Robotic Versus Conventional Latissimus Dorsi-Flap Harvested for Immediate Breast Reconstruction
Article Information
Gilles Houvenaeghel1*, Sandrine Rua2, Julien Barrou1, Aurore Van Troy2, Sophie Knight2, Monique Cohen2, Marie Bannier2
1Department of surgical oncology, Paoli Calmettes Institute and CRCM, CNRS, INSERM, Aix Marseille Université., 13009 Marseille, France
2Department of surgical oncology, Paoli Calmettes Institute, 13009 Marseille, France
*Corresponding author: Gilles Houvenaeghel, Department of surgical oncology, Paoli Calmettes Institute and CRCM, CNRS, INSERM, Aix Marseille Université. 13009 Marseille, France.
Received: 21 October 2021; Accepted: 30 October 2021; Published: 09 December 2021
Citation: Houvenaeghel G, Rua S, Barrou J, Troy AV, Knight S, Cohen M, Bannier M. Robotic Versus Conventional Latissimus Dorsi-Flap Harvested for Immediate Breast Reconstruction. Journal of Surgery and Research 4 (2021): 749-764.
Share at FacebookAbstract
Importance
Latissimus Dorsi-Flap (LDF) is a reliable and reproducible technique for Immediate Breast Reconstruction (IBR) that requires a dorsal scar with the conventional open technique. Robotic-LDF dissection recently described, avoids making a dorsal scar.
Objective
The primary objective of this prospective study was to compare results of R-LDF and conventional LDF (C-LDF) in terms of dorsal complication rate, secondary objectives were to compare length of hospital stay (LHS), length of anesthesia and undertake a cost evaluation.
Design
All patients undergoing LDF-IBR with or without implant reconstruction were analyzed. Complication rate was determined using Clavien-Dindo grading. A cost evaluation was performed. An a priori hypothesis of 100 R-NSM and 100 C-NSM was planned.
Results
204 LDF-IBR were performed, 126 R-LDF (61.8%) and 78 C-LDF, by five surgeons. Several significant differences were reported between the two groups: higher rates of previous radiotherapy, neo-adjuvant chemotherapy (NAC) and NAC with neo-adjuvant radiotherapy in C-LDF group, higher median age and higher rate of nipple sparing mastectomy in R-LDF group. LDF-IBR was associated with implant-IBR in 24.0% of patients (49/204). Duration of surgery was not significantly different for R-LDF versus C-LDF (OR=1.712, 95%CI 0.822-3.566, p=0.151). Crude dorsal complication rate was 35.3% (72/204) including 65 seromas (90.3% complications). There was no significant difference in complication rates between both groups, however Grade 2-3 dorsal complications were associated with LDF with implant reconstruction (OR=5.661, 95%CI 1.146-27.97, p=0.033). A significantly higher cost was observed for R-LDF, with a 27.2% median total cost difference (2108 Euros).
Keywords
Breast reconstruction, Cost, Complications, Latissimus dorsi-flap, Robotic surgery
Breast reconstruction articles; Cost articles; Complications articles; Latissimus dorsi-flap articles; Robotic surgery articles.
Breast reconstruction articles Breast reconstruction Research articles Breast reconstruction review articles Breast reconstruction PubMed articles Breast reconstruction PubMed Central articles Breast reconstruction 2023 articles Breast reconstruction 2024 articles Breast reconstruction Scopus articles Breast reconstruction impact factor journals Breast reconstruction Scopus journals Breast reconstruction PubMed journals Breast reconstruction medical journals Breast reconstruction free journals Breast reconstruction best journals Breast reconstruction top journals Breast reconstruction free medical journals Breast reconstruction famous journals Breast reconstruction Google Scholar indexed journals Complications articles Complications Research articles Complications review articles Complications PubMed articles Complications PubMed Central articles Complications 2023 articles Complications 2024 articles Complications Scopus articles Complications impact factor journals Complications Scopus journals Complications PubMed journals Complications medical journals Complications free journals Complications best journals Complications top journals Complications free medical journals Complications famous journals Complications Google Scholar indexed journals Latissimus dorsi-flap articles Latissimus dorsi-flap Research articles Latissimus dorsi-flap review articles Latissimus dorsi-flap PubMed articles Latissimus dorsi-flap PubMed Central articles Latissimus dorsi-flap 2023 articles Latissimus dorsi-flap 2024 articles Latissimus dorsi-flap Scopus articles Latissimus dorsi-flap impact factor journals Latissimus dorsi-flap Scopus journals Latissimus dorsi-flap PubMed journals Latissimus dorsi-flap medical journals Latissimus dorsi-flap free journals Latissimus dorsi-flap best journals Latissimus dorsi-flap top journals Latissimus dorsi-flap free medical journals Latissimus dorsi-flap famous journals Latissimus dorsi-flap Google Scholar indexed journals Robotic surgery articles Robotic surgery Research articles Robotic surgery review articles Robotic surgery PubMed articles Robotic surgery PubMed Central articles Robotic surgery 2023 articles Robotic surgery 2024 articles Robotic surgery Scopus articles Robotic surgery impact factor journals Robotic surgery Scopus journals Robotic surgery PubMed journals Robotic surgery medical journals Robotic surgery free journals Robotic surgery best journals Robotic surgery top journals Robotic surgery free medical journals Robotic surgery famous journals Robotic surgery Google Scholar indexed journals implant reconstruction articles implant reconstruction Research articles implant reconstruction review articles implant reconstruction PubMed articles implant reconstruction PubMed Central articles implant reconstruction 2023 articles implant reconstruction 2024 articles implant reconstruction Scopus articles implant reconstruction impact factor journals implant reconstruction Scopus journals implant reconstruction PubMed journals implant reconstruction medical journals implant reconstruction free journals implant reconstruction best journals implant reconstruction top journals implant reconstruction free medical journals implant reconstruction famous journals implant reconstruction Google Scholar indexed journals nipple sparing mastectomy articles nipple sparing mastectomy Research articles nipple sparing mastectomy review articles nipple sparing mastectomy PubMed articles nipple sparing mastectomy PubMed Central articles nipple sparing mastectomy 2023 articles nipple sparing mastectomy 2024 articles nipple sparing mastectomy Scopus articles nipple sparing mastectomy impact factor journals nipple sparing mastectomy Scopus journals nipple sparing mastectomy PubMed journals nipple sparing mastectomy medical journals nipple sparing mastectomy free journals nipple sparing mastectomy best journals nipple sparing mastectomy top journals nipple sparing mastectomy free medical journals nipple sparing mastectomy famous journals nipple sparing mastectomy Google Scholar indexed journals adjuvant chemotherapy articles adjuvant chemotherapy Research articles adjuvant chemotherapy review articles adjuvant chemotherapy PubMed articles adjuvant chemotherapy PubMed Central articles adjuvant chemotherapy 2023 articles adjuvant chemotherapy 2024 articles adjuvant chemotherapy Scopus articles adjuvant chemotherapy impact factor journals adjuvant chemotherapy Scopus journals adjuvant chemotherapy PubMed journals adjuvant chemotherapy medical journals adjuvant chemotherapy free journals adjuvant chemotherapy best journals adjuvant chemotherapy top journals adjuvant chemotherapy free medical journals adjuvant chemotherapy famous journals adjuvant chemotherapy Google Scholar indexed journals ipsilateral breast local recurrence articles ipsilateral breast local recurrence Research articles ipsilateral breast local recurrence review articles ipsilateral breast local recurrence PubMed articles ipsilateral breast local recurrence PubMed Central articles ipsilateral breast local recurrence 2023 articles ipsilateral breast local recurrence 2024 articles ipsilateral breast local recurrence Scopus articles ipsilateral breast local recurrence impact factor journals ipsilateral breast local recurrence Scopus journals ipsilateral breast local recurrence PubMed journals ipsilateral breast local recurrence medical journals ipsilateral breast local recurrence free journals ipsilateral breast local recurrence best journals ipsilateral breast local recurrence top journals ipsilateral breast local recurrence free medical journals ipsilateral breast local recurrence famous journals ipsilateral breast local recurrence Google Scholar indexed journals prophylactic mastectomy articles prophylactic mastectomy Research articles prophylactic mastectomy review articles prophylactic mastectomy PubMed articles prophylactic mastectomy PubMed Central articles prophylactic mastectomy 2023 articles prophylactic mastectomy 2024 articles prophylactic mastectomy Scopus articles prophylactic mastectomy impact factor journals prophylactic mastectomy Scopus journals prophylactic mastectomy PubMed journals prophylactic mastectomy medical journals prophylactic mastectomy free journals prophylactic mastectomy best journals prophylactic mastectomy top journals prophylactic mastectomy free medical journals prophylactic mastectomy famous journals prophylactic mastectomy Google Scholar indexed journals
Article Details
1. Introduction
Despite a great increase of breast conservative surgery over the past few decades, especially with the development of oncoplastic techniques [1,2], mastectomy remained necessary for patients with multifocal disease, large tumors without indication of neo-adjuvant chemotherapy, ipsilateral breast local recurrence [3,4], prophylactic mastectomy [5] and patient’s wish. Immediate breast reconstruction (IBR), whether using a skin sparing (SSM) or a nipple sparing mastectomy (NSM) technique, results in better cosmetic outcome and quality of life than mastectomy without IBR [6]. It should therefore be discussed with the patient whenever possible. The main technics used for IBR are implants (definitive implant or expander). Since first described in 1994 [7] distal inferior epigastric perforator (DIEP) flap has been extensively developed during recent years, particularly for delayed reconstruction [8]. However, latissimus dorsi-flap (LDF) remains a reproducible technic, with a high reliability, which can be offered according to patient’s wishes, previous treatment, patient’s morphology, breast cup-size and ptosis. In a French multicentric study [6] LDF-IBR was performed in 46.9% of patients. The conventional open technique requires a dorsal scar which can be avoided with the help of endoscopic techniques. Endoscopic non-robotic LDF dissections have been reported in various centers [9-14] however due to the 2-dimensional vision and the non-flexible instruments this procedure is technically challenging. Consequently, due to the development of robotic surgery in other specialties, few cases of robotic-LDF (R-LDF) have been reported [15-22]. Due to our experience of robotic surgery in oncological gynecology since January 2007, we decided to develop robotic breast and LDF surgery. The primary objective of this study was to compare results of R-LDF and conventional LDF (C-LDF) in terms of dorsal complication rate, secondary objectives were to compare length of hospital stay (LHS), length of anesthesia and undertake a cost evaluation.
2. Methods
2.1 Patients
All patients undergoing LDF-IBR between January 2016 and July 2020 were included in this study. A strong decrease in breast cancer surgery and particularly mastectomy with LDF-IBR was observed due to COVID-19 pandemic with only 3 LDF-IBR after the 11th of February 2020. We included patients operated for mastectomy with IBR, with or without robotic assistance for LDF dissection (R-LDF: robotic-LDF and C-LDF: conventional-LDF) with or without implant reconstruction. The main objective was to compare R-LDF and C-LDF in terms of complication and 200 patients were planned to achieve this comparison, with a first hypothesis of 100 R-NSM and 100 C-NSM. Secondary aims were to compare post-operative LHS and duration of the procedure and undertake a cost evaluation. LDF-IBR were performed by five surgeons: R-LDF by 4 surgeons and C-LDF by 5 surgeons. All patients were informed of robotic assistance before surgery. The selection criteria between C-NSM and R-NSM were determined by the choice of surgeons but also on the choice of patients: for NSMs which did not require the addition of a skin paddle, the choice was not to impose a dorsal scar whenever possible and for SSM the choice of patients was determined between the absence of dorsal scar or the removal of a dorsal skin paddle allowing a reconstruction of the nipple areolar complex at the same operative time. Intraoperative antibiotic therapy was systematically administered and preoperative search for nasal carriage of staphylococcus with a preoperative decontamination in case of positive result was performed. Our institutional ethical committee approved robotic breast surgery procedures and data were collected in the institutional breast cancer data-base (NCT02869607).
2.2 Analysis criteria
Patient characteristics (age, body mass index (BMI), tobacco use, diabetes, ASA status, breast cup-size), previous treatment for BC (sentinel lymph node biopsy, axillary lymph node dissection (ALND), neo-adjuvant chemotherapy, previous breast radiotherapy), indications for mastectomy (primary BC, local recurrence or prophylactic surgery), reconstruction with autologous LDF (muscle with fat around muscle) or non-autologous LDF (without fat around muscle), association of breast implant, year of treatment (2016, 2017, 2018 and 2019-2020). Complication rate was established using Clavien-Dindo grading [23] during a post-operative period of 30 days. Re-operation rate, type of complication and number of LHS days were analyzed. Time between surgery and adjuvant chemotherapy (AC) or post-mastectomy radiotherapy (PMRT) for patients without AC was recorded.
2.3 R-LDF procedure
A standardized technique was established and previously reported, using da Vinci Si Ò Surgical system XI or SI [24]. In summary, for skin sparing mastectomy (SSM), total mastectomy, axillary surgery and R-LDF dissections were performed through the incision around nipple areolar complex and for Nipple Sparing Mastectomy (NSM), total mastectomy, axillary surgery and R-LDF were performed through a short axillar or external incision whose length depended on the breast volume in order to allow the extraction of the specimen (5-7 centimeters). We began with the dissection of the sub-cutaneous plan of LD muscle and a dissection along the anterior axillary line of about 6-7cm up to the inferior mammary fold in order to introduce a robotic trocar (8 mm). A GelpointÒ Path mono-trocar was introduced through the axillar incision with one trocar for 0° camera, one robotic trocar and one trocar for AirsealÒ device insufflation. We used a low pressure (7mm Hg) and two robotic instruments, monopolar scissors and bipolar clamp. After having completed total mastectomy and axillary node surgery, the robotic procedure started with superficial LD muscle dissection from the middle of the muscle to the inferior part (5-7 centimeters under the inferior mammary fold) and to the superior part with a total section of the tendinous insertion. Dissection then continued to the deep side of the LD muscle and the section of posterior and inferior insertions of the muscle with monopolar scissors. Then a rotation of the muscle attached by its vascular pedicle allowed its mobilization to the mastectomy site. Two drains were placed in the dorsal area through the inferior scar used for the robotic trocar and one or two drains were placed in mastectomy site. C-LDF procedure: In all cases, LDF were harvested through dorsal scar with skin flap taken in all these cases.
2.4 Cost evaluation
A cost evaluation, in euros, was undertaken including cost of duration of anesthesia (length of operating room occupation), length of hospital stay (number of days), cost of robotic instrumentation and other surgical devices (Gelpoint and Airseal), breast implant and cost of re-operation (duration of anesthesia and LHS in case of re-hospitalization). Purchase and maintenance costs of Da Vinci systems were not included as they are in relation with the number of procedures per-year for breast surgery and other indications of robotic procedures for urologic, gynecologic and digestive tumors. All costs for breast reconstruction were covered by the national insurance and costs of robotic procedure were supported by the institution. Patients had no pay out-of-pocket. Statistics: Main characteristics were reported using median, mean, confidence intervals 95% (CI 95) for quantitative criteria. Comparisons were performed using Chi2, t-test and followed by binary logistic regression adjusted on significant univariate variables, using SPSS 16.0 (SPSS Inc., Chicago, Illinois).
3.Results
3.1 Patients
204 LDF-IBR were performed, comprising 126 R-LDF (61.8%) and 78 C-LDF. Patient characteristics are reported in Table 1.
|
C-LDF |
R-LDF |
Chi2 |
Total |
||||||
|
Nb |
% |
Nb |
% |
p |
Nb |
% |
|||
|
Number |
78 |
38.2 |
126 |
61.8 |
|||||
|
Breast cup size* |
A-B |
37 |
47.4 |
55 |
43.7 |
0.869 |
92 |
45.1 |
|
|
C |
25 |
32.1 |
43 |
34.1 |
68 |
33.3 |
|||
|
>C |
16 |
20.5 |
28 |
22.2 |
44 |
21.6 |
|||
|
BMI* |
<= 24.9 |
50 |
64.1 |
81 |
64.3 |
0.752 |
131 |
64.2 |
|
|
25-29.99 |
21 |
26.9 |
30 |
23.8 |
51 |
25 |
|||
|
>= 30 |
7 |
9 |
15 |
11.9 |
22 |
10.8 |
|||
|
ASA status |
1 |
24 |
30.8 |
46 |
36.5 |
0.573 |
70 |
34.3 |
|
|
2 |
53 |
67.9 |
77 |
61.1 |
130 |
63.7 |
|||
|
3 |
1 |
1.3 |
3 |
2.4 |
4 |
2 |
|||
|
Tobacco |
No |
64 |
82.1 |
92 |
73 |
0.094 |
156 |
76.5 |
|
|
Yes |
14 |
17.9 |
34 |
27 |
48 |
23.5 |
|||
|
Diabetes |
No |
78 |
100 |
120 |
95.2 |
0.053 |
198 |
97.1 |
|
|
Yes |
0 |
0 |
6 |
4.8 |
6 |
2.9 |
|||
|
Previous RTH |
No |
29 |
37.2 |
73 |
57.9 |
0.003 |
102 |
50 |
|
|
Yes |
49 |
62.8 |
53 |
42.1 |
102 |
50 |
|||
|
NAC |
No |
40 |
51.3 |
93 |
73.8 |
0.001 |
133 |
65.2 |
|
|
Yes |
38 |
48.7 |
33 |
26.2 |
71 |
34.8 |
|||
|
NAC+N-RTH |
No |
45 |
57.7 |
105 |
83.3 |
<0.0001 |
150 |
73.5 |
|
|
Yes |
33 |
42.3 |
21 |
16.7 |
54 |
26.5 |
|||
|
Axillary surgery |
No |
39 |
50 |
56 |
44.4 |
0.596 |
95 |
46.6 |
|
|
SLNB |
18 |
23.1 |
37 |
29.4 |
55 |
27 |
|||
|
ALND |
21 |
26.9 |
33 |
26.2 |
54 |
26.5 |
|||
|
Indication |
Prophylactic |
0 |
0 |
2 |
1.6 |
0.419 |
2 |
1 |
|
|
Primary |
63 |
80.8 |
95 |
75.4 |
158 |
77.5 |
|||
|
Local recurrence |
15 |
19.2 |
29 |
23 |
44 |
21.6 |
|||
|
LDF |
autologous |
67 |
85.9 |
81 |
64.3 |
0.001 |
148 |
72.5 |
|
|
no autologous |
11 |
14.1 |
45 |
35.7 |
56 |
27.5 |
|||
|
Implant |
No |
69 |
88.5 |
86 |
68.3 |
0.001 |
155 |
76 |
|
|
Yes |
9 |
11.5 |
40 |
31.7 |
49 |
24 |
|||
|
Mastectomy |
NSM |
5 |
6.4 |
76 |
60.3 |
<0.0001 |
81 |
39.7 |
|
|
SSM |
69 |
88.5 |
50 |
39.7 |
119 |
58.3 |
|||
|
Standard |
4 |
5.1 |
0 |
0 |
4 |
2 |
|||
|
All complications |
No |
33 |
42.3 |
61 |
48.4 |
0.24 |
94 |
46.1 |
|
|
Yes |
45 |
57.7 |
65 |
51.6 |
110 |
53.9 |
|||
|
Grade complication |
0 |
33 |
42.3 |
59 |
46.8 |
0.104 |
92 |
45.1 |
|
|
1 |
39 |
50 |
45 |
35.7 |
84 |
41.2 |
|||
|
2 |
1 |
1.3 |
6 |
4.8 |
7 |
3.4 |
|||
|
3 |
5 |
6.4 |
16 |
12.7 |
21 |
10.3 |
|||
|
Dorsal complication |
No |
47 |
85 |
0.310 |
132 |
64.7 |
|||
|
Yes |
31 |
39.7 |
41 |
32.5 |
72 |
35.3 |
|||
|
Dorsal complication |
0 |
47 |
60.3 |
85 |
67.5 |
0.572 |
132 |
64.7 |
|
|
1 |
28 |
35.9 |
37 |
29.4 |
65 |
31.9 |
|||
|
2 |
0 |
0 |
1 |
0.8 |
1 |
0.5 |
|||
|
3 |
3 |
3.8 |
3 |
2.4 |
6 |
2.9 |
|||
|
Years |
2016 |
21 |
26.9 |
20 |
15.9 |
0.037 |
41 |
20.1 |
|
|
2017 |
18 |
23.1 |
41 |
32.5 |
59 |
28.9 |
|||
|
2018 |
22 |
28.2 |
49 |
38.9 |
71 |
34.8 |
|||
|
2019-2020 |
17 |
21.8 |
16 |
12.7 |
33 |
16.2 |
|||
|
Length hospital stay |
<= 3 |
37 |
47.4 |
50 |
39.7 |
0.173 |
87 |
42.6 |
|
|
> 3 |
41 |
52.6 |
76 |
60.3 |
117 |
57.4 |
|||
|
Histology |
DCIS |
10 |
12.8 |
24 |
19 |
0.136 |
34 |
16.7 |
|
|
NST |
57 |
73.1 |
69 |
54.8 |
126 |
61.8 |
|||
|
Lobular |
9 |
11.5 |
27 |
21.4 |
36 |
17.6 |
|||
|
others |
1 |
1.3 |
3 |
2.4 |
4 |
2 |
|||
|
Benign* |
1 |
1.3 |
3 |
2.4 |
4 |
2 |
|||
|
implant size |
<= 250 |
4 |
44.4 |
6 |
15 |
0.087 |
10 |
20.4 |
|
|
255-350 |
4 |
44.4 |
18 |
45 |
22 |
44.9 |
|||
|
> 350 |
1 |
11.1 |
16 |
40 |
17 |
34.7 |
|||
|
BMI: body mass index, RTH: radiotherapy, NAC: neo-adjuvant chemotherapy, N-RTH: neo-adjuvant radiotherapy, LDF: latissimus dorsi-flap, DCIS: ductal carcinoma in-situ, NST: non-specific tumor (ductal invasive carcinoma). 81 NSM: 57 Robotic-NSM and 24 non-robotic-NSM. Benign histology: 2 prophylactic mastectomies and 2 patients with complete resection on pre-operative per-cutaneous biopsy. * Breast cup-size and BMI were significantly associated (p<0.0001). |
|||||||||
Table 1: Characteristics of patients according to R-LDF (robotic latissimus dorsi-flap) and C-LDF (conventional latissimus dorsi-flap).
Autologous LDF were performed in 64.3% of R-LDF and 85.9% of C-LDF (p<0.0001) with association of breast implant in 31.7% and 11.5% respectively (p=0.001). Several significant differences were reported between the two groups, namely higher rates of previous radiotherapy, NAC and NAC with N-RTH (neo-adjuvant radiotherapy) in the C-LDF group, higher median age and higher rate of NSM in the R-LDF group (Tables 1,2). Number of R-LDF and C-LDF for the five surgeons is reported in Figure 1.
|
C- LDF |
R-LDF |
t-test: p |
Total |
||
|
Age |
median |
50.5 |
54.5 |
0.007 |
52 |
|
CI 95% |
47.53-53.06 |
52.94-57.44 |
51.56-55.08 |
||
|
BMI |
median |
23.7 |
23.51 |
0.311 |
23.52 |
|
CI 95% |
23.41-25.06 |
24.04-25.69 |
24.03-25.22 |
||
|
Anesthesia |
median |
317 |
378 |
<0.0001 |
349.5 |
|
Duration |
CI 95% |
304.9-332.7 |
370.4-398.0 |
348.1-370.1 |
|
|
Surgery |
median |
262.5 |
294.5 |
<0.0001 |
279 |
|
Duration |
CI 95% |
243.3-269.7 |
292.9-318.9 |
277.0-297.0 |
|
|
Mastectomy |
median |
389 |
359 |
0.4 |
375 |
|
Weight |
CI 95% |
361.2-459.8 |
392.1-492.5 |
394.0-466.2 |
|
|
Implant size |
median |
280 |
340 |
0.002 |
330 |
|
CI 95% |
180-304 |
307-355 |
291-339 |
||
|
Length hospital stay |
median |
4 |
4 |
0.183 |
4 |
|
CI 95% |
3.45-4.01 |
3.74-4.24 |
3.7-4.1 |
||
|
Total cost |
median |
7737 |
9845 |
<0.0001 |
8900 |
|
CI 95% |
7352-8224 |
9875-10921 |
8999-9801 |
||
|
mean |
7788 |
10398 |
9400 |
||
|
BMI: body mass index |
|||||
Table 2: Median and 95%CI results according to R-LDF (robotic latissimus dorsi-flap) and C-LDF (conventional latissimus dorsi-flap).
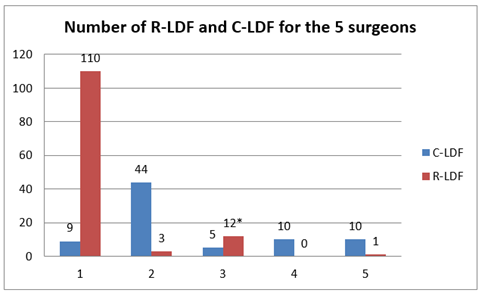
*10 R-LDF performed by surgeon 3 and 1 concomitantly.
Figure 1: Number of R-LDF (robotic latissimus dorsi-flap) and C-LDF (conventional latissimus dorsi-flap) for the five surgeons.
3.2 Type of reconstruction
LDF-IBR was associated with implant-IBR in 24.0% patients (49/204) with a significant association between breast cup-size and LDF-IBR with implant: 13.0% (12/92) for cup-sizes A-B, 30.9% (21/68) for cup-size C and 36.4% (16/44) for cup-sizes > C (p=0.003). Higher rate of LDF-IBR with implant was observed for patients with previous radiotherapy (30/102: 29.4% with radiotherapy versus 19/102: 18.6% without radiotherapy, p=0.071). In binary logistic regression, LDF-IBR with implant was significantly associated with previous radiotherapy (OR: 2.031, CI95% 1.028-4.011, p=0.041) and breast cup-size (Cup-size C: OR=3.181, CI95% 1.419-7.131, p=0.005 and for breast cup-size >C: OR=4.077, CI95% 1.695-9.803, p=0.002). Higher mean implant sizes were used for R-LDF versus C-LDF (p=0.002) (Table 2).
3.3 Breast cancer and treatment
166 patients had invasive breast cancer (Table 1). Neo-adjuvant chemotherapy was administered in 71 patients (34.8%) with a significantly higher rate for C-LDF versus R-LDF (Table 1), and adjuvant chemotherapy was administered in 34 patients (16.7%), with no difference between both groups (17.5%: 22/126 for R-LDF and 15.4%: 12/78 for C-LDF, p=0.699). 48 patients received radiotherapy before surgery (32/126: 25.4% for R-LDF and 16/78: 20.5% for C-LDF), NAC with N-radiotherapy was administered in 54 patients (21/126: 16.7% for R-LDF and 33/78: 42.3% for C-LDF), 29 patients received post mastectomy radiotherapy (25/126: 19.8% for R-LDF and 4/78: 5.1% for C-LDF) and 80 patients did not receive radiotherapy (48/126: 38.1% for R-LDF and 25/78: 32.1% for C-LDF) (p<0.0001). Endocrine therapy was administered in 126 patients (61.8%), with no difference between the two groups (89/126: 70.6% for R-LDF and 49/78: 62.8% for C-LDF, p=0.246).
3.4 Durations of surgery
Included all procedures and several installations from skin incision to the end of skin suture. Median duration of surgery and anesthesia was higher for R-LDF (Table 2). Significantly higher rates of duration of anesthesia more than median duration (349.5 minutes) were observed for non-autologous LDF, association with breast implant, NSM and R-LDF in univariate analysis (Table 3).
Table 3: Anesthesia duration and length of hospital stay (LHS).
In binary logistic regression, adjusted on R-LDF or C-LDF, NSM or SSM, autologous LDF or not, association with breast implant or not, BMI with 3 categories and year of treatment, there was no significant association with R-LDF or C-LDF (OR: 1.571, 95%CI 0.729-3.382, p=0.249), with autologous LDF versus non autologous (OR: 1.228, 95%CI 0.519-2.904, p=0.641) and a significant association with LDF with implant versus no-implant (OR: 5.003, 95%CI 1.942-12.89, p=0.001), with BMI > 30 (OR: 3.002, 95%CI 1.049-8.589, p=0.040), with SSM versus NSM (OR: 0.284, 95%CI 0.130-0.622, p=0.002) and with year of treatment from 2016 to 2020 (OR: 0.696, 95%CI 0.496-0.978, p=0.037). For distinction between years, there was a significant association with years 2019-2020 versus 2016 (OR: 0.310, 95%CI 0.098-0.976, p=0.045) with no significant difference for year 2017 and year 2018. In the same statistic model applied for R-LDF alone, there was a significant association between duration of anesthesia and year of treatment from 2016 to 2020 (OR: 0.596, 95%CI 0.369-0.962, p=0.034), with according to years of treatment a significant association with year 2019-2020 (OR: 0.161, 95%CI 0.029-0.902, p=0.038), without significant difference for year 2017 (OR: 0.307, 95%CI 0.071-1.328, p=0.114) and near significant result for year 2018 (OR: 0.233, 95%CI 0.053-1.024, p=0.054). In the model applied for C-LDF alone, there was no significant association with year of treatment (OR: 0.800, 95%CI 0.464-1.379, p=0.422).
3.5 Post-operative LHS
was not significantly different between both groups, with a median of 4 days (Table 2). In univariate analysis, significantly higher rates of LHS of more than 4 days were observed for BMI 25-29.99 and > 30 (Table 3) and all 7 patients with grade 2-3 dorsal complications had LHS higher than 3 days with a significant difference in comparison with patients without dorsal complications or grade 1 complications (55.8%: 110/197 with LHS > 3 days) (p=0.020). Overall crude complication rate was 53.9% : 84 grade 1 (75% of complications), 7 grade 2 and 21 grade 3 (21 re-operations). Implant loss rate was 20.4% (10/49) with no significant difference between R-LDF and C-LDF (10/40: 8/23 NSM and 2/17 SSM versus 0/9 SSM respectively, p=0.173), no difference between previous radiotherapy or not (6/30 versus 4/19, p=0.929) and no difference for smokers vs non-smokers (4/12: 33.3% versus 6/37: 16.2%, respectively, 0.233). Higher implant loss rate was significantly associated with NSM (8/23: 34.8%) versus SSM (2/26: 7.7%) (p=0.022) (OR: 6.4, CI95% 1.20-34.28). Crude dorsal complication rate was 35.3% (72/204): 65 dorsal seroma grade 1 complications (90.3% of complications: 29.4% (37/126) for R-LDF and 35.9% (28/78) for C-LDF, p=0.35), 1 grade 2 complication consisting in a dorsal infection for a R-LDF and 6 grade 3 complications (re-operation for: 4 dorsal bleedings, 1 dorsal infection, 1 partial LDF necrosis), 3 in each group R-LDF and C-LDF. There was no significant difference between both groups, R-LDF and C-LDF (Table 1). In univariate analysis, the only significant association for dorsal complications was BMI > 25 (p=0.022) and Grade 2-3 dorsal complications were significantly associated with ASA status > 1 (p=0.014) and implant associated with LDF (p=0.037) (Table 4). In binary logistic regression, Grade 2-3 dorsal complications were associated with LDF-implant versus no implant (OR: 5.661, CI95% 1.146-27.97, p=0.033).
Table 4: Dorsal complications and Grade 2-3 dorsal complications.
3.6 Interval time between surgery and adjuvant therapy
was not different between both groups (median: 54 days for R-LDF and 48 days for C-LDF, p=0.234) and between adjuvant chemotherapy or post mastectomy radiotherapy (48 days vs 59 days, respectively, p=0.063). Interval time was 54 days for patients with no or grade 1 dorsal complications in comparison with 91.5 days for grade 2-3 dorsal complications (p=0.030).
3.7 Cost evaluation
Significantly higher costs were observed for R-LDF in comparison with C-LDF, with a median total cost difference of 27.2% (2108 Euros) (Table 2). In binary logistic regression, cost > 8900 Euros (total median cost) was significantly associated with LDF-IBR and breast implant versus no implant (OR: 7.941, CI95% 2.664-23.67, p<0.0001) R-LDF versus C-LDF (OR: 6.968, CI95% 2.855-17.00, p<0.0001) and year of treatment 2017 (OR: 0.201, 95%CI 0.069-0.589, p=0.003), year 2018 (OR: 0.371, 95%CI 0.134-1.025, p=0.056), years 2019-2020 (OR: 0.126, 95%CI 0.036-0.444, p=0.001), with no significant association between autologous LDF versus non-autologous LDF (OR: 2.271, CI95% 0.882-5.851, p=0.089), SSM versus NSM (OR: 0.823, CI95% 0.366-1.855, p=0.639), BMI 25-29.99 (OR: 1.468, CI95% 0.667-3.232, p=0.340) and BMI > 30 (OR: 2.232, CI95% 0.718-6.933, p=0.165) versus BMI < 24.9. In the same statistic model applied for R-LDF alone, there was a significant association between cost > 8900 Euros and years 2019-2020 (OR: 0.164, 95%CI 0.031-0.875, p=0.034) without significant association for year 2018 (OR: 0.592, 95%CI 0.136-2.568, p=0.483) and year 2017 (OR: 0.316, 95%CI 0.074-1.352, p=0.120) versus year 2016. In the model applied for C-LDF alone, there was no significant association for each years in comparison with year 2016.
4. Discussion
Crude dorsal complication rate was 35.3% with 3.5% grade 2-3 complications; there was no significant difference in complication rates and notably in seroma rate between both groups. There was no difference in terms of operative time between both groups. However longer operative time was observed for LDF-IBR associated with breast implant reconstruction or NSM. There was also no difference in LHS and interval time to adjuvant treatment between both groups, with longer LHS and interval time for patients with grade 2-3 dorsal complications. R-LDF has the advantage of avoiding an approximately 9-12 cm dorsal scar that is required when performing a C-LDF. The tension on the dorsal scar can be responsible for discomfort and pain as well as a depression in the back scar. However, C-LDF allows reconstruction of nipple areolar complex using part of the dorsal skin flap for patients with SSM. A limitation of this technique lies in the cost of robotic procedures, with a median difference of around 2000 euros, due to the cost of robotic instruments. The first publications on R-LDF dissections were reported in order to assess feasibility, reproducibility and standardize the technique [15-22,24-26]. To our knowledge, no study has yet compared R-LDF and C-LDF dissections for IBR. A study by Winocour et al. [27] compared 25 R-LDF and 27 C-LDF for delayed breast reconstruction through previous mastectomy incision with significantly shorter median LHS and significantly longer duration of surgery for R-LDF. In this study, the authors reported a revision rate of 24% (6/25) for R-LDF in comparison with our rate of 2.38% (3/126). Various studies have evaluated duration of surgery for R-LDF-IBR: a mean operative time of 400 minutes for Chung et al [17], 440 and 300 minutes for two cases reported by Lai et al (18) and median duration of 366 minutes for 25 R-LDF performed for delayed breast reconstruction by Winocour et al. [27]. About 60-90 minutes for dorsal surgery with R-LDF or C-LDF, as reported by Clemens et al. (average time of 92 minutes) [16] and 15 to 20 minutes for docking and positioning of the robotic instruments as robotic set up time averaged 23 minutes in Selber et al study [15]. A short learning curve of R-LDF, using a standardized technique, with the help of a double console for new surgeons on a team, represents an advantage over conventional endoscopic surgery, which is more difficult to perform. Robotic nipple sparing mastectomy [24,26] appears as a more complex procedure to perform than R-LDF. A reduction in anesthesia duration in multivariate analysis with an OR of 0.161 for years 2019-2020, shows an improvement in the length of the procedure over the years in relation with the learning curve for surgeons and the whole team. In contrast, there was no decrease in duration of anesthesia for C-LDF according to years of treatment. We reported a high rate of radiotherapy performed prior to the mastectomy with LDF-IBR (50%) for patients with ipsilateral local recurrence after breast conservative treatment with whole breast radiotherapy, for patients with previous radiotherapy for Hodgkin disease and for patients with neo-adjuvant chemotherapy and radiotherapy [28]. In these cases, LDF nourishes and protect the thin skin envelope [4]. Determinant factors to offer LDF-IBR are usually, previous radiotherapy, large breast size, ptotic breasts and patient’s wishes, particularly in recent years for patients who do not want implant IBR. Winocour et al. [27] reported 72% previous radiotherapy (18/25) for R-LDF performed for delayed breast reconstruction. Many centers offer implant-IBR with acellular dermal matrix or synthetic mesh, but the use of these matrixes could increase surgical complication rate, such as infections and seromas, with higher risk of re-operation and removal of implant [29-34]. Additional procedures such as lipofillings are often required after LDF-IBR in order to obtain sufficient volume and a best-preserved curve. However, with preservation of the skin envelope and nipple areolar complex when tumor distance is more than 1 or 2 centimeters, a large reconstruction volume is required for breast cup-sizes > B and LDF-IBR is proposed in association with breast implant. Despite the preventive post-operative infection measures, a high rate of implant removal for these patients at high risk of complications was observed. This rate was higher than reported in our experience for implant-IBR (7.8%) and in literature (1.0% to 9.9%). Autologous reconstruction with DIEP is an alternative that should be offered, however due to the complexity of the procedure it is more frequently offered for delayed reconstructions [8]. Cost evaluation has been reported for implant-IBR with robotic surgery, with higher cost in comparison with conventional implant-IBR [35,36] and also for robotic gynecologic oncologic surgery [37]. The same finding was observed in this study for R-LDF. The uses of specific devices for robotic procedures explain this cost difference. However, in correlation with a decrease of anesthesia duration cost > 8900 Euros decreased significantly during the last period (2019-2020) for R-LDF. To our knowledge, this is the only study comparing R-LDF and C-LDF and the largest study of R-LDF for IBR. However, some limitations can be pointed out: it is a monocentric study, without satisfaction evaluation, functional results and pain evaluation for dorsal surgery, and without oncologic outcome evaluation. However, this non-randomized study including all patients with LDF-IBR in usual clinical practice in a breast unit put the basis for more robust data in the next future.
5. Conclusion
In conclusion, there was no difference between R-LDF and C-LDF, except for a higher cost with R-LDF. Duration of anesthesia and cost decreased significantly over time throughout the learning curve of the team. LDF-IBR associated with breast implant reconstruction, for patients at high risk of complications, is associated with longer duration of surgery and a higher rate of implant removal. Further research should compare robotic to conventional surgery in terms of complication rates but should also evaluate other aspects such as quality of life, satisfaction, pain and functional results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
GH conceived the study and participated in its design, GH, SK, MC, MB drafted the manuscript, GH, SR, JB analyzed and interpreted the data, GH performed statistical analyses, GH, SR, JB, MC MB provided administrative, technical, or material support, GH supervised the statistical analysis, GH, SR, SK, MC, MB participated in critical revisions of the manuscript with respect to important intellectual content, GH supervised the study. All authors contributed to the article and approved the submitted version.
Funding
None
Acknowledgments
None
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
References
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 6 (2005): 145-157.
- Holmes DR, Schooler W, Smith R. Oncoplastic approaches to breast conservation. Int J Breast Cancer (2011): 303879.
- Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol 5 (1998): 620-626.
- Simon P, Barrou J, Cohen M, et al. Types of mastectomies and immediate reconstructions for ipsilateral breast local recurrences. Frontiers in oncology (2020).
- G Houvenaeghel, M Cohen, MA Dammacco, et al. Prophylactic nipple sparing mastectomy with immediate breast reconstruction: results of a French prospective trial. Br J Surg (2020).
- Dauplat J, Kwiatkowski F, Rouanet P, et al. Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg 104 (2017): 1197-1206.
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 32 (1994): 32-38.
- Hunsinger V, Hivelin M, Derder M, et al. Long-term follow-up of quality of life following DIEP flap Breast Reconstruction. Plast Reconstr Surg 137 (2016): 1361-1371.
- MC Missana, Pomel C. Endoscopic latissimus dorsi flap harvesting. Am J Surg 194 (2007): 164-169.
- Dejode M, Barranger E. Endoscopic 3D latissimus dorsi flap harvesting for immediate breast reconstruction, Gynecol. Obstet. Fertil 44 (2016): 372-374.
- Iglesias M, Gonzalez-Chapa DR. Endoscopic latissimus dorsi muscle flap for breast reconstruction after skin-sparing total mastectomy: report of 14 cases. Aesthet Plast Surg 37 (2013): 719-727.
- Xu S, Tang P, Chen X, et al. Novel technique for laparoscopic harvesting of latissimus dorsi flap with prosthesis implantation for breast reconstruction: a preliminary study with 2 case reports, Medicine (Baltim.) 95 (2016): 5428.
- Nakajima H, Fujiwara I, Mizuta N, et al. Clinical outcomes of video-assisted skin-sparing partial mastectomy for breast cancer and immediate reconstruction with latissimus dorsi muscle flap as breast-conserving therapy. World J Surg 34 (2010): 2197-2203.
- Yuan H, Xie D, Xiao X, et al. The clinical application of mastectomy with single incision followed by immediate laparoscopic-assisted breast reconstruction with latissimus dorsi muscle flap. Surg Innovat 24 (2017): 349-352.
- Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg 129 (2012): 1305-1312.
- Clemens MW, Kronowitz S, Selber JC. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg. Févr 28 (2014): 20-25.
- Chung JH, You HJ, Kim HS, et al. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg 68 (2015): 966-972.
- Lai HW, Lin SL, Chen ST, et al. Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest - Technique and preliminary results. J Plast Reconstr Aesthet Surg 71 (2018): 59-61.
- Houvenaeghel G, Cohen M, Ribeiro SR, et al. Robotic Nipple-Sparing Mastectomy and Immediate Breast reconstruction with robotic latissimus dorsi flap harvest: Technique and Results. Surg Innov (2020): 155.
- Houvenaeghel G, Bannier M, Rua S, et al. Skin sparing mastectomy and robotic latissimus dorsi-flap reconstruction through a single incision. World J Surg Oncol 17 (2019): 176.
- Houvenaeghel G, Bannier M, Rua S, et al. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg Oncol 31 (2019): 38-45.
- Houvenaeghel G, El Hajj H, Schmitt A, et al. Robotic-assisted skin sparing mastectomy and immediate reconstruction using latissimus dorsi flap a new effective and safe technique: A comparative study. Surg Oncol 35 (2020): 406-411.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240 (2004): 205-213.
- Houvenaeghel G, Bannier M, Rua S, et al. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol 17 (2019): 27.
- Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg 28 (2012): 457-464.
- Lai HW, Toesca A, Sarfati B, et al. Consensus Statement on Robotic Mastectomy-Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019: Annals of Surgery (2020).
- Winocour S, Tarassoli S, Chu CK, et al. Comparing outcomes of robotic-assisted latissimus dorsi harvest to traditional open approach in breast reconstruction. Plast Reconstr Surg (2020).
- Zinzindohoué C, Bertrand P, Michel A, et al. A Prospective study on skin-sparing mastectomy for immediate breast reconstruction with latissimus dorsi flap after neoadjuvant chemotherapy and radiotherapy in Invasive Breast Carcinoma. Ann Surg Oncol 23 (2016): 2350-2356.
- Chun YS, Verma K, Rosen H, et al., Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications, Plast. Reconstr. Surg 125 (2010): 429e36.
- Hoppe IC, Yueh JH, Wei CH, et al., Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty 11 (2011): 40.
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 129 (2012): 28e41.
- Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/- implant breast reconstruction. Ann Plast Surg 64 (2010): 674e8.
- Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 125 (2010): 1606e14.
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 128 (2011): 1162-1169.
- Dikmans RE, Negenborn VL, Bouman MB, et al. Two-stage implant-based breast reconstruction compared with immediate one-stage implant-based breast reconstruction augmented with an acellular dermal matrix: an open-label, phase 4, multicentre, randomised, controlled trial. Lancet Oncol 18 (2017): 251-258.
- Lai HW, Chen ST, Mok CW, et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer- A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg 73 (2020): 1514-1525.
- Houvenaeghel G, Barrou J, Jauffret C, et al. Robotic versus conventional nipple sparing mastectomy with immediate breast reconstruction. Frontiers in oncology 11 (2021): 637049.
- Marino P, Houvenaeghel G, Narducci F, et al. Costeffectiveness of conventional vs robotic-assisted laparoscopy in gynecologic oncologic indications. Int J Gynecol Cancer 25 (2015): 1102-1108.
