Severe Cryoglobulinemic Vasculopathy and Underlying Monoclonal Gammopathy: The Importance of Identifying and Targeting the Underlying Plasma Cell Clone in Diagnosis and Treatment
Article Information
Lisa B Leypoldt1*, Susanne Ghandili1, Stefan W Schneider2, Christof Iking-Konert3, Carsten Bokemeyer1, Katja C Weisel1
1Department of Hematology, Oncology and Bone Marrow Transplantation with Section of Pneumology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
2Department of Dermatology and Venereology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
3Department of Nephrology, Section of Rheumatology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
*Corresponding author: Dr. Lisa B Leypoldt, Department of Hematology, Oncology and Bone Marrow Transplantation with Section of Pneumology, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, Tel: +49 15222831701; Fax: +4940-7410-58581
Received: 11 June 2021; Accepted: 23 June 2021; Published: 21 July 2021
Citation: Lisa B Leypoldt, Susanne Ghandili, Stefan W Schneider, Christof Iking-Konert, Carsten Bokemeyer, Katja C Weisel. Severe Cryoglobulinemic Vasculopathy and Underlying Monoclonal Gammopathy: The Importance of Identifying and Targeting the Underlying Plasma Cell Clone in Diagnosis and Treatment. Archives of Clinical and Biomedical Research 5 (2021): 556-568.
Share at FacebookAbstract
Cryoglobulinemia often occurs secondary to an underlying monoclonal gammopathy (MG). MG is a common finding in the elderly population with a prevalence of 5% in persons aged 70 years and older and can range from asymptomatic monoclonal gammopathy of unknown significance (MGUS) to symptomatic multiple myeloma (MM) for which treatment is clearly indicated. However, rare forms of MG lead to clinical symptoms and are summarized as monoclonal gammopathy of clinical significance (MGCS). Here, no clear treatment recommendations exist, treatment ranges from symptomatic treatment of paraneoplasia to causal treatment of the underlying plasma cell clone. Here, we report an exemplary case of a 74-year-old female with MG and cryoglobulinemic vasculopathy seriously affecting skin and peripheral nervous system accompanied by severe pain. First- and second-line treatments with conventional immunosuppressive agents and B cell depleting therapy did not lead to sustained remission. After identifying MG as the potential underlying cause MGCS was considered. As symptoms were markedly affecting quality of life, myeloma-derived treatment with anti-CD38 antibody daratumumab, lenalidomide and dexamethasone was initiated and subsequently clinical symptoms and laboratory cryoglobulinemia resolved. In conclusion, this case report shows for the first time that daratumumab-based treatment of MGCS can be effective and is well-tolerated.
Keywords
Monoclonal Gammopathy of Clinical Significance; Cryoglobulinemia; Vasculopathy; Polyneuropathy; Daratumumab; Lenalidomide
Article Details
1. Introduction
Monoclonal gammopathy is a frequent disorder in the older population and is characterized by the presence of a small fraction (< 10%) of monoclonal plasma cells in the bone marrow leading to production of a monoclonal protein. It is present in about 5.3% of the population ≥ 70 years of age [1, 2]. In most cases, there is no immediate clinical impact of this finding and clinical surveillance is sufficient, leading to the definition of a monoclonal gammopathy of unknown significance (MGUS) [3, 4]. MGUS has a risk of about 1% per year to progress into Smoldering Myeloma (SM) or Multiple Myeloma (MM) [4], being defined by the presence of ≥ 10% monoclonal plasma cells (PC) in the bone marrow in combination without (SM) or with (MM) organ dysfunctions or certain laboratory abnormalities indicating a high risk of progression (SLiM-CRAB criteria) (Table 1) [5]. Anti-myeloma treatment is undoubtedly indicated once a diagnosis of MM is reached, with additional growing evidence for early treatment initiation also in high-risk SM patients although there is no approved treatment indication yet [6].
|
MGUS |
MGCS |
SMM |
MM |
|
|
Monoclonal protein |
< 3g/dl (serum) and < 500mg/24h (urine) |
< 3g/dl (serum) and < 500mg/24h (urine) |
³ 3g/dl (serum) and/or ³ 500mg/24h (urine) |
detectable |
|
and / or |
and / or |
and / or |
and |
|
|
Monoclonal plasma cell bone marrow infiltration |
< 10% |
10-60% |
³ 10% |
|
|
and |
and |
and |
||
|
End organ damage SLiM-CRAB criteria (60% bone marrow infiltration, SFLC ratio ³ 100, > 1 focal lesion ³ 5 mm on MRI, hypercalcemia, renal insufficiency (creatinine ³ 2mg/dl or GFR < 40ml/min), anemia (Hb <10 g/dl or ³ 2g/dl below lower limit of normal), bone lesions (³ 1 osteolytic lesions on imaging) |
no evidence |
Evidence of end-organ damage related to mono-clonal gammopa-thy (e.g. histopa-thologically, clinically [vascu-litis, neuropathy, nephropathy]) |
no evidence |
evidence |
GFR: glomerular filtration rate; Hb: haemoglobin; MGCS: monoclonal gammopathy of clinical significance; MGUS: monoclonal gammopathy of unknown significance; MM: Multiple Myeloma; MRI: magnetic resonance imaging; SFLC: serum free light chain; SMM: Smoldering Multiple Myeloma;
Table 1: Types of monoclonal gammopathies according to IMWG criteria and Ferman et al. [5, 7].
A small percentage of patients with MGUS are presenting with various clinical symptoms. This disorder has been characterized as monoclonal gammopathy of clinical significance (MGCS), most often of renal significance (MGRS), where the underlying PC clone causes organ damage due to the toxicity of its monoclonal immunoglobulins (Igs) [7]. Recommendations on the diagnosis and management of monoclonal gammopathies have been made by the European Myeloma Network (EMN) and others [8, 9]. Nonetheless, identification of monoclonal gammopathy as the underlying cause for various clinical phenomena is often challenging and due to potential toxicities, patients and physicians often refrain from specific anti-myeloma treatment. Here, we present the case of a 74-year-old woman with MGCS resulting in severe cryoglobulinemic occluding vasculopathy with ulcera and polyneuropathy who highly benefitted from anti-myeloma treatment with the combination of the anti-CD38 antibody daratumumab with lenalidomide and dexamethasone, after several immunosuppressive treatments beforehand had failed.
2. Case Report
A 74-year-old female patient was first diagnosed with cryoglobulinemia in December 2018 when she presented at the rheumatology department with raynaud’s phenomenon, purpura and severe pain of the lower extremities, aggravated by cold exposure as well as peripheral polyneuropathy. Physical examination showed bilateral livedo and finely spotted non-palpable purpura of the lower limbs as well as symmetrical sensory deficit of the feet but was otherwise normal. A full rheumatologic workup showed positive results for monoclonal cryoglobulins as well as a concomitant monoclonal gammopathy type IgG kappa, other tests remained normal. Monoclonal protein level was not quantified, serum free light chains (SFLC) were within normal ranges (kappa: 18,77 mg/l, lambda: 7,65 mg/l) and bone marrow infiltration was slightly elevated but otherwise without convincing correlate for pathological monoclonal gammopathy (8% CD38- and CD138-positive and CD56-negative plasma cells, normal SFLC ratio). Since none of the SLiM-CRAB criteria were fulfilled, MGUS was diagnosed. In addition, there was no evidence of chronic or past infection with hepatitis B or C or human immunodeficiency virus (HIV) and no signs of autoimmune disease (e.g. negative results for ANAs, ANCAs, complement C3 and C4, rheumatoid factor). A computerized tomography (CT) excluded osteolyses or other underlying neoplastic disease. A diagnosis of a MGUS associated cryoglobulinemic occluding vasculopathy was made.
Symptomatic treatment with corticosteroids (20 mg prednisone/d) was initiated and resulted in quick clinical response, especially pain markedly improved. However, due to progressive polyneuropathy, treatment was intensified by addition of cyclophosphamide (500 mg i.v. every two weeks) in January 2019. Prednisone down tapering took place in parallel. After six planned doses of cyclophosphamide (equalling 3 g in total) until May 2019, neurological examination showed stable but not improved polyneuropathy. Cryoglobulins were still highly positive. In addition, cyclophosphamide treatment led to significant side effects with severe nausea and was overall not well tolerated. Subsequently, immunosuppressive treatment was changed to azathioprine in June 2019 at a starting dose of 50 mg/d orally for the first two weeks and then raised to 100 mg/d, with a final dose of 125 mg/d after another month. Treatment with azathioprine was well tolerated, the disease and symptoms temporarily sufficiently controlled.
In January 2020, the patient presented with again worsening of now severe polyneuropathy with paraesthesia and loss of fine motor skills. In parallel, she developed life-quality affecting myalgic pain and new skin lesions. Neurologic workup showed mononeuritis multiplex with maximum manifestation of Nn. ulnares (left > right), for the first time in this patient marking neuropathy of the upper extremities. Azathioprine treatment was discontinued and an emergency seven-day course of prednisone 60 mg/d was initiated. Decision was made to initiate treatment with rituximab as monoclonal antibody directed against CD20 to eliminate pathological B cell clones and was first given in January 2020 (rituximab 1000 mg i.v.), followed by a second dose two weeks later. Prednisone doses were tampered down in parallel. The treatment was well tolerated and led to a temporary clinical improvement of polyneuropathy.
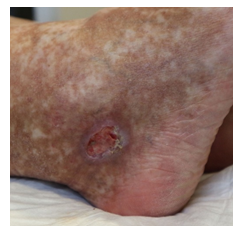
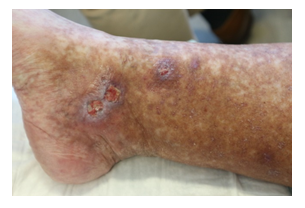
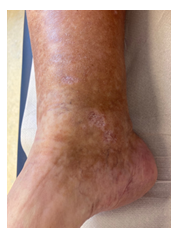
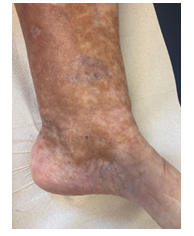
Once again though, in May 2020, symptoms worsened. Physical examination showed livedo and purpura of the legs bilaterally, apart from polyneuropathy of all extremities. Dermatologic lesions were also progressing into ulcera of the lower limbs around the Malleoli mediales bilaterally with severe pain. The patient was transferred to our haematology-oncology department for further diagnostic and therapy under the rationale of the monoclonal gammopathy being of clinical importance. Full diagnostic workup to assess both floridity of the vasculopathy and also a possible progression into Smoldering Myeloma or Multiple Myeloma was done. Electroneurography confirmed markedly worsened, severe axonal-motoric and sensitive neuropathy of the lower and axonal-sensorimotoric neuropathy of the left arm, corresponding to the patient’s symptoms. In addition, several large ulcerations on the lower extremities were noted in the physical examination (Figure 1A-C).
Cryoglobulinemic precipitation was highly positive and consisted fully (100 %) of monoclonal protein IgG kappa, indicating the clinical impact of the monoclonal protein. Immunofixation in the serum was positive for the known IgG kappa monoclonal protein, serum electrophoresis showed low level monoclonal protein (3 g/l). 24-hour urine analysis showed normal results; SFLC were slightly elevated (kappa 27,53 mg/l, ratio 7,71). MRI of the whole body revealed several older lesions of degenerative nature but no clear bone lesions or extramedullary manifestations typical for myeloma. A fresh bone marrow aspiration and biopsy showed less than 10 % plasma cell infiltration, but these were of monoclonal origin as confirmed by chromogenic in situ hybridization (CISH) analysis showing clonal kappa light chain restriction. Thus, the monoclonal plasma cell disorder was formally still characterized as MGUS. However, due to the clinical course of the patient with insufficient success of general immunosuppressive treatment and the cryoglobulinemic precipitation consisting fully of the pathologic Ig, we reasoned the cryoglobulinemic occluding vasculopathy being caused and driven by the monoclonal gammopathy of clinical significance. Thus, a myeloma-directed treatment with the monoclonal antibody daratumumab directed against CD38 in combination with the immunomodulatory drug lenalidomide and dexamethasone (DRd) as introduced for treatment of transplant-ineligible newly diagnosed MM patients in the pivotal MAIA trial was initiated in August 2020 [10].
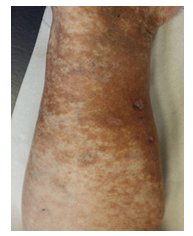
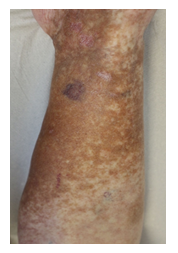
Within the first cycle, a pruritic rash of the upper body and head occurred as a common side effect of lenalidomide. It was handled with temporary discontinuation of lenalidomide, application of anti-histaminic drugs and subsequent dose reduction of lenalidomide. With this adapted dose regimen, DRd treatment was well tolerated without any further complications or side effects. Monoclonal protein dissolved, SFLC normalized within weeks and cryoglobulin testing became negative for the first time. Moreover, and most importantly, the patient benefitted enormously clinically within short notice as shown in Figure 2A-C with complete wound healing of the leg ulcerations and improvement of polyneuropathy. The patient’s performance status has constantly improved; she continues DRd treatment to date, lesions have fully healed (Figure 3).
3. Discussion
Cryoglobulinemia is a disorder characterized by the presence of cryoprecipitation e.g. the precipitation of blood proteins called cryoglobulins at temperatures below 37°C [11, 12]. These cryoglobulins are abnormal immunoglobulins generated by clonal B cell expansion, prevalence of cryoglobulinemia is estimated at 1 in 100,000 [12, 13]. There are three main categories of cryoglobulinemia based on the Ig composition (Table 2) [11]: in type I cryoglobulinemia, as is present in the depicted case, cryoglobulins consist of monoclonal Igs and thus this type often develops secondary to MGUS, MGCS, SM, MM or other B cell neoplasia [11, 12, 14]. Type I accounts for about 5-25% of cryoglobulinemias. Type II and III cryoglobulinemia are characterized by mixed cryoglobulins consisting of either a mixture of monoclonal IgM and polyclonal IgG (type II) or completely polyclonal Igs (type III) [11]. Both type II and III are strongly associated with infectious diseases, most often hepatitis C virus infection, or autoimmune disorders like Sjögren’s syndrome (Table 2) [12, 13].
The mere presence of circulating cryoglobulins does not always cause symptoms, but up to 50% of patients eventually develop clinical signs, most frequently skin changes, arthralgia, myalgia and weakness [14, 15], as was the case in our patient depicted above. Dermatologic symptoms most often present as livedo reticularis, purpura, Raynaud’s phenomenon, acrocyanosis or even ulcera of the extremities [14, 16, 17]. In case of a palpable purpura, skin lesions indicate a small vessel vasculitis whereas ulcerations and purpura mainly indicate an occluding vasculopathy of small or medium-sized vessels. Pathophysiological this is caused by a precipitation of monoclonal cryoglobulins thereby obstructing blood vessels, as typically seen in type I, as was the case in the patient described above. Palpable purpura indicate a small vessel vasculitis as in immune complex-mediated vasculitis in mixed cryoglobulinemia, type II/III [12].
The typical symptom complex in occluding cryoglobulinemic vasculopathy of Type I cryoglobulinemia consists of purpura, severe pain (often reaching 10/10 in pain scale) and ulceratic lesions, but livedoid pigmentation and gangrene can also occur [18]. Joint pain or arthralgia marks another typical symptom in up to 50% of patients, mainly affecting bilateral wrists or hands and knees without signs of overt arthritis [13]. Improvement of pain is typically the first indicator of successful and effective treatment and may thus be used for treatment evaluation [18]. Neurologic symptoms are common in cryoglobulinemia, particularly involvement of peripheral nerves as sensory or sensorimotoric polyneuropathy in up to 50% of patients [13, 14, 17]. Paraesthesiae with burning sensation in the lower limbs typically occurs first but may progress into motor loss in later stages.
Other clinical presentations include renal involvement or rarely gastrointestinal, cardiac or central nervous involvement or hyperviscosity syndrome [13, 16]. The latter occurs mainly in type I cryoglobulinemia and due to urgent need for treatment to prevent further organ damage its identification is of high importance [12, 16]. Typical symptoms include headache, dizziness, confusion, visual changes, hearing loss, or epistaxis. If cryoglobulinemia is suspected due to typical clinical presentation, a full diagnostic workup should be initiated to confirm diagnosis, rule out differential diagnoses and identify a possible underlying cause. Primarily, cryoglobulins need to be detected and characterized for which delicate handling of the probe is essential to prevent false-negative results. The probe needs to be taken and constantly kept at body temperature (37°C) until and including centrifugation in the laboratory, otherwise cryoglobulins may precipitate beforehand, leading to false-negative tests. After detection of cryoglobulins, these should be further characterized by (immuno) electrophoresis to identify the exact type of cryoglobulinemia [13]. Once the type of cryoglobulin is identified (type I-III), adequate diagnostic workup should be initiated, including testing for hepatitis C (or other infections) and autoimmune diseases in types II and III, respectively. For type I cryoglobulinemia, a search for underlying hematologic malignancies is warranted. This should especially include full workup for monoclonal gammopathies and myeloma, as depicted in Table 3.
Treatment recommendations typically include general immunosuppressive treatment with steroids and alkylating agents such as cyclophosphamide, especially in situations of high disease activity to quickly alleviate symptoms and prevent further organ damage [12, 14, 17]. More recently, B cell depleting antibody treatment with rituximab was introduced to eliminate the pathological antibody-producing B cell clones [12, 14, 17]. In cases of associated hematologic disease, treatment in type I cryoglobulinemia should be directed at the underlying malignancy including chemotherapy combinations in non-hodgkin lymphoma. In monoclonal plasma cell disorders, several case reports and some (retrospective) clinical trials showed effectiveness of proteasome inhibition, particularly bortezomib, or immunomodulatory drugs such as thalidomide and lenalidomide [14, 17, 19]. In mixed cryoglobulinemia, the underlying disease should be targeted, too.
In case of hepatitis C virus infection, adequate antiviral treatment is recommended and successful treatment generally also leads to improvement of cryoglobulinemia. However, in case of persisting or severe symptoms, general immunosuppressive treatment with rituximab or alkylating agents and corticosteroids are still valuable options [20].
|
type I |
type II |
type III |
|
|
Cryoglobulin |
Monoclonal immunoglobulins |
Monoclonal lgM and polyclonal lgG |
Polyclonal lgM and polyclonal lgG |
|
Type of monoclonal Ig |
IgM >IgG >IgA |
IgM+++ (Kappa >> Lambda) |
|
|
Associated underlying disorders |
hematologic malignancies (B cell neoplasia, MGUS, M. Waldenström, MM and others) |
Hepatitis C hepatitis B, HIV and others |
autoimmune disease (Sjögre-n’s syndrome, Systemic Lup-us erythematodes and others) |
HIV: human immunodeficiency virus; Ig: immunoglobulin; MGUS: monoclonal gammopathy of unknown significance; MM: Multiple Myeloma
Table 2: Types of cryoglobulinemia [11-13].
|
Diagnostics |
|
|
Blood |
Complete blood count incl. differential blood counts · Electrolytes incl. Ca2+(corrected) · Renal function parameter (GFR according to CKD-EPI or MDRD) · LDH, β2-microglobulin, albumin, whole protein, transaminases, CRP · Serum protein electrophoresis and immunofixation · Immunoglobulins: IgG, IgA, IgM (IgD in case of IgD-MM) · SFLC (κ und λ) and ratio · peripheral blood smear (cytomorphology) |
|
24-hour-urine |
Proteinuria (whole protein, albumin) · Urine protein electrophoresis and immunofixation |
|
Bone marrow |
Aspiration: cytomorphology, flow cytometry, cytogenetics (FISH for at least t(4;14), del17p13, gain 1q21, t(14;16)) · Biopsy (histology) |
|
Imaging |
Osteo-CT (Low-dose whole body-CT) · Whole body-MRI |
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CRP: C reactive protein; CT: computerized tomography; FISH: fluorescence in situ hybridization; GFR: glomerular filtration rate; LDH: lactate dehydrogenase; MDRD: modified diet in renal disease; MRI: magnetic resonance imaging; SFLC: serum free light chain
Table 3: Suggested workup for diagnosis of monoclonal plasma cell disorder (5).
4. Conclusion
Even a monoclonal gammopathy that can hardly be identified as such due to very low-level presence may cause severe clinical problems and organ dysfunction secondary to cryoglobulinemic vasculopathy and should then be classified as MGCS. In the above presented case, initial treatment approaches with immunosuppressive treatment failed as well as the biological approach with B cell depletion with rituximab or showed only short-term temporary improvement. By identifying the underlying MG as likely cause of the severe symptoms, treatment directly targeted at the plasma cell clone was initiiated, even though the monoclonal disorder did not fulfil any treatment indication according to IMWG criteria. Established treatment options with bortezomib and thalidomide, however, were no feasible options due to their significant risk of polyneuropathy in this patient with already existing severe polyneuropathy. Thus, we decided to initiate treatment with the monoclonal antibody daratumumab directed at CD38 on plasma cells, in combination with lenalidomide and dexamethasone. To our knowledge, this is the first report of a patient treated successfully with daratumumab for cryoglobulinemic vasculopathy due to MGCS. As reported above, DRd treatment resulted in quick and impressive clinical response and was well-tolerated, making it an easy and effective treatment option. Thus, therapy targeted at specifically erasing the malignant plasma cell clone with substances used in MM treatment such as monoclonal anti-CD38 antibodies should be initiated early on to alleviate clinical symptoms and prevent further organ damage in MGCS patients.
Conflicts of Interest
Dr. Leypoldt reports non-financial support and personal fees from GSK, non-financial support from Abbvie, outside the submitted work.
Dr. Ghandili has nothing to disclose.
Prof. Dr. Schneider has nothing to disclose
Dr. Iking-Konert reports has nothing to disclose.
Prof. Dr. Bokemeyer reports personal fees from Sanofi Aventis, personal fees from Merck KgA, personal fees from Bristol-Myers Squibb, personal fees from Merck Sharp & Dohme, personal fees from Lilly Imclone, personal fees from Bayer Healthcare, personal fees from GSO Contract research, personal fees from AOK-Rheinland-Hamburg, personal fees from Novartis, outside the submitted work.
Prof. Dr. Weisel reports grants, personal fees and non-financial support from Amgen, personal fees and non-financial support from BMS, grants, personal fees and non-financial support from Celgene | A Bristol Myers Squibb Company, personal fees from Adaptive Biotech, grants, personal fees and non-financial support from Janssen, personal fees and non-financial support from GSK, personal fees from Karyopharm, grants, personal fees and non-financial support from Sanofi, personal fees and non-financial support from Takeda, personal fees from Oncopeptides, personal fees from Roche, outside the submitted work.
References
- Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 375 (2010): 1721-1728.
- Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. Massachusetts Medical Society 354 (2006): 1362-1369.
- Kyle RA. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med 64 (1978): 814-826.
- Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, et al. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. Massachusetts Medical Society 378 (2018): 241-249.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Bladé J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15 (2014): e538-e548.
- Mateos M-V, Kumar S, Dimopoulos MA, Gonzalez-Calle V, Kastritis E, Hájek R, et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer Journal Springer US 16 (2020): 1-11.
- Fermand J-P, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood 132 (2018): 1478-1485.
- van de Donk NWCJ, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica 99 (2014): 984-996.
- Go RS, Rajkumar SV. How I manage monoclonal gammopathy of undetermined significance. Blood 131 (2018): 163-173.
- Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med 380 (2019): 2104-2115.
- Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 57 (1974): 775-788.
- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet 379 (2012): 348-360.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: An update in 2019. Joint Bone Spine 86 (2019): 707-713.
- Harel S, Mohr M, Jahn I, Aucouturier F, Galicier L, Asli B, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. John Wiley & Sons, Ltd 168 (2015): 671-678.
- Trejo O, Ramos-Casals M, García-Carrasco M, Yagüe J, Jiménez S, la Red de G, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore) 80 (2001): 252-262.
- Rossa Della A, Tavoni A, Bombardieri S. Hyperviscosity syndrome in cryoglobulinemia: clinical aspects and therapeutic considerations. Semin Thromb Hemost 29 (2003): 473-477.
- Sidana S, Rajkumar SV, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. John Wiley & Sons, Ltd 92 (2017): 668-673.
- Benjamin Terrier, Alexandre Karras, Jean-Emmanuel Kahn, Guillaume Le Guenno, Isabelle Marie, Lucas Benarous, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore) 92 (2013): 61-68.
- Calabrese C, Faiman B, Martin D, Reu F, Calabrese LH. Type 1 cryoglobulinemia: response to thalidomide and lenalidomide. J Clin Rheumatol 17 (2011): 145-147.
- Dammacco F, Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med. Massachusetts Medical Society 369 (11): 1035-1045.