno DW, et al. Calcium/Calmodulin-Dependent Kinase II Inhibition in Smooth Muscle Reduces Angiotensin II–Induced Hypertension by Controlling Aortic Remodeling and Baroreceptor Function. Journal of the American Heart Association 4 (2015): e001949.
- Chaudhari N, Roper SD. The cell biology of taste. Journal of Cell Biology 190 (2010): 285-296.
