Revisiting a Meta-analysis Shows that Hydroxychloroquine with Azithromycin may be Efficient in Covid-19 patients
Article Information
Valère Lounnas1, Alexis Lacout2*, Xavier Azalbert3, Christian Perronne4
1EMBL Heidelberg alumni, Meyerhofstraße 1, 69117, Heidelberg, Germany
2Centre de diagnostic ELSAN, Centre médico –chirurgical, Aurillac, France
3Toulouse School of Economics, 1988 Econometrics, France
4Service des Maladies Infectieuses et Tropicales, University of Versailles Saint Quentin – Paris Saclay, University Hospital Raymond Poincaré, Garches, France
*Corresponding Author: Alexis Lacout, Centre de diagnostic ELSAN, Centre Médico–Chirurgical, 83 avenue Charles de Gaulle, 15000, Aurillac, France
Received: 22 January 2021; Accepted: 02 February 2021; Published: 09 February 2021
Citation:
Valère Lounnas, Alexis Lacout, Xavier Azalbert, Christian Perronne. Revisiting a Meta-analysis Shows that Hydroxychloroquine with Azithromycin may be Efficient in Covid-19 patients. Archives of Microbiology & Immunology 5 (2021): 154-175.
Share at FacebookAbstract
Objective: To analyze the impact of study selection on the results of a recently published meta-analysis of the efficacy of hydroxychloroquine (HCQ) and hydroxychloroquine plus azithromycin (AZI) in Covid-19 patients.
Methods: 31 studies were reviewed looking for critical bias. Combined hazard ratios and confidence intervals were calculated for both treatments using a fixed effects size model and a random effects model. Quantitative analysis regarding the toxicity of the association HCQ plus AZI is made.
Results: Meta-analyses performed on the 11 studies we deem critically unbiased show a mortality reduction of 55% for HCQ and 66% for HCQ plus AZI. For both treatments, our meta-analysis indicates a significant efficacy in reducing mortality in hospitalized Covid-19 patients.
Keywords
Covid-19; Meta-analysis; Critical bias; Clinical studies; Hydroxychloroquine; Azithromycin
Covid-19 articles; Meta-analysis articles; Critical bias articles; Clinical studies articles; Hydroxychloroquine articles; Azithromycin articles
Article Details
1. Introduction
The article [1]: “Effect of hydroxychloroquine (HCQ) with or without azithromycin (AZI) on the mortality of coronavirus disease 2019 (Covid-19) patients: a systematic review and meta-analysis”, published on August 26, 2020 in Clinical Microbiology and Infection, concludes to the inefficacy of hydroxychloroquine in the treatment of hospitalized Covid-19 patients. However, this study presents many weak points and inconsistencies. Firstly, the statistical methodology, which raises concerns, provides results which fuel controversies among clinicians. Secondly, and more importantly, crucial information were neglected to the sole profit of a statistical approach. Neglected data were: patients clinical status, disease stage, study conditions, posology indications on the treatments under investigation (HCQ or HCQ plus AZI). This, in a meta-analysis, does not allow to draw conclusions on clinical practice with Covid-19 patients. Despite the authors claim of having followed a well established methodology to identify critical bias, their article is astonishingly lacking explicit explanation on why they have specifically retained 17 studies among 31 preselected ones. Despite the meta-analysis authors list a number of hard limitations in their discussion, they rely blindly on their calculations to firmly suggest that: (1) HCQ alone does not show efficacy against Covid-19; and (2) any patient treated by HCQ and AZI, at any stage of the disease, would develop a high risk of cardiac failure subsequent to treatment intake.
2. Methods
We have reviewed the 31 preselected studies [2-32] looking for critical bias not allowing some of these studies to be retained in the meta-analysis calculation. Following Fiolet et al., we have excluded the study of Kuderer [15] because it was performed on the same cancer registry (CCC19) as the study of Rivera [24].
2.1 Efficacy of HCQ with or without AZI
In our meta-analysis, we calculated the combined hazard ratio (HR) using both a fixed effects size model and a random effects model, according to Borenstein [33] (Introduction to Meta-Analysis, 2007). The variance of each study was retro-calculated using the published adjusted hazard ratio (aHR) and 95% confidence interval (95% CI). Contrary to the logrank method, this approach does not require a hypothesis on the expected mortality in the treatment and control arms. Details of the calculations are provided in Figures 1a and 1b. We have also used the logrank method to calculate the variances of an expected mortality of 26% [1,34].
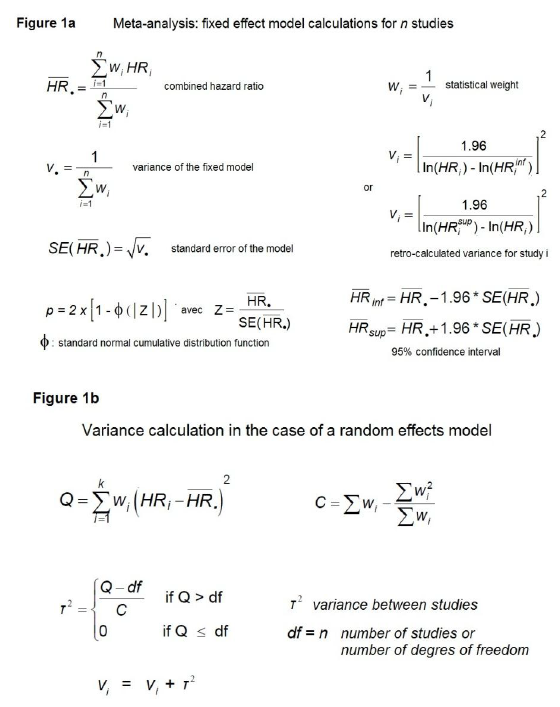
Four meta-analyses were performed on:
- the 31 preselected studies, excluding Kuderer [15], (29 studies for HCQ and 11 for HCQ plus AZI),
- the 17 studies retained in the meta-analysis [1],
- our 11 studies we deemed unbiased (Table 1),
- our 11 unbiased studies plus 2 unfavorable.
2.2 Regarding the toxicity of HCQ combined with AZI
Quantitative analysis was made on the arguments presented in their discussion, notably regarding the toxicity of the association HCQ plus AZI.
3 Results
3.1 Efficacy of HCQ with or without AZI
Among the 31 preselected studies, we disagree concerning the presence or not of critical bias in 18 of them (Table1). Among the 18 studies (including Kuderer) deemed critically unbiased, we found 12 studies with bias which are critical. Conversely, among the 13 studies considered as critically biased, we found 7 studies without bias sufficiently significant to prevent them from entering in the meta-analysis.
In total, we have selected 11 studies that we deemed free of critical bias for HCQ (9 studies) and HCQ plus AZI (4 studies). Meta-analyses performed on these 11 studies (Table 2) show a mortality reduction of 55% for HCQ and 66% for HCQ plus AZI. For comparison purpose, the combined HRs and 95% CIs calculated for the 31 preselected studies (except Kuderer) and for the 17 studies selected by Fiolet et al. are presented in Table 2.
The effect of heterogeneity in HR and 95% CI calculation, and the differences between the statistical weights obtained with respect to the method used for evaluating variances is illustrated (Table 3) with combined HRs remaining favorable to both treatments.
3.2 Quantitative analysis of the toxicity of HCQ with or without AZI
In the study by Bessière [35], 40 patients in intensive care unit (ICU) have received HCQ or HCQ plus AZI, 20 of whom (50%) have also received another treatment known for causing QTc prolongation on the electrocardiogram. In total, 14 patients presented prolongations of QTc ≥ 60 ms, of whom only 7 (17%) a prolongation of QTc ≥ 500 ms, after 2 to 5 days of treatment. No patient died from cardiac arrest and no ventricular arrhythmia or torsade de pointe was recorded. Bessière admits that his results cannot be generalized outside the ICU setting.
The study by Rosenberg [26] reports the raw rate of cardiac deaths with respect to the number of deceased patients: 35/118 (29.7%) for HCQ plus AZI; 14/38 (36.8%) for HCQ alone; 5/17 (29.4%) for AZI alone and 7/20 (35.0%) for the control group. After adjustment with a logistic regression model, Rosenberg obtains a risk ratio of cardiac arrest of 2.13 [1.12-4.05] for HCQ plus AZI compared with control. This result takes into account all observed cardiac failures in addition to those leading to death. The raw data correspond to a doubling of cardiac failures in the HCQ plus AZI (15.5%) arm with respect to control without treatment (6.8%). However, Rosenberg admits that the patients treated with HCQ or HCQ plus AZI were sicker at the time of their inpatient admission than those in the control arm. This has introduced a selection bias (Table 1) that Fiolet does not take into account in his discussion. The study of Rosenberg presents many limitations: (a) the readmission of patients in other hospitals is not accounted for; (b) mortality is calculated on all hospitalized patients whereas some have been hospitalized only 24 hours and lost to follow-up thereafter; (c) inflammatory markers associated with disease severity were not measured; (d) in some cases, the extremely short delay between inpatient hospital admission and ICU transfer, often concomitantly to treatment initiation with HCQ, does not allow treatment efficacy to be correctly assessed.
Among the 11 preselected studies dealing with the HCQ plus AZI combination, 5 provide quantitative information recorded on the cardiac toxicity. They are without consequence on mortality: (1) Arshad [4]: 783 patients, none with torsade de pointe; (2) Cavalcanti [7]: 217 patients, QTc prolongation observed in 116 patients (QTc > 480 ms within 7 days in 17 patients), no toxic death; (3) Lagier [16]: 3119 patients, QTc prolongation (> 60 ms) observed in 25 patients (0.67%) leading to treatment discontinuation (3 cases with QTc > 500 ms), no torsade de pointe or sudden death; (4) Mahévas [20]: 8 patients (10%) presented an electrocardiographic change with QTc prolongation > 60 ms (1 patient with QTc prolongation > 500 ms); (5) Rosenberg [26]: no significant electrocardiographic difference recorded according to their logistic regression adjusted model.
Table 1: Critical bias analysis
|
Table 2: Fiolet et al. revisited meta-analysis |
||
|
Hydroxychloroquine |
Hydroxychloroquine + Azithromycin |
|
|
Meta-analysis model1 |
HR IC 95%CI |
HR IC 95%CI |
|
Fiolet et al. 31 preselected studies (except Kuderer ) |
||
|
fixed effect |
0.95 [0.89 - 1.01] |
1.04 [0.93 - 1.15] |
|
random effects |
0.91 [0.77 - 1.05] |
1.05 [0.77 - 1.34] |
|
random effects2 |
0.95 [0.82 - 1.08] |
1.02 [0.76 - 1.27] |
|
Fiolet et al. 17 finally selected studies for their meta-analysis |
||
|
fixed effect |
0.96 [0.88 - 1.03] |
1.27 [1.12 - 1.41] |
|
random effects |
0.93 [0.76 - 1.10] |
1.33 [1.06 - 1.60] |
|
random effects2 |
0.94 [0.79 - 1.09] |
1.31 [1.08 - 1.55] |
|
Our 11 studies we consider unbiased |
||
|
fixed effect |
0.45 [0.31 - 0.59] |
0.34 [0.06 – 0.61] |
|
random effects |
0.45 [0.31 - 0.59] |
0.34 [0.06 - 0.61] |
|
random effects2 |
0.46 [0.36 - 0.56] |
0.36 [0.17 - 0.54] |
1p values > 0.001 are indicated
2variance calculated with logrank and an expected 26% mortality rate
|
Table 3: Fiolet et al. revisited meta-analysis |
||||||
|
Hydroxychloroquine |
Hydroxychloroquine + Azithromycin |
|||||
|
Selected studies1 |
Patients2 (N) |
HR 95%CI |
Weight3 (% / %) |
Patients2 (N) |
HR 95%CI |
Weight3 (% / %) |
|
Arshad |
1202 |
0.34 [0.25 - 0.46] |
18.6 / 18.8 |
783 |
0.29 [0.21 - 0.40] |
56.2 / 46.2 |
|
Ayerbe |
1857 |
0.42 [0.32 - 0.54] |
25.2 / 12.1 |
|||
|
Bousquet |
27 |
0.49 [0.19 - 1.29] |
6.4 / 3.5 |
|||
|
Cravedi4 |
101 |
1.53 [0.84 - 2.80]5 |
4.8 / 1.9 |
|||
|
Lagier |
503 |
0.49 [0.25 - 0.97] |
12.7 / 24.5 |
|||
|
Lecronier |
38 |
0.58 [0.27 - 1.24]5 |
3.0 / 0.9 |
|||
|
Magagnoli4 |
198 |
1.83 [1.16 - 2.89] |
8.3 / 8.1 |
214 |
1.31 [0.80 - 2.15] |
23.9 / 23.9 |
|
Membrillo |
123 |
0.07 [0.01 - 0.40] |
0.6 / 1.2 |
|||
|
Mikami |
2813 |
0.53 [0.41 - 0.67] |
28.6 / 41.8 |
|||
|
Paccoud |
38 |
0.89 [0.23 - 3.47] |
0.9 / 1.3 |
|||
|
Rogado |
18 |
0.02 [0.01 - 0.73] |
0.9 / 1.9 |
|||
|
Sanchez |
629 |
0.47 [0.28 - 0.79] |
6.4 / 10.7 |
|||
|
Yu |
48 |
0.36 [0.18 - 0.75] |
3.4 / 2.7 |
|||
|
Meta-analysis fixed effect |
7047 |
0.62 [0.48 - 0.75] p < 0.001 |
100 / 100 |
1545 |
0.57 [0.33 - 0.81] p < 0.001 |
100 / 100 |
|
Meta-analysis random effects6 |
0.73 [0.40 - 1.06] p < 0.001 |
- |
- |
0.63 [0.14 - 1.13] p = 0.012 |
- |
|
|
Meta-analysis random effects7 |
0.70 [0.39 - 1.02] p < 0.001 |
- |
- |
0.60 [0.16 - 1.03] p = 0.007 |
- |
|
1our selection of 11 unbiased studies plus 2 unfavorable biased studies
2 number of patients in the treatment arm
3relative weight calculated from the variance : retro-calculated vs. logrank
4studies with critical bias
5HR not adjusted
6 model results with retro-calculated variances using the adjusted HR IC 95%
7model results with variances calculated with the logrank method under the assumption of an expected 26% mortality rate
4. Discussion
4.1 Comparing the meta-analyses results
Fiolet et al. briefly review other published meta-analyses they deemed of poor quality because of: (a) integrating too few studies, (b) lacking a comparator group, (c) lacking sub-group analysis and sensitivity analysis, and above all, (d) not having studied the sources of heterogeneity in the data published. But the latter point may characterize their study as well.
Surprisingly, despite a statistically significant mortality reduction of 17% (HR = 0.83 [0.65 -1.06] p < 0.01) calculated on the 17 studies they selected, they conclude to the inefficacy of HCQ.
On the same article selection, our method gives the same trends but with higher hazard ratios, HR = 0.93 [0.76 – 1.10] for HCQ and HR = 1.33 [1.06 – 1.60] for HCQ plus AZI, to be compared with their HR = 1.24 [1.04 – 1.54]. This shows that the statistical method employed can influence up to +/- 0.1 the value of the calculated HR and that results should be regarded cautiously when HR values are close to 1. Fiolet et al. have used the method of DerSimonian [36] for their meta-analysis which resulted in statistical weights differing from ours.
Performed on the 11 studies we deem free of critical bias, our meta-analyses shows a very significant efficacy of both treatments with HR = 0.45 [0.31 – 0.59] for HCQ and HR = 0.34 [0.06 – 0.61] for HCQ plus AZI. Overall, our calculations demonstrate that bias analysis is substantially more important than the mathematical technique.
Table 3 shows the effect of heterogeneity on the combined HR and 95% CI. It shows that statistical weights are more important than HR values. Despite HRs markedly > 1., the statistical weight of the unfavorable studies does not exceed 13% (HCQ) and 24% (HCQ plus AZI), so that results are, in this example, still in line with mortality reduction.
4.2 Review of the meta-analysis methodology
4.2.1 The domain of application of the random effects methodology not fully matched
We agree that a random effects model should be applied when combining several studies with heterogeneous results. However, this heterogeneity is not principally due to systematic errors, or statistical bias, but to intrinsic differences between population samples. Main differences are: old or young patients, with or without comorbidities, presenting physiological variations that may influence the effect of treatment when appertaining to different human population groups, or different socio-economical groups. Variation of the clinical practice between institutions may also cause differences in the measured effect for a given treatment. In that case, each study should correspond to a well defined group or type of patients, or a single institution, and the measured average treatment effect (ATE) will be situated around a value corresponding to the real ATE for the group of patients considered. For a different type of patients, the real ATE (mean ATE over all institutions) may be different. Conversely for a different institution, the real ATE (mean ATE over all groups of patients) may differ as well. Subsequently, the random effects model gets closer to the real ATE value, by encompassing all types of patient groups or, alternatively, all institutions. But mixing all types of patient groups with all clinical practices is disastrous for the result of a meta-analysis, when hazard ratios HRs range from 0.4 (treatment completely beneficial) to 2 (treatment totally harmful) for a same treatment.
In the ideal case, where all studies have measured a near statistically significant benefit, a mean treatment effect is produced as well as a reduced confidence interval and a strengthened statistical power.
In the case where the treatment brings a benefit to some types of patients, or within the framework of an institution, but not to other types of patients or not in other institutions, the meta-analysis will get closer to the overall mean value of the ATE. This overall effect of a treatment may be beneficial or null, and sometimes intrinsically harmful due to exacerbated adverse reactions in certain groups of patients, or harmful due to a deleterious clinical practice. We see that the reasons for a treatment not to be beneficial are diverse and unrelated to its intrinsic curative potential. In any case, the result of a meta-analysis does not mean that an overall null or unfavorable effect abrogates the curative effect measured in certain groups of patients or when the treatment is combined with an adequate clinical practice.
For instance, in the case of the severe acute respiratory syndrome (SARS) due to the infectious Covid-19 disease, the timing of administration of HCQ or HCQ plus AZI as soon as the first day of hospitalization was crucial, as well as appropriate co-medications to fight adverse physiological effects such as coagulation disorders. Observational studies allow clinicians to rapidly report curative tactics developed on patient samples of intermediate sizes (100 to 200 patients). This form of publication allows the medical community to improve its practice for the benefit of patients. For instance, Bousquet [6] conducted on 108 patients a study aimed at measuring the treatment effect of HCQ plus AZI. They concluded to the efficacy of HCQ plus AZI (HR = 0.49) in univariate analysis. Multivariate analysis could not be performed because of the reduced number of patients included in the study; but the severity of the disease was a well established parameter and the strategy of treatment uniquely defined. This study was eliminated from the meta-analysis we review due to a confounding co-variable invoked but not specified. We suppose the authors have probably considered that 93/108 patients having received an anticoagulant was a confounding factor. This understanding was incorrect because administrating an anticoagulant was an adequate co-medication potentiating the treatment effect.
4.2.2 A bias analysis not explicit and quite erroneous
The bias analysis was presented in a 6 item summary table in appendix A of the article with no explicit statements accompanying it.
All preselected studies were either published (21) or deposited (10) without peer review on the site of MedRxiv at the University of Yale. Some present bias that make their evaluation very difficult (Table 1) such as the studies by Horby [13], Sbidian [28], Singh [29] and Wang [31].
Similarly to the study by Wang, the retrospective studies on large samples of patients entering the meta-analysis may mix Covid-19 patients, either hospitalized with patients requiring only ambulatory care. In addition, patient heterogeneity, diversity of clinical and individual clinician practice, severity of the disease, age and comorbidities constitute a broad spectrum of medical conditions.
The 3 randomized studies (Table 1) that the meta-analysis authors consider free of critical and serious bias actually cannot be taken into account for simple reasons.
(a) The first one (Horby [13]) conducted on hospitalized patients conceals an over-dosage of HCQ [38] that has most probably impacted the survival chances of the patients (Table 1). Unfortunately, due to its very small variance, the study has a statistical weight that dominates the meta-analysis.
(b) The second one (Cavalcanti [7]) mixes inpatients and outpatients and the primary endpoint was not the efficacy of HCQ. The published adjusted HR = 1.47 for HCQ is unrealistically unfavorable in front of a raw 3% death rate in the HCQ arm compared with 10% in the control arm. It is also unrealistic with respect to HCQ + AZI with a HR = 0.64 and a similar 2.5% death rate (Table 1). The internal inconsistency of this randomized study is not discussed. Fiolet et al. categorize it as being very reliable although it demonstrates the benefit of HCQ+ AZI, contrary to their conclusion.
(c) Finally, the third one (Skipper [30]) was remotely conducted on patients staying home with very mild diseases. Over 14 hospitalized patients, only one died in the placebo group. Last but not least, adding to the confusion, only 34% of those patients received appropriate PCR SARS-CoV-2 testing. Surprisingly, they write they have excluded two Chinese studies because no death were reported but they take into account the study of Skipper on the ground it is a randomized study free of bias.
Fiolet et al. did not have access to any patient file, which prevented them from conducting a rigorous meta-analysis. They lacked systematically the necessary information such as disease severity, dosage and number of days treatments were administered.
They claim they have used the ROBIN-I [39] (non randomized studies), and Rob2 [40] (randomized studies) bias evaluation tools as well as the Cochrane on line recommendations [37,41] concerning the conduct of meta-analyses. Although providing useful indications on the nature of classically encountered bias, these tools do not allow the automatic knowledge and detection of all possible bias. They advocate them but do not explicitly explain any of their study selection. Over the 14 studies they eliminated, they invoked the presence of confounding variables in 11 of them without stating them. Strange enough, they included in their calculation the study by Ayerbe they deemed as having a critical bias, and excluded the study by Wang that does not present critical bias according to them.
The study by Rosenberg [26] was categorized as being at low risk of bias (Table 1), although it has several serious limitations (see results section), among which the fact that patients are not consecutive. A drastic random reduction by 70% of the patients took place and, subsequently, over the 2362 remaining patients, 887 were eliminated because the review of their files was incomplete.
Some retained studies (Ip [14] and Geleris [11]) with HR close to 1. did not address HCQ efficacy as primary or secondary endpoints and have overwhelming statistical weight due to their large number of included patients (> 1000), abrogating the potential benefit of HCQ and HCQ plus AZI. We find these studies should have been excluded from the meta analysis (Table 1).
Finally, studies with beneficial effects of HCQ and HCQ plus AZI [4,6,16,17,25] (Table 1) were eliminated although they are characterized by clearly defined treatment strategies, homogeneous patient selection and performed in single institutions or in a connected network of collaborative institutions (Arshad).
4.3 A meta-analysis disconnected from the patient
Covid-19 is a disease with two successive phases: a phase of viral multiplication followed by an inflammatory phase (cytokine storm) where the viral load decreases, while lungs are impaired. Antiviral treatments such as HCQ and/or AZI should be prescribed as early as possible during the first phase, whereas corticosteroids and oxygenation must take place at the very beginning of the second phase. Timing is crucial and may vary according to the groups of patients. Oxygenation may occur by non invasive means or via tracheal intubation and therapeutic indications may vary according to the medical team. It has evolved according to experience and recommendations, as the clinical aspects of the disease became better known. The timing and dose of the anti-coagulants prescription is an increasing factor of heterogeneity of care, as well as nursing and medical support care.
4.4 Concerning the harmfulness of the association HCQ plus AZI
Any active medication necessarily conceals adverse effects. Physicians are always dealing with them to obtain a therapeutic effect beneficial to the patient. This implies weighting the associated risk. When patients are hospitalized, they easily benefit from clinical monitoring. For instance, routine electrocardiograms allow the early detection of possible cardiac rhythm disorder (e.g. prolongation of QTc interval resulting from a specific treatment toxicity or from unexpected drug interactions); hypokalemia may favor a possible torsade de pointe.
Fiolet et al. cite several studies that would have, according to them, demonstrated the cardiac harmfulness of HCQ plus AZI, but they did not analyze the clinical context and other medicament associations. They don't discuss the inconsistencies inside the studies they selected. For instance, in the study by Magagnoli [19], the cardiac toxicity is suggested, referring to the Rosenberg study, to explain an increased mortality in the HCQ plus AZI arm (HR = 1.31 (p=0.28). But for HCQ alone, mortality increase is even more pronounced (HR = 1.83 (p=0.009), which is inconsistent with the hypothesis of an exacerbated toxicity of the association HCQ plus AZI. The same problem is found in the study of Cavalcanti [7] where HR = 0.64 [0.18-2.21] for HCQ plus AZI whereas HR = 1.47 [0.48-4.53] for HCQ. There are probably some bias of patient selection in these studies: the median hospitalization times reported by Magagnoli indicates that possibly only more severely affected patients have received HCQ or HCQ plus AZI (Table 1).
As ultimate proof of their conclusion, they cite the World Health Organization statistics [42] on adverse reactions recorded in 167,000 patients with auto-immune chronic diseases (lupus, rheumatoid arthritis) receiving long-term HCQ and/or AZI. The measured risk ratio of QTc prolongation, torsade de pointe and ventricular tachycardia is 2.48 [95% CI, 1.28–4.79] for HCQ plus AZI, but event frequencies are very low with 0.3% for HCQ, 0.8% for AZI and 1.5% for their combination. In 263 adverse reactions recorded among 76,215 patients, only 7 patients died (less than 1/10 000) due to torsade de pointe and none following QTc prolongation. This data are for long-term treatments, whereas in the case of Covid-19, the treatment usually lasts 10 days for HCQ and 5 days for AZI, in monitored patients.
In the retrospective article by Harvey Risch [43], we learn that HCQ plus AZI was used in the USA as standard care on more than 300,000 aged patients with multiple comorbidities, 0.047% of whom have developed arrhythmia due to the treatment. Only 9 patients per 100,000 (0.009%) died, which has to be compared with the 10,000 Americans weekly dying of the disease. Lagier et al. [16] have observed QTc > 600 ms in 0.67 % of the patients, without torsade de pointe nor sudden death.
5. Conclusion
Generally speaking, meta-analyses cannot reliably be applied to non randomized heterogeneous studies with hidden multiple bias due to complex confounding factors difficult to identify. This is particularly the case for the studies on Covid-19. Regardless the statistical methodology used, meta-analyses may unavoidably lead to results with poor or no scientific significance if not rigorously conducted. After thorough discussion of the bias, the results of the meta-analysis remains in favor of the efficacy of HCQ alone or combined with AZI for the treatment of Covid-19. These medications did not demonstrate any significant cardiac toxicity, and were overall well tolerated.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was entirely benevolent. The authors declare that this study received funding from Association Bon Sens to cover the publication fees. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Ethical Approval
Approval was not required for this work.
Acknowledgments
Thanks to all members of the France Soir - Citizen Circle (scientists, medical practitioners, lecturers and jurists) for the many public audience articles they wrote or help publish in France Soir, with scientific insight and dedicated efforts in critically analyzing the published literature on hydroxychloroquine and azithromycin during the Covid-19 pandemic.
References
- Fiolet T, Guihur A, Rebeaud ME, et al. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clinical Microbiology and Infection 27 (2021): 19-27.
- Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney International 98 (2020): 20-26.
- Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. Journal of Thrombosis and Thrombolysis (2020): 1-4.
- Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. International Journal of Infectious Diseases 97 (2020): 396-403.
- Barbosa J, Kaitis D, Freedman R, et al. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. N Engl J Med 1 (2020): 8882.
- Bousquet G, Falgarone G, Deutsch D, et al. ADL-dependency, D-Dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with Covid-19. Aging (Albany NY) 12 (2020): 11306.
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. New England Journal of Medicine 383 (2020): 2041-2052.
- Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. American Journal of Transplantation 20 (2020): 3140-3148.
- Fontana F, Alfano G, Mori G, et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. American Journal of Transplantation 20 (2020): 1902-1906.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents 56 (2020): 105949.
- Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. New England Journal of Medicine 382 (2020): 2411-2418.
- Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Internal Medicine 180 (2020): 1436-1446.
- Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse A. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. MedRxiv (2020).
- Ip A, Berry DA, Hansen E, et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—An observational study. PloS One 15 (2020): e0237693.
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet 395 (2020): 1907-1918.
- Lagier JC, Million M, Gautret P, et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Medicine and Infectious Disease 36 (2020): 101791.
- Lecronier M, Beurton A, Burrel S, et al. Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis. Critical Care (London, England) 24 (2020): 418.
- Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Annals of Oncology 31 (2020): 1386-1396.
- Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med 1 (2020): 114-127.
- Mahévas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 369 (2020).
- Membrillo FJ, Ramírez-Olivencia G, Estébanez M, et al. Early Hydroxychloroquine Is Associated with an Increase of Survival in COVID-19 Patients: An Observational Study. Preprints (2020).
- Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID-19 in New York City. Journal of General Internal Medicine (2020): 1-10.
- Paccoud O, Tubach F, Baptiste A, et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital. Clinical Infectious Diseases (2020).
- Rivera DR, Peters S, Panagiotou OA, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discovery 10 (2020): 1514-1527.
- Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clinical & Translational Oncology (2020): 1-5.
- Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 323 (2020): 2493-2502.
- Sánchez-Álvarez JE, Fontán MP, Martín CJ, et al. Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrología (English Edition) 40 (2020): 272-278.
- Sbidian E, Josse J, Lemaitre G, et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. MedRxiv (2020).
- Singh S, Khan A, Chowdhry M, et al. Outcomes of hydroxychloroquine treatment among hospitalized COVID-19 patients in the United States-real-world evidence from a federated electronic medical record network. MedRxiv (2020).
- Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Annals of Internal Medicine 173 (2020): 623-631.
- Wang AL, Zhong X, Hurd Y. Comorbidity and sociodemographic determinants in COVID-19 mortality in an US urban healthcare system. MedRxiv (2020).
- Yu B, Li C, Chen P, et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Science China Life Sciences 63 (2020): 1515-1521.
- Borenstein M, Hedges L, Rothstein H. Meta-analysis: Fixed effect vs. random effects. Meta-Analysis. com (2007).
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369 (2020).
- Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiology 5 (2020): 1067-1069.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 7 (1986): 177-188.
- Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions. Part 3: Special topics. 13.6.2.2 Combining studies (version 6) 2019. https://handbook-5-1.cochrane.org/chapter_13/13_6_2_2_combining_studies.htm (accessed July 28, 2020).
- Borba MG, Val FF, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open 3 (2020): e208857.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355 (2016).
- Sterne JA, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366 (2019).
- Cochrane Training. Cochrane Handbook for Systematic Reviews of Interventions. Part 3: Special topics. https://handbook-5-1.cochrane.org/chapter_13/13_5_1_1_sources_of_bias_in_non_randomized_studies.htm
- Nguyen LS, Dolladille C, Drici MD, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization Pharmacovigilance Database. Circulation 142 (2020): 303-305.
- Risch HA. Early outpatient treatment of symptomatic, high-risk coronavirus disease 2019 patients that should be ramped up immediately as key to the pandemic crisis. American Journal of Epidemiology.
