Role of Epstein-Barr Virus Reactivation in Malaria Severity
Article Information
Ide Armelle Djuidje Chatue1,2, Palmer Masumbe Netongo*,2,3,4, Severin Donald Kamdem2,5, Maloba Franklin2,4, Ngum Lesley Ngum2,3,8, Anselme Michel Yawat Djogang6,7, Pierre René Fotsing Kwetché6,7, Maximilienne Ascension Nyegue1
1Department of Microbiology, University of Yaounde I, Yaounde, Cameroon
2Molecular Diagnostics Research Group, Biotechnology Centre-University of Yaounde I (BTC-UYI), Yaounde, Cameroon
3Department of Biochemistry, Faculty of Science, University of Yaounde I (UY1), Yaounde, Cameroon
4Biology Program, School of Science, Navajo Technical University, Crownpoint, New Mexico, USA
5Department of Pathology, University of Utah School of Medicine, United State of America
6School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes, Bangangte, Cameroon
7Laboratory of Microbiology, Universite des Montagnes Teaching Hospital, Bangangte, Cameroon
8 Institute of Medicine and Medicinal Plants Studies, IMPM, Yaounde, Cameroon
*Corresponding author: Palmer Masumbe Netongo, Department of Biochemistry, University of Yaounde I, Po Box 812 Yaounde Cameroon
Received: 17 June 2022; Accepted: 28 June 2022; Published: 18 July 2022
Citation:
Ide Armelle Djuidje Chatue, Palmer Masumbe Netongo, Severin Donald Kamdem, Maloba Franklin, Ngum Ngum Lesly, Anselme Michel Yawat Djogang, Pierre René Fotsing Kwetché, Maximilienne Ascension Nyegue. Role of Epstein - Barr virus Reactivation in Malaria Severity. Archives of Clinical and Biomedical Research 6 (2022): 612-622
Share at FacebookAbstract
Epstein-Barr Virus (EBV) known as human gammaherpesvirus is latently present in more than 95% of the world adult population. The virus undergoes lytic replication and latent cycle to insure its spread and the episomal persistence of the viral genome respectively. Under certain conditions that dysregulate the host immune system, latent EBV infection reactivates. EBV reactivation has been involved in the pathogenesis of a variety of autoimmune diseases and cancers. Many studies describe the association between Epstein-Barr virus reactivation and malaria severity. It is suggested that the reactivation of EBV infection (lytic replication) impairs the immune response to malaria, exacerbates its pathogenesis, and increases the frequency and susceptibility to severe malaria. However, the immunological mechanisms are not clear. A thorough understanding of the specific role of EBV reactivation in the severity of malaria, is crucial to the development of effective vaccine. In this review, we provide a summary of the impact of Epstein-Barr Virus reactivation on malaria in the current scientific literature.
Keywords
Epstein-Barr Virus; reactivation; malaria; severity; immune response (MeSH)
Epstein-Barr Virus articles, reactivation articles, malaria articles, severity articles, immune response (MeSH) articles
Article Details
1. Introduction
Epstein-Barr Virus (EBV) known as human herpesvirus 4 (HHV-4) is an oncogenic virus that belongs to the herpesvirus family. It is a ubiquitous virus that infects 95% of the world’s adult population at an early age establishing long-term persistent infection [1, 2]. The virus has two life cycles, namely, the lytic replication that is important for the spread of the virus whereas latency facilitates the episomal persistence of the viral genome [1,3,4]. Sometimes, under psychological and physiological stress, EBV reactivates. During pregnancy, which is assumed to be an immunosuppressed state, nearly 35% of latent EBV infection becomes reactivated [5]. Maternal EBV reactivation has been associated with adverse offspring development, including severe symmetrical fetal growth restriction, lower birth weight, and leukemia [6]. EBV infection in children usually don’t cause symptoms but in adolescents and young adults, it may cause infectious mononucleosis, with symptoms like fatigue, malaise, pharyngitis, fever, abdominal discomfort, lymphadenopathy and headache. Some patients may develop complications such as autoimmune diseases, splenic rupture and neurological complications [7].
In sub-Saharan Africa, Epstein-Barr virus (EBV) and Plasmodium falciparum have similar geographical distribution [8-10]. The co-infection with these two infectious agents has been proven to be more dangerous than each infection separately [11]. Various epidemiological studies have long established an etiology link between Epstein-Barr virus (EBV), Plasmodium, and endemic Burkitt Lymphoma (eBL). Endemic Burkitt Lymphoma is the most common paediatric malignant tumour in children aged 5-9 years livings in sub-Saharan Africa and Papua New Guinea [12-14]. Previous studies have demonstrated that acute Epstein-Barr virus infection can suppress the development of humoral and adaptive immunity against malaria, becoming a risk factor for severe malaria [11, 15]. In a well-defined mouse model, Matar et al have shown that acute infection with murine gammaherpesvirus 68 (MHV68) suppresses humoral antimalarial responses by disrupting B-cell formation and differentiation into antibody-producing plasma cells at the germinal centre in the spleen [16]. However, the reactivation of EBV infection has been involved in the pathogenesis of numerous diseases in general [17]. Recently, it has been revealed that reactivated EBV can infect human brain microvascular endothelial cells (HBECs), increase RBC adhesion on ECs, and thus aid in exacerbation of cerebral malaria pathology [18]. Nevertheless, little is known about the association of EBV reactivation and the severity of malaria. Furthermore, the mechanisms by which reactivated EBV infection leads to severe malaria are not clear. The aim of this article is to review the most recent document on the association of EBV reactivation and the severity of malaria. We will also examine and discuss the possible mechanisms via which EBV may alter the development of an effective immune response and control against malaria.
2. Biology and Epidemiology of Epstein-Barr virus
Epstein-Barr Virus (EBV) was discovered in 1964 by Epstein et al. in a culture of Burkitt lymphoma cells. Its genome has a double-stranded DNA genome of about 170 kbp that is maintained in the nucleus of a host cell as an episome [19]. In sub-Saharan Africa, children get infected early in life (from 6 months of age) while in developed countries infections occur in adolescence and adulthood [20, 21]. Once the virus penetrates in the host, it interacts with the CDß1 molecule present on the cell plasma membrane and persists preferentially in B-lymphocytes and oropharyngeal epithelial cells for life [22]. However, the virus is also capable of infecting Dendritic Cells (DC), T-lymphocytes, neutrophils (N), macrophages (MA), and monocytes (MO) [23-26]. The main route of transmission is oral via direct contact with saliva. Nevertheless, infections through blood transfusion, sexual contact, organ transplantation, breast milk, and during childbirth have been reported [27-30].
Generally, the primary EBV infection is asymptomatic but in immunocompromised conditions, EBV DNA loads increase may cause various complications progressing to lymphoid or epithelial malignancies [31, 32]. EBV can undergo two life cycles, namely latent (non-productive) or lytic replication (reproductive) (Figure 1) [1]. The virus spreads from cell to cell and from host to host thanks to the lytic replication whereas latency facilitates the episomal persistence of the viral genome [3, 4]. However, under certain physiological conditions, the virus may undergo lytic reactivation leading to the expression of lytic proteins, followed by the assembly and exit of virions capable of infecting other cells and infecting other hosts [33].
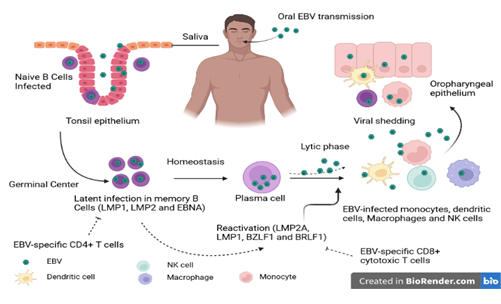
Figure 1: Schematic representation of the EBV life cycle. EBV is transmitted mainly by the oral route. Once in the oral cavity, the virus spreads through saliva and infects epithelial cells and naive B cells (CD21 and CD35). The naive B cells that have been in contact with the antigen transform into active lymphoblast cells capable of presenting the antigen to the CD4+ T cells of the germinal center. In the germinal center, the infected B cells will undergo a latent phase through the expression of latency genes. This phase favors the persistence of the virus in the host. Under certain conditions, EBV present in reactive memory B cells induces a lytic phase of replication characterized by release of numerous virions and expression of immediate, early and late lytic genes. Subsequently, memory B cells may migrate to the oral compartment and excrete the virus. This phase allows the transmission of the virus. Source: Own elaboration (Created with BioRender.com).
3. Stimulators factors of EBV reactivation
The reactivation of the lytic infection from latently infected cells in an infected host is most often due to an alteration of the cellular immune response. During the reactivation, early viral genes are expressed mainly, BamHI Z fragment leftward open reading frame 1 (BZLF1) (also known as Zta, Z, ZEBRA, EB1) and BRLF1 (also known as Rta, R, EB2) two transactivators [33]. Many factors are involved in the reactivation process.
3.1 Physiological and Environmental Inductors
Studies have shown that chemical and biological agents like 12-O-tetradecanoylphorbol-13-acetate (TPA), sodium butyrate, anti-Ig M, transforming growth factor-beta (TGF-β), calcium ionophores, histone deacetylase inhibitors (HDAC) and Inhibitors of DNA methyltransferase can trigger EBV reactivation by the two immediate-early transactivators (EI) induced, Zt and Rta [34-36]. It has been revealed that E. tirucalli can reactivate EBV thanks to the 4-deoxyphorbol ester, the latter is related to the TPA which is present in the milky, rubbery sap of this plant [37]. B-cell receptor (BCR) stimulation and epithelial cell differentiation are also known to activate the lytic replication form of EBV infection from latency in the host [4, 38]. Signaling pathways mediated by cellular stress such as DNA damage, PKC, MAPK (ERKs, JNKs, and p38), and PI3K pathways appear to be involved in the reactivation of lytic infection [39]. Previous research has shown that EBV uses the autophagy mechanism to promote viral replication, yet in the host, this mechanism serves as a defence against viral infection [40,41]. Other studies reveal that activation of autophagic mechanisms through Rta-mediated ERK and PKCθ-p38 signaling promotes the reactivation of EBV [40, 42]. According to Pei Tung Yiu and colleagues in 2019, an iron chelator, C7, activates the lytic cycle of Epstein-Barr virus by activating the ERK1/2 autophagy pathway in epithelial cancers [43].
3.2 Psychological Stress
Previous evidence suggested a link between the reactivation of Epstein-Barr virus and chronic stress due to a decrease in cellular immune responses [44]. Among the various types of stress, psychological stress like pregnancy-related stress, student exam stress, marital stress, attachment anxiety or fear of abandonment can reduce immune responses [45-50]. People with more attachment anxiety have been shown to have higher VCA EBV IgG antibody titers than those with less attachment anxiety [48]. In recent years, the association between EBV reactivation and depression during pregnancy has been the subject of some studies and it has been found that women with depression are more likely to have EBV reactivation [51]. In addition, Purtilo and Sakamoto, 1982 reported an increase in several serological markers of reactivation during pregnancy and suggested that pregnant women have a higher incidence of EBV reactivation because cellular immune responses to EBV appear to be suppressed during pregnancy [52]. In 2010, Haeri et al showed that among 98% of women with EBV, 35% showed EBV reactivation during pregnancy in the second trimester [5]. With regard to physical stress, it has not been implicated in the reactivation of EBV or altered response to the disease [53].
3.3 Immunosuppression and tobacco consumption
EBV-positive individuals treated with immunosuppressive drugs, individuals with congenital or acquired immune deficiencies, or individuals who have undergone organ or stem cell transplants, are more susceptible to viral reactivation of EBV [54, 55]. The association between tobacco consumption and the reactivation of EBV has been much studied. It is known that smoking decreases oxygen and increases hypoxia. Previous studies have suggested that smoking may significantly increase the long-term risk of Nasopharyngeal Carcinoma (NPC) by reactivating EBV infection [56]. Eleanor V. et al also suggest that there is a relationship between Hodgkin's lymphoma and smoking and the reactivation of Epstein-Barr virus [57]. In vitro experiments conducted by Feng-Hua Xu, et al 2012 demonstrate that cigarette smoke promotes the expression of the immediate and early transcriptional activators Zta and Rta. Indeed, these transactivators promote the lytic replication of EBV [58].
4. EBV reactivation and malaria outcomes
4.1 The link between EBV reactivation and malaria
Coinfection between EBV and Plasmodium is common in Africa because of their high geographical distribution [8-10]. In equatorial Africa, these two pathogens have long been associated with the genesis of endemic Burkitt lymphoma (eBL), the most prevalent paediatric cancer in children aged 5-9 years [12, 13]. In this region, the age of EBV seropositivity in children (early at 6 months) coincides with the decline in protective maternal antibodies and increased susceptibility to primary Plasmodium infection [20]. The association of P. falciparum malaria infection and EBV reactivation has been the subject of many studies. It was reported that acute Plasmodium falciparum infection affects the persistence of EBV by increasing the levels of circulating EBV and antibody titers to viral capsid antigen (VCA) and the Z Epstein-Barr replication activator (ZEBRA) protein in peripherical blood [59-62]. Donati et al showed that EBV loads can drop to undetectable levels after antimalaria treatment in children living in malaria-endemic areas (85% of the cases [60]. The mechanisms by which acute malaria reactivate EBV lytic infection is not fully understood, but prolonged and intense exposure to P. falciparum malaria leads to a loss of viral control by compromising T-cell immunity (CD8+ T cells) to EBV [13, 63, 64]. Other studies suggest that apart from the inhibitory effect of Plasmodium on EBV-specific T cell responses, P. falciparum affects the B cell compartment by the expansion of EBV-carrying B cells; induction of apoptosis in the infected B cell pool, with consequent release of virus/viral DNA; or increase of viral replication [60, 65-68]. Additionally, Cysteine-rich inter-domain region 1α (CIDR1α) of the Plasmodium falciparum membrane protein 1 a polyclonal B cell activator has been showed to directly induce EBV reactivation during malaria infection increases the risk of BL development for children living in malaria-endemic areas [59].
Cases of co-infection with EBV and other Plasmodium species have been observed in recent years. The first case of P. vivax malaria and EBV coinfection was reported in 2013 in a 5-year old Turkish boy from the Southeast Anatolia and Cukurova regions of Turkey where P. vivax is the most common cause of malaria [69]. Recently in the United States, an 11-year-old boy was diagnosed with malaria and EBV coinfection. The patient had traveled for a 1-month visit to Sierra Leone (his native country and an endemic area for chloroquine-resistant malaria). The current symptoms were pancytopenia with neutropenia. In this country, co-infection with malaria and EBV is extremely rare [70].
4.2 EBV reactivation as a predisposing factor to severe malaria
To date, a handful of studies have already been carried out on the influence of EBV infection on the innate or adaptative immune response against malaria (Table 1). However, these have focused only on acute infection. This is the case of the work carried out by Wedderburn et al. in 1988 on the impact of Epstein-Barr virus and P. brasilianum coinfection on the development of glomerulonephritis in infected marmoset mice. In a well-developed model, the latter observed an increase in the severity and mortality of the disease in animals infected simultaneously with both agents. It is also noted that animals co-infected with P. brasilianum and EBV had a more prolonged parasitaemia compared to those mono-infected with P. brasilianum, suggesting that co-infection could lead to the etiology of quarantine malarial nephropathy [71]. A few years ago, Caline M. et al, 2015 demonstrated the immunosuppressive effect of acute EBV infection on the development of humoral antimalarial immunity [16]. In 2018, a study conducted in Brazil on two distinct malaria area, one from the Amazon region (sporadically exposed to malaria transmission) and the other from the agricultural area of Rio Pardo (long term exposure to malaria) suggested that EBV infection influence the morbidity to P. vivax malaria with altered haematological parameters, including low haemoglobin, haematocrit, and platelet levels; and impaired the long-term acquired antibody responses to P. vivax proteins (PvDBPII and PvAMA1) [72]. These results demonstrate that acute EBV infection disrupts the malaria immune response. Given that under the influence of some risk factors mentioned above, EBV can undergo a reactivation of its lytic infection inside latent cells leading to the expression of many lytic genes, it is plausible that reactivated EBV similarly to acute infection alters malaria immunity leading to the exacerbation of malaria.
The impact of EBV reactivation on others diseases has long been studied. Indeed, it has already been shown that chronic and uncontrolled EBV reactivation may exacerbate certain diseases and be a risk factor in the etiology of many cancers and tumours such as nasopharyngeal carcinoma, and post-transplant lymphoproliferative diseases [73, 74]. According to Ramroodi N. et al., the reactivation of EBV also aggravates the pathogenesis of multiple sclerosis. People with multiple sclerosis subtypes and patients with relapses of the disease (exacerbations) had strong reactivation of Epstein-Barr virus with high viral DNA loads. This suggests that EBV reactivation exacerbates multiple sclerosis and promotes relapses [75]. Similarly, in the article written by Larry Beresford in (2020) in the journal the Rheumatologist, Dr. James, co-author of the study, published in Annals of the Rheumatic Diseases, reveals that reactivation of lytic EBV infection is a risk factor for the development of Systemic Lupus Erythematosus (SLE). This is thought to be due to increased EBV reactivation (increase in EBV viral capsid antigen (VCA) and IgG against early antigens). Previous research work conducted by Keiko Nagata et al in 2011 suggests that reactivation of EBV is associated with the etiology of Graves' disease, through stimulation of antibody-producing B cells predisposed to make TRAb (Thyroid Autoantibody), which could contribute to or exacerbate the disease [76].
A first study on the impact of EBV reactivated on malaria severity, has recently been studied by Indari et al., the results revealed that during malaria, the reactivated EBV can infect human brain microvascular endothelial cells (HBECs) and modify the blood-brain barriers microenvironment (BBB). Addionally EBV infection of HBECs significantly elevated inflammatory cytokines (TNFα, CCL2) and endothelial markers (integrin β3, PECAM, VEGFA, VWF, claudin-5, cx37). EBV and P. falciparum co-infection significantly (P < 0.05) enhanced RBC adhesion to HBECs compared to mono-infection, this could facilitate iRBCs sequestration at the BBB and exacerbation of CM pathology [18]. Based on the results of these studies, malaria immune response may be impaired during the reactivation of lytic EBV infection, which could worsen the clinical condition of co-infected individuals.
Table 1: Reported experimental and epidemiological studies on the impact of acute and reactivated EBV infection on malaria
|
Authors |
Type of Study |
Country and Study population |
Key findings |
|
Omkar Indari et al 2021 |
Experimental |
India Blood sample collection from the volunteers. |
This study revealed that during malaria onset, the reactivated EBV can infect human brain microvascular endothelial cells (HBECs). EBV infection of HBECs significantly elevated several inflammatory (TNFα, CCL2) and endothelial (integrin β3, PECAM, VEGFA, VWF, claudin-5, cx37) markers. The EBV-infected HBECs secretion significantly reduced migration of HBECs, glial and neuronal cells. They showed that EBV-P. falciparum co-infection significantly (P < 0.05) enhanced RBC adhesion to HBECs compared to mono-infection scenarios. The results support the role of EBV at the BBB and exacerbation of cerebral malaria. |
|
Insani Budiningsih et al 2021 |
Cross-sectional |
Indonesia Venous blood samples of 68 patients with acute malaria from Sumba Island in East Nusa Tenggara and 27 healthy controls in Surabaya (East-Java), Indonesia. |
They demonstrated a relationship between EBV reactivation and cytokine activity during acute P. falciparum malaria. Indeed, the author observed significantly elevated EBV DNA loads in P. falciparum and P. vivax infections (P<0.05) compared to controls. Levels of the cytokines TNF-α and IL-10 were higher in malaria cases than in controls. TNF-α levels in P. falciparum cases showed a clear correlation with elevated EBV DNA levels (R2=0.8915) |
|
Dias Micelle Hallais Franca et al 2018 |
Cross-sectional |
Brazil Blood sample from 154 individuals sporadically exposed to malaria and 541 individuals with long-term exposure to malaria in the Amazon region (P. vivax infected (n=38) and not infected (n= 503)) |
During acute malaria infection, EBV positivity is associated with altered hematological parameters: decreased hemoglobin, hematocrit and platelet level. No association between EBV DNA and clinical manifestations of malaria in individuals living in the sporadic malaria transmission zone. In contrast, in individuals with long-term exposure to malaria, a positive association between EBV DNA was detected. Detection of viral DNA appears to influence, positively or negatively, the long-term acquired antibody responses to different blood stage P. vivax proteins (PvDBPII and, PvAMA1, respectively). They suggest that EBV infection may influence P. vivax morbidity and immunity. |
|
Bashiru, S. 2017 |
Cross-sectional |
Ghana Blood sample of 80 children below 5 years with malaria. |
The overall EBV seroprevalence was 26.25%. In children with severe malaria (31 of 80), EBNA-1 seroprevalence was 32.78%. There was no significant association (p=0.123) between severe malaria and EBV infection. However, children with EBV were more likely to develop severe malaria (hazard ratio 2.024, 95% CI 0.8249 to 4.9686, p = 0.1236). |
|
Caline G. Matar et al 2015 |
Experimental |
United State of America Animals (Female C57BL/6 mice (6-8 weeks), µMT and RAG2-/- mice) |
This work demonstrated that acute infection with murine gammaherpesvirus 68 (MHV68) is capable of transforming simple, lethal malaria. This result can be explained by a defect in the maintenance of germinal center B cells and follicular helper T cells (Tfh) in the spleen. Furthermore, the author identified a viral protein, MHV68 M2, and its role in altering the anti-malarial immune response. Indeed, the M2 protein plays an essential role in the suppression of anti-malarial humoral responses during infection. |
|
Ibrahim I Daud et al 2015 |
Prospective |
Western Kenya Venous blood sample of 200 pregnant women. Pregnant women from Chulaimbo sub-district hospital. |
Increase in malaria load correlated with increase in EBV load (p < 0.0001). Pregnant women who had malaria detected during pregnancy were more likely to have detectable EBV DNA than pregnant women who had no evidence of malaria infection during pregnancy (64 vs. 36 %, p = 0.01). Frequency and EBV load were significantly higher in pregnant women with P. falciparum malaria than in women without evidence of malaria infection (p = 0.01) regardless of gestational age of the pregnancy. |
|
Clarisse LRP Yone et al 2006 |
longitudinal matched-pair study |
Gabon 60 children Plasma and whole blood samples (a subset of 200 original participants in the 1-95/C study 1995-2002). |
They demonstrated that EBV is reactivated during acute P. falciparum malaria. EBV activity persists at a higher frequency in the blood of children with a history of severe malaria compared to those with mild malaria when they were healthy and parasite free (67% vs. 39%; P = 0.013). High EBV DNA loads were associated with more malaria attacks and with elevated plasma concentrations of both TNF-_ and IL-12p40. Suggesting that higher peripheral blood EBV DNA loads are associated with susceptibility to more frequent P. falciparum episodes and with altered cytokine activity. |
|
A. HAQUE et al 2004 |
Experimental |
Gabon 60 children Plasma and whole blood samples (a subset of 200 original participants in the 1-95/C study 1995-2002) |
They demonstrated that coinfection with malaria and MHV-68 induced more severe disease than either agent alone. Significant mortality of co-infected mice and aggressive lung inflammation with a marked influx of neutrophils and megakaryocytes were observed. On the other hand, acute malaria reduced the capacity of anti-MHV-68 CD8+ cells in these animals. |
|
Lam, K.M.C. et al 1991 |
Cross sectional |
Gambia Blood samples from Gambian children with acute malaria; UK patients with infectious mononucleosis (IM) and healthy UK adults. Cross sectional study |
Globally, the results showed a much higher number of virus-infected cells in acute malaria patients and in UK patients with infectious mononucleosis than in convalescent malaria patients and in healthy UK adult controls. Suggesting that acute malaria is associated with an increase in the load of EBV and EBV-infected B lymphocytes in the circulation. |
Source: Own elaboration
4.3 Impairment of antimalaria humoral response during reactivated EBV infection
The reactivation of EBV during acute malaria has been reported in several studies [59, 60]. Humoral response plays an important role in the mechanisms to control peripheral parasitaemia in human malaria infection and is essential in the natural acquisition of partial immunity to malaria [77-79]. It consists of blocking parasite invasion of red blood cells (RBCs) by blocking ligand-receptor interactions on the surface of RBCs, promoting the formation of antibody- dependent cytotoxicity on Plasmodium infected RBCs (iRBC), blocking the activity of parasite toxins, and inhibiting the development of intraerythrocytic parasites [80]. Thus, a disruption of this immune response would aggravate the malaria infection. It has been observed that during acute gammaherpesvirus infection in animals co-infected with P. yoelii XNL, these animals developed severe malarial anaemia due to a reduction in the antibody response (IgM and total IgG levels) compared to those infected individually with P. yoelii XNL [16]. In addition, the authors showed that increased replication of MHV68 contributes to the lethality of P. yoelii in co-infected mice [16].
The reactivation of EBV infection has been associated with increased susceptibility and frequency of malaria episodes in children in Gabon, due to altered cytokine activity [81]. Cytokines are important modulators of lymphocytes. There are essential for generating many of the known subsets of CD4 T cells (Th1, Th2, Th17, and iTreg). Studies have shown that the combined absence of IL-6 and IL-21 leads to a reduction in the differentiation of Tfh cells and a reduction in the expression of the Bcl6 protein [82]. Direct induction of Bcl6, a critical regulator of germinal centres (GCs) by cytokines is sufficient to generate the Tfh subset [83,84]. It is clear that cytokines contribute to Tfh differentiation at the germinal centre and ensure that the humoral immune response is effective [85]. Furthermore, alteration of cytokine (IL-10, IL-12p70, IL-12p40, TNF-α, and IFN-γ) activity during persistent EBV has been suggested to be one of the immunomodulatory mechanisms influencing pathogen interactions [81].
A change in the activity of these cytokines could lead not only to a decrease in the differentiation of Tfh cells from the germinal centre to the spleen but also to a reduction in GC B cells, which would disrupt the humoral response against secondary infections by the mechanisms induced [86]. It has been shown that a defect in the formation and maintenance of total Tfh cells (CD4+ CXCR5+ PD-1+), activated/antigen-specific Tfh cells (CD4+ PD-1+ CD44hi CXCR5+) and germinal centre Tfh cells (GL7+ CXCR5+) suppress the humoral immune response against malaria in animals infected with MHV68 [16,87]. Kaneko et al. has showed abnormally high concentrations of the cytokine TNF- α could block the differentiation of T cells into Bcl-6 helper follicular T cells [88]. We hypothesize that during EBV reactivation, the alteration of cytokine activity may disrupt the production of anti-malarial antibodies through a defect in the maintenance of Tfh cells in patients with malaria (figure 2). Nevertheless, further studies need to be carried out to elucidate these mechanisms during EBV reactivation and malaria coinfection.
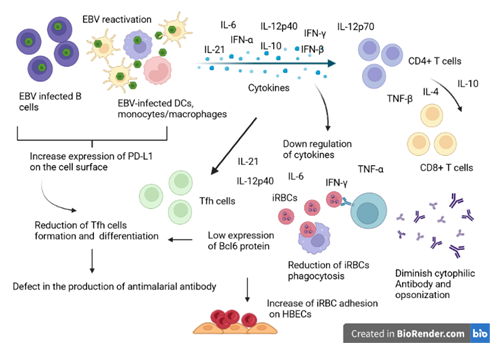
Figure 2: Illustrative schema of the interference model of EBV reactivation during the antimalarial immune response. During EBV reactivation, infected B cells and other EBV-infected immune cells secrete large amounts of inflammatory and immunosuppressive cytokines. To regulate, the immunosuppressive cytokines will inhibit the production of IFN-alpha, IL-6 and TNF-beta cytokines by helper T cells (CD4+) and cytotoxic T cells (CD8+). Overall, the reduction of these cytokines will negatively affect the clearance of Plasmodium-infected red blood cells and the production of T cell-dependent cytotoxic antibodies. The reduction of Il-6 reduces the expression of the transcription factor BCl-6 important in the regulation of T-helper cell proliferation. On the other hand, Il-10 hypersecretion also affects the formation and differentiation of Tfh, important in the production of antibodies against malaria. Source: Own elaboration (Created with BioRender.com).
EBV reactivation has been correlated with the expression of PD-1/PD-L1 antigens in patients with proliferative glomerulonephritis [89]. PD-1, also known as CD279, is a cell surface receptor that, in humans, is expressed on T cells, B cells, monocytes/macrophages, and certain cancer cells [90,91]. PD-L1/L2-PD-1 signaling has been shown to influence germinal centre responses, thus an increased expression of PD-L1 negatively regulates the expansion of Tfh [92]. According to Matar et al, this is one of the mechanisms necessary to hinder humoral immunity to malaria during acute gammaherpesvirus infection [16]. We think that the expression of PD-L1 on the cell surface of T cells, B cells, and monocytes/macrophages infected with the virus may contribute to the disruption of humoral antimalarial immunity.
4.4 Disruption of innate immune cells infected by EBV during malaria
Innate immunity plays a key role in parasitaemia control thanks to the phagocytic activity of monocytes and macrophages. Many studies have described the essential role of activated monocytes/macrophages and Dendritic Cells (CDs) function in the reduction of parasite burden through plasmodium-infected red blood cells (iRBCs) and free merozoites, cytokine production, and antigen presentation [93]. Previous studies have shown that Epstein-Barr virus is capable of affecting the functional capacities of the cells it infects, thus reducing their phagocytic potential to respond to the challenges of other pathogens such as bacteria and inhibiting the secretion of key cytokines (TNF-α) [94, 95]. EBV can also inhibit the development of dendritic cells by promoting the apoptosis of their monocytic precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4 [96].
Early production of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, and other inflammatory cytokines by innate immune cells allows faster inhibition and elimination of the parasite and stimulation of the phagocytosis [97, 98]. Smith CM et al (2007) demonstrated that Dendritic Cells (DCs) infected with the gammaherpesvirus MHV68 are unable to present the antigen as effectively as those not infected with MHV68 [99]. Reactivated EBV infection has been shown to alter the activity of cells present in the brain-blood barriers microenvironment (BBB) and increase RBC adhesion on ECs (Endothelial Cells). These change lead to an overexpression of endothelial and surface markers, and an elevation of several inflammatory cytokines (TNFα, CCL2), exacerbating cerebral malaria pathology [18].
The Fc-gamma receptors antibodies to P. falciparum merozoites are important for the opsonic phagocytosis of merozoites [100]. It will be interesting to study the cellular characteristic and membrane receptor markers for phagocytosis (CD16, CD32, CD64) of monocyte/macrophage with EBV. Since pregnancy, immunosuppression, and tobacco consumption are risk factors for reactivation of EBV, it will be also interesting to study in vivo the activity of macrophages against iRBCs during the co-infection in these patient groups. Complementary studies are required.
4.5 Modulation of INF-type I and IL-10 during the EBV/Plasmodium coinfection
Adaptive immunity during malaria infection involve Interferon-γ (IFN-γ) the production and antibodies under the coordination of CD4 cells [101]. It is well known that IFN-α and IFN-β can inhibit the development of P. falciparum in the liver and blood stages. The regulation of the interferons activities is an important aspect of establishing an effective and controlled adaptive immune response. This mechanism includes interferon regulatory factors (IRFs). It has been suggested that inhibition of IRF prevents the production of key inflammatory cytokines such as IFN type I (IFN-α and IFN-β) [102]. Hwang S et al, 2009, have reported that ORF36, a kinase of murine gammaherpesvirus 68 (MHV-68) is capable of inhibiting IFN by binding to interferon regulatory factor 3 (IRF-3) [103, 104]. Furthermore, chronic IFN type I signaling has an important role in the severity of malaria anaemia (SMA) due to the ability to reduce the production of haematopoietic stem cells [105].
Given that EBV inhibits IRF and TLR signaling pathways in infected B lymphocytes, this may influence humoral responses during subsequent infection with an infectious agent like malaria. Rangaswamy et al, revealed that viral protein M2 is capable of promoting BCR signals and inducing IL-10 secretion from EBV- infected B cells [106, 107]. IL-10 produced is capable of negatively regulating the survival of T follicular helper cells (Tfh) and consequently affecting the maintenance and development of the germinal centre. Concerning IL-10 producing T cells, these cells have been found to exacerbate P. yoelii parasitaemia in mice in mouse models [108, 109]. It is thought that stimulation of BCR signaling pathways by the EBV viral protein M2 may reactivate EBV infection and contribute to increased production of IL-10 which will aggravate malaria. The same observation was made by Saraiva M & O'Garra A. 2010, who found in rodent malaria models that homologous EBV-encoded viral IL-10 can impair control of Plasmodium parasitaemia in the peripheral circulation [110, 111].
5. Conclusion
In this review, the association between EBV reactivation and malaria severity was demonstrated. Studies showed that lytic EBV infection can be reactivated during malaria coinfection, pregnancy, physiological and environmental factors, stress, and immunosuppression. Reactivated EBV infection can negatively impact the pathogenesis of malarial infection and thus can be considered as a risk factor for severe malaria.
Different immunological mechanisms have been incriminated in this process namely: (1) the reduction of the production of antibodies against Plasmodium via the alteration of inflammatory cytokines (2) impairment of the function of innate immune cells (monocyte, macrophage, dendritic cells, B cells, and T cells) latently infected by the virus, and (3) inhibition of germinal centre (GC) regulators (Bcl6). The limit of these studies is that most are in vitro and there is very little human evidence. More immunological and epidemiological studies addressing the influence of reactivated EBV infection on the development of innate and adaptative immune response to Plasmodium in patients should be carried and further studies on the secretome of EBV-infected monocyte and macrophage cells should be also investigated.
Contributions of the authors
All the authors cited have made a substantial, direct, and intellectual contribution to the work and have approved its publication.
Declaration of conflict of interest
The authors state that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Acknowledgements
This work is supported by the University of Yaounde I, Cameroon.
References
- Drouet E. The Role of the Epstein - Barr virus Lytic Cycle in Tumor Progression: Consequences in Diagnosis and Therapy. Hum Herpesvirus Infect Featur Transm Symptoms, Diagnosis Treat (2019).
- Hudnall SD, Chen T, Allison P, Tyring SK, Heath A. Herpesvirus prevalence and viral load in healthy blood donors by quantitative real-time polymerase chain reaction. Transfusion 48 (2008): 1180-7.
- Kenney SC, Mertz JE. Regulation of the latent-lytic switch in Epstein-Barr virus. In: Seminars in cancer biology. Elsevier (2014): 60-8.
- Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 79 (2005): 1296-307.
- Haeri S, Baker AM, Boggess KA. Prevalence of Epstein-Barr Virus Reactivation in Pregnancy. Am J Perinatol. 12.04.2010 27 (2010): 715-20.
- Eskild A, Bruu A, Stray-Pedersen B, Jenum P. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. BJOG An Int J Obstet Gynaecol 112 (2005): 1620-4.
- Hess RD. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol 42 (2004): 3381-7.
- Epstein MA. Burkitt’s lymphoma: clues to the role of malaria. Nature 312 (1984): 398.
- Facer CA, Playfair JH. Malaria, Epstein-Barr virus, and the genesis of lymphomas. Adv Cancer Res 53 (1989): 33-72.
- Geser A, Brubaker G. A preliminary report of epidemiological studies of Burkitt’s lymphoma, Epstein-Barr virus infection and malaria in North Mara, Tanzania. IARC Sci Publ 60 (1985): 205-15.
- Matar CG, Jacobs NT, Speck SH, Lamb TJ, Moormann AM. Does EBV alter the pathogenesis of malaria? Parasite Immunol 37 (2015): 433-45.
- Mawson AR, Majumdar S. Malaria, Epstein-Barr virus infection and the pathogenesis of Burkitt’s lymphoma. Int J cancer 141 (2017): 1849-55.
- Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis 24 (2011): 435.
- Lavu E, Morewaya J, Maraka R, Kiromat M, Ripa P, Vince J. Burkitt lymphoma in Papua New Guinea—40 years on. Ann Trop Paediatr 25 (2005): 191-7.
- Bashiru S. Correlation between Epstein-Barr Virus Infection and The Severity of Malaria in Ghanaian Children Under Five (5) Years of Age. University of Ghana (2017).
- Matar CG, Anthony NR, O’Flaherty BM, Jacobs NT, Priyamvada L, Engwerda CR, et al. Gammaherpesvirus co-infection with malaria suppresses anti-parasitic humoral immunity. PLoS Pathog 11 (2015).
- Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol 72 (2019): 651-8.
- Indari O, Chandramohanadas R, Jha HC. Epstein-Barr virus infection modulates blood-brain barrier cells and its co-infection with Plasmodium falciparum induces RBC adhesion. Pathog Dis 79 (2021): ftaa080.
- Epstein MA. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1 (1964): 702-3.
- Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, Moormann AM, et al. Early age at time of primary Epstein-Barr Virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the Etiology of Endemic Burkitt Lymphoma. J Infect Dis 205 (2012): 906-13.
- Biggar RJ, Henle W, Fleisher G, Böcker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J cancer 22 (1978): 239-43.
- Cohen JI. Epstein-Barr virus infection. N Engl J Med 343 (2000): 481-92.
- Martorelli D, Muraro E, Merlo A, Turrini R, Fae DA, Rosato A, et al. Exploiting the interplay between innate and adaptive immunity to improve immunotherapeutic strategies for Epstein-Barr-virus-driven disorders. Clin Dev Immunol (2012).
- Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournié J-J. Acquisition of viral receptor by NK cells through immunological synapse. J Immunol 170 (2003): 5993-8.
- Savard M, Bélanger C, Tremblay MJ, Dumais N, Flamand L, Borgeat P, et al. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J Immunol 164 (2000): 6467-73.
- Auclair H. Etude des homologies phénotypiques et fonctionnelles des lymphocytes B en latence III de l’EBV avec les cellules B régulatrices, implication de l’axe PD-1/PD-L1. Limoges (2017).
- Papesch M, Watkins R. Epstein-Barr virus infectious mononucleosis. Clin Otolaryngol Allied Sci 26 (2001): 3-8.
- Alfieri C, Tanner J, Carpentier L, Perpete C, Savoie A, Paradis K, et al. Epstein-Barr virus transmission from a blood donor to an organ transplant recipient with recovery of the same virus strain from the recipient’s blood and oropharynx (1996).
- Trottier H, Buteau C, Robitaille N, Duval M, Tucci M, Lacroix J, et al. Transfusion-related Epstein-Barr virus infection among stem cell transplant recipients: a retrospective cohort study in children. Transfusion 52 (2012): 2653-63.
- Daud II, Coleman CB, Smith NA, Ogolla S, Simbiri K, Bukusi EA, et al. Breast milk as a potential source of Epstein-Barr virus transmission among infants living in a malaria-endemic region of Kenya. J Infect Dis 212 (2015): 1735-42.
- Daud II, Ogolla S, Amolo AS, Namuyenga E, Simbiri K, Bukusi EA, et al. Plasmodium falciparum infection is associated with epstein-barr virus reactivation in pregnant women living in malaria holoendemic area of Western Kenya. Matern Child Health J 19 (2015): 606-14.
- Jha HC, Pei Y, Robertson ES. Epstein-Barr virus: diseases linked to infection and transformation. Front Microbiol 7 (2016): 1602.
- Longnecker RM, Kieff E, Cohen JI. Epstein-barr virus. In: Fields Virology: Sixth Edition. Wolters Kluwer Health Adis (ESP) (2013).
- Murata T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol Immunol 58 (2014): 307-17.
- Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. Lytic Cycle Switches of Oncogenic Human Gammaherpesviruses1. Adv Cancer Res 97 (2007): 81-109.
- Countryman J, Gradoville L, Bhaduri-McIntosh S, Ye J, Heston L, Himmelfarb S, et al. Stimulus duration and response time independently influence the kinetics of lytic cycle reactivation of Epstein-Barr virus. J Virol 83 (2009): 10694-709.
- Mannucci S, Luzzi A, Carugi A, Gozzetti A, Lazzi S, Malagnino V, et al. EBV Reactivation and Chromosomal Polysomies: Euphorbia tirucalli as a Possible Cofactor in Endemic Burkitt Lymphoma. Adv Hematol (2012): 149780.
- Crawford DH, Ando I. EB virus induction is associated with B-cell maturation. Immunology 59 (1986): 405.
- Li H, Liu S, Hu J, Luo X, Li N, Bode AM, et al. Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. Int J Biol Sci 12 (2016): 1309.
- Hung C-H, Chen L-W, Wang W-H, Chang P-J, Chiu Y-F, Hung C-C, et al. Regulation of autophagic activation by Rta of Epstein-Barr virus via the extracellular signal-regulated kinase pathway. J Virol 88 (2014): 12133-45.
- De Leo A, Colavita F, Ciccosanti F, Fimia GM, Lieberman PM, Mattia E. Inhibition of autophagy in EBV-positive Burkitt’s lymphoma cells enhances EBV lytic genes expression and replication. Cell Death Dis 6 (2015): e1876-e1876.
- Gonnella R, Granato M, Farina A, Santarelli R, Faggioni A, Cirone M. PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim Biophys Acta (BBA)-Molecular Cell Res 1853 (2015): 1586-95.
- Yiu SPT, Hui KF, Münz C, Lo K-W, Tsao SW, Kao RYT, et al. Autophagy-dependent reactivation of epstein-barr virus lytic cycle and combinatorial effects of autophagy-dependent and independent lytic inducers in nasopharyngeal carcinoma. Cancers (Basel) 11 (2019): 1871.
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130 (2004): 601.
- Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun 26 (2012): 1280-7.
- Zhu P, Chen Y-J, Hao J-H, Ge J-F, Huang K, Tao R-X, et al. Maternal depressive symptoms related to Epstein-Barr virus reactivation in late pregnancy. Sci Rep 3 (2013): 3096.
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med 8 (1985): 249-60.
- Fagundes CP, Jaremka LM, Glaser R, Alfano CM, Povoski SP, Lipari AM, et al. Attachment anxiety is related to Epstein-Barr virus latency. Brain Behav Immun 41 (2014): 232-8.
- Jaremka LM, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Attachment anxiety is linked to alterations in cortisol production and cellular immunity. Psychol Sci 24 (2013): 272-9.
- Glaser R, Friedman SB, Smyth J, Ader R, Bijur P, Brunell P, et al. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav Immun 13 (1999): 240-51.
- Johnson N, Haeri S, Baker A, Raines C, Barrow D, Boggess K. 826: Depression in pregnancy: is there an association with Epstein-Barr virus (EBV) reactivation? Am J Obstet Gynecol 204 (2011): S322.
- Purtilo DT, Sakamoto K. Reactivation of Epstein-Barr virus in pregnant women: social factors, and immune competence as determinants of lymphoproliferative diseases—a hypothesis. Med Hypotheses 8 (1982): 401-8.
- Aubrecht TG, Weil ZM, Abi Salloum B, Ariza ME, Williams M, Reader B, et al. Chronic physical stress does not interact with Epstein-Barr virus (EBV)-encoded dUTPase to alter the sickness response. J Behav Brain Sci 5 (2015): 513.
- Cesarman E, Mesri EA. Kaposi sarcoma-associated herpesvirus and other viruses in human lymphomagenesis. Kaposi Sarcoma Herpesvirus New Perspect (2007): 263-87.
- Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med 378 (2018): 549-62.
- Hu T, Lin C, Xie S, Chen G, Lu Y, Ling W, et al. Smoking can increase nasopharyngeal carcinoma risk by repeatedly reactivating Epstein-Barr Virus: An analysis of a prospective study in southern China. Cancer Med 8 (2019): 2561-71.
- Willett E V, O’Connor S, Smith AG, Roman E. Does smoking or alcohol modify the risk of Epstein-Barr virus-positive or-negative Hodgkin lymphoma? Epidemiology (2007): 130-6.
- Xu F-H, Xiong D, Xu Y-F, Cao S-M, Xue W-Q, Qin H-D, et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J Natl Cancer Inst 104 (2012): 1396-410.
- Chêne A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog 3 (2007): e80.
- Donati D, Espmark E, Kironde F, Mbidde EK, Kamya M, Lundkvist Å, et al. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J Infect Dis 193 (2006): 971-7.
- Rasti N, Falk KI, Donati D, Gyan BA, Goka BQ, Troye-Blomberg M, et al. Circulating epstein-barr virus in children living in malaria-endemic areas. Scand J Immunol 61 (2005): 461-5.
- Piriou E, Kimmel R, Chelimo K, Middeldorp JM, Odada PS, Ploutz-Snyder R, et al. Serological evidence for long-term epstein-barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol 81 (2009): 1088-93.
- Chattopadhyay PK, Chelimo K, Embury PB, Mulama DH, Sumba PO, Gostick E, et al. Holoendemic malaria exposure is associated with altered Epstein-Barr virus-specific CD8+ T-cell differentiation. J Virol. 2013;87(3):1779-88.
- Njie R, Bell AI, Jia H, Croom-Carter D, Chaganti S, Hislop AD, et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis 199 (2009): 31-8.
- Lam KMC, Syed N, Crawford DH, Whittle H. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet 337 (1991): 876-8.
- Whittle HC, Brown J, Marsh K, Greenwood BM, Seidelin P, Tighe H, et al. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum Nature 312 (1984): 449-50.
- Whittle HC, Brown J, Marsh K, Blackman M, Jobe O, Shenton F. The effects of Plasmodium falciparum malaria on immune control of B lymphocytes in Gambian children. Clin Exp Immunol 80 (1990): 213-8.
- Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol 3 (2005): 182-7.
- Akin F, Kocaoglu C, Solak ES, Ozdemir H, Pektas B, Arslan S. Coinfection of Plasmodium vivax and Epstein-Barr virus: case report. Asian Pacific J Trop Dis [Internet] 3 (2013): 74-5.
- Wilkerson H, Maniam G, Dean RE, Bewaji T, Okotcha E, Mattamal R. Pediatric coinfection with malaria and Epstein-Barr virus. Consultant (2020).
- Wedderburn N, Davies DR, Mitchell GH, Desgranges C, de Thé G. Glomerulonephritis in common marmosets infected with Plasmodium brasilianum and Epstein-Barr virus. J Infect Dis 158 (1988): 789-94.
- Dias MHF. Influência da infecção pelo Epstein-Barr vírus na malária humana causada por Plasmodium vivax (2018).
- Das B, Morrow R, Huang R, Fixler D. Persistent Epstein-Barr viral load in Epstein-Barr viral naïve pediatric heart transplant recipients: risk of late-onset post-transplant lymphoproliferative disease. World J Transplant 6 (2016): 729.
- Chen Y, Xu Y, Zhao W, Xiao X, Zhou X, Lin L, et al. Lack of association between cigarette smoking and Epstein Barr virus reactivation in the nasopharynx in people with elevated EBV IgA antibody titres. BMC Cancer 18 (2018): 1-7.
- Ramroodi N, Niazi AA, Sanadgol N, Ganjali Z, Sarabandi V. Evaluation of reactive Epstein-Barr Virus (EBV) in Iranian patient with different subtypes of multiple sclerosis (MS). Brazilian J Infect Dis 17 (2013): 156-63.
- Nagata K, Fukata S, Kanai K, Satoh Y, Segawa T, Kuwamoto S, et al. The influence of Epstein-Barr virus reactivation in patients with Graves’ disease. Viral Immunol 24 (2011): 143-9.
- Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45 (1991): 297-308.
- Osier FHA, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12 (2014): 1-15.
- BOWEN TJ, WEDGWOOD RJ, OCHS HD, HENLE W. Transient immunodeficiency during asymptomatic Epstein-Barr virus infection. Pediatrics 71 (1983): 964-7.
- Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 9 (2008): 725-32.
- Yone CLRP, Kube D, Kremsner PG, Luty AJF. Persistent Epstein-Barr viral reactivation in young African children with a history of severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 100 (2006): 669-76.
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 6 (2011): e17739.
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29 (2008): 138-49.
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science (80- ) 325 (2009): 1001-5.
- Jandl C, Loetsch C, King C. Cytokine expression by T follicular helper cells. In: Germinal Centers. Springer (2017): 95-103.
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol 6 (2005): 353-60.
- Crotty S. Follicular helper CD4 T cells (Tfh). Annu Rev Immunol 29 (2011): 621-63.
- Kaneko N, Kuo H-H, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 183 (2020): 143-57.
- Grywalska E, Smarz-Widelska I, Korona-Glowniak I, Mertowski S, Gosik K, Hymos A, et al. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int J Mol Sci 21 (2020): 8001.
- Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med 212 (2015): 1125-37.
- Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162 (2015): 1242-56.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26 (2008): 677-704.
- Dobbs KR, Crabtree JN, Dent AE. Innate immunity to malaria—the role of monocytes. Immunol Rev 293 (2020): 8-24.
- Savard M, Gosselin J. Epstein-Barr virus immunossuppression of innate immunity mediated by phagocytes. Virus Res 119 (2006): 134-45.
- Lin Y, Li M. Human cytomegalovirus and Epstein-Barr virus inhibit oral bacteria-induced macrophage activation and phagocytosis. Oral Microbiol Immunol 24 (2009): 243-8.
- Abrial C. Caractérisation de la polarisation des macrophages pulmonaires humains et voies de régulation. Université de Versailles-Saint Quentin en Yvelines (2014).
- Ayimba E, Hegewald J, Segbena AY, Gantin RG, Lechner CJ, Agosssou A, et al. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol 166 (2011): 218-26.
- Oyegue-Liabagui SL, Bouopda-Tuedom AG, Kouna LC, Maghendji-Nzondo S, Nzoughe H, Tchitoula-Makaya N, et al. Pro-and anti-inflammatory cytokines in children with malaria in Franceville, Gabon. Am J Clin Exp Immunol 6 (2017): 9.
- Smith CM, Gill MB, May JS, Stevenson PG. Murine gammaherpesvirus-68 inhibits antigen presentation by dendritic cells. PLoS One 2 (2007): e1048.
- Richards JS, Stanisic DI, Fowkes FJI, Tavul L, Dabod E, Thompson JK, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51 (2010): e50-60.
- Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol 4 (2004): 169-80.
- Tarumi T, Sawada K, Sato N, Kobayashi S, Takano H, Yasukouchi T, et al. Interferon-alpha-induced apoptosis in human erythroid progenitors. Exp Hematol 23 (1995): 1310-8.
- Hwang S, Kim KS, Flano E, Wu T-T, Tong LM, Park AN, et al. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 5 (2009): 166-78.
- Mounce BC, Tsan FC, Droit L, Kohler S, Reitsma JM, Cirillo LA, et al. Gammaherpesvirus gene expression and DNA synthesis are facilitated by viral protein kinase and histone variant H2AX. Virology 420 (2011): 73-81.
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med 15 (2009): 696-700.
- de Miranda MP, Alenquer M, Marques S, Rodrigues L, Lopes F, Bustelo XR, et al. The Gammaherpesvirus m2 protein manipulates the Fyn/Vav pathway through a multidocking mechanism of assembly. PLoS One 3 (2008): e1654.
- Rangaswamy US, Speck SH. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog 10 (2014): e1003858.
- Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, et al. IL-10 from CD4+ CD25− Foxp3− CD127− adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4 (2008): e1000004.
- Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun 68 (2000): 4399-406.
- Saraiva M, O’garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10 (2010): 170-81.
- Miyazaki I, Cheung RK, Dosch H-M. Viral interleukin 10 is critical for the induction of B cell growth transformation by Epstein-Barr virus. J Exp Med 178 (1993): 439-47.
