Red Blood Cell Transfusion and Neurodevelopmental Outcome in Preterm Infants
Article Information
Shahana Akter1*, Mohammad Golam Sadik2, Md. Arif Hossain1, M A Mannan3
1Medical Officer, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Junior Consultant (Paediatrics), Upazila Health Complex, Saturia, Manikganj, Bangladesh
3Professor, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
*Corresponding Author: Shahana Akter, Medical Officer, Department of Neonatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Received: 28 April 2022; Accepted: 09 May 2022; Published: 12 May 2022
Citation: Shahana Akter, Mohammad Golam Sadik, Md. Arif Hossain, M A Mannan. Red Blood Cell Transfusion and Neurodevelopmental Outcome in Preterm Infants. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 269-284.
Share at FacebookAbstract
Background: Preterm neonates are the most commonly transfused group of patients and about 70-80% of preterm low birth weight infants receive transfusion. Blood transfusions are a common form of supportive therapy for sick neonatesand remain as an important life-saving intervention for neonatal intensive care patients. Red blood cell (RBC) transfusions provide an immediate increase in tissue oxygenation.
Objective: To assess the neurodevelopmental outcome of preterm infants who receive packed red blood cell transfusion.
Methodology: This prospective observational study was conducted in the Department of Neonatology, BSMMU, Dhaka, Bangladesh after approval by Institutional Review Board, over eighteen-month period. All Preterm low birth weight neonate satisfying the inclusion criteria were enrolled in the study. All patient managed as per standard clinical guidelines of NICU, BSMMU, Dhaka, Bangladesh. PRBC transfusion was given according to institutional protocol. We included total 60 infants, were divided in to two groups on the basis of PRBC transfusion. 29 infants were in PRBC group and 31 in non PRBC group. The hospital outcomes were recorded as necrotizing enterocolitis (NEC), Broncho pulmonary dysplasia (BPD) and Retinopathy of prematurity (ROP). Detailed neurodevelopmental assessment was done at 6 and 9 months of postnatal age, using Bayley Scales of Infant Development Third edition, in the Institute of Pediatric Neurodisorder and Autism (IPNA). Data were analyzed using the statistical package for social sciences (SPSS) version 25. P value < 0.05 is considered as statistically significant.
Results: Total 60 preterm low birth weight patients completed neurodevelopmental follow up. Among them 29 (48.3%) patients who received PRBC and 31 (51.7%) patients who did not receive PRBC transfusion. Mean gestational age was 32.75 weeks and mean birth weight was 1503.33 g
Keywords
Red Blood Cell, Neurodevelopmental, Outcome, Preterm Infants
Red Blood Cell articles; Neurodevelopmental articles; Outcome articles; Preterm Infants articles
Article Details
1. Introduction
Blood component transfusion plays an important role in modern transfusion. Preterm neonates are the most heavily transfused group of patients.70-80% of VLBW and about 85% of ELBW new-borns require at least one transfusion by the end of their hospital stay. Blood transfusions are a common form of supportive therapy for sick infants, and the use of red blood cell (RBC) transfusions has undoubtedly been one of the key factors enabling the increased survival of critically ill premature babies. Premature and low birth weight (LBW) neonates account for nearly three quarters of neonatal RBC transfusions. The reasons for increased transfusion are immature haematopoiesis, poor comp-ensatory hematological mechanisms and frequent sampling Strauss et al. [1]. Recent global estimates suggest that more than 1 in 10 or an estimated 15 million babies born in 2010 were preterm, of which more than 1 million died as a result of preterm birth and related complications Blencowe et al. [2]. The absolute numbers and rates of preterm birth have increased during this period Howson et al. [3].
Although neonatal mortality rates have fallen globally between 1990 and 2009 Oestergaard et al. [4]. Pre-term birth complications account for 35% of the estimated 3.1 million global neonatal deaths and are the second leading cause of death in children under 5 years of age Liu et al. [5]. The vast majority (85%) of global preterm births occur in Asia and Africa Beck et al. [6], where health systems are weak and access to and utilization of health services are limited, contributing to the higher risks of death and disa-bilities in preterm babies World Health Organization, [7] Lawn et al. [8]. Approximately one-third of preterm survivors suffer from severe long-term neurological disabilities, such as cerebral palsy or mental retardation Lawn et al. [9] Furthermore, preterm infants carry increased risk of a range of neurodevelopmental impairments and disabilities, including behavioural problems, school learning difficulties, chronic lung disease, retinopathy of prematurity, hearing impairment, and lower growth attainment Saigal et al. [10].
Preterm neonates are the most heavily transfused group of patients and about 85% of extremely low birth weight (ELBW) new-borns receive transfusion by the end of their hospital stay Madan et al. [11] & Lin et al. [12]. The reasons for increased transfusion in preterm neonates are immature haematopoiesis, poor compensatory hematological mechanisms, blood losses from frequent laboratory testing, sepsis, necro-tizing enterocolitis (NEC), bleeding, and consumptive coagulopathy Strauss et al. [13]. It has been reported that between 50% and 94% of the very low birth weight (VLBW) infants (birth weight < 1500 g) and as high as 95% of extremely low birth weight (ELBW) infants (birth weight < 1000 g) receive at least one transfusion during their hospital stay Whyte et al. [14]. Blood transfusions are a common form of supportive therapy for sick neonates. Red blood cell (RBC) transfusion in anemic patients provides an immediate increase in tissue oxygenation. It is common practice in critically ill patients with cardiopulmonary comp-romise. Neonatal patients, with their relatively small blood volume and immature hematopoietic system, are among the most heavily transfused populations Strauss, [15] The smaller the infants are, the more likely they are to receive frequent transfusions. Upon RBC transfusion, improvement in cerebral blood flow and oxygenation have been observed, while a more liberal transfusion policy may be associated with a better developmental outcome New et al. [16]. How-ever, in the past 2 decades, there have been increasing concerns of possible deleterious effects from RBC transfusion in patients under critical care.Blood transfusion may introduce some inflammatory media-tors, such as interleukin (IL)-1β, IL-8, tumor necrosis factor-α, and monocyte chemoattractant protein Ho et al. [17] & Keir et al. [18].
Furthermore, the ability of RBCs to deliver oxygen may be diminished by changes in the cell membranes that alter RBC deformability and decrease their ability to scavenge nitric oxide as well as by biochemical changes such as a reduction in 2,3-diphosphoglycerate levels Donadee et al. [19] Tinmouthet al. [20]. Histological studies have suggested perturbation of microcirculatory regulation by RBC transfusion is involved, hence compromising tissue oxygenation Somani et al. [21]. It also may induce immunologic reactions in the intestine and affect regulation of mesenteric blood flow and tissue oxygenation, increa-sing the risk of tissue ischemia and/or reperfusion injury La Gamma and Blau, [22]. PRBC transfusions have been implicated in the development of Broncho pulmonary dysplasia (BPD) Collard, [25] necrotizing enterocolitis (NEC) Mally et al. [26] and retinopathy of prematurity (ROP) Fortes et al. [27]. Preterm infants are particularly vulnerable to tissue injuries related to a disturbed oxygen delivery. Unique and serious complications of premature birth are often attributed to such unbalanced tissue oxygenation. Research studies over the past several years have documented in both animal and human studies that erythropoietin has substantial neuroprotective pro-perties Nopoulos et al. [23, 24, 25]. Transfusing infants to improve oxygen-carrying capacity and restricting RBCT to avoid transfusion-associated risks and costs may both potentially impair long-term development.
2. Materials and Methods
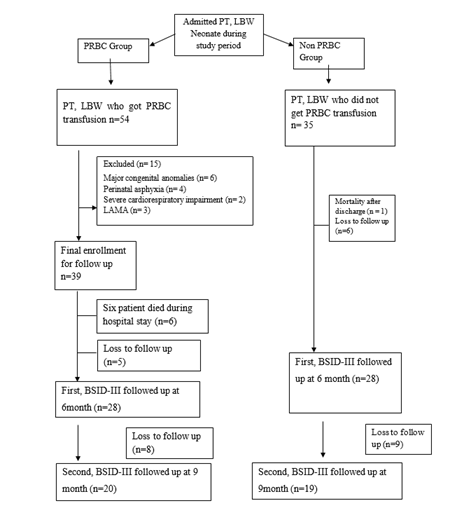
This prospective observational study was conducted in the Department of Neonatology, BSMMU, Dhaka, Bangladesh after approval by Institutional Review Board, over eighteen-month period. All Preterm low birth weight neonate satisfying the inclusion criteria were enrolled in the study. All patient managed as per standard clinical guidelines of NICU, BSMMU, Dhaka, Bangladesh. PRBC transfusion was given according to institutional protocol. We included total 60 infants, were divided in to two groups on the basis of PRBC transfusion. 29 infants were in PRBC group and 31 in non PRBC group. The hospital outcomes were recorded as necrotizing enterocolitis (NEC), Broncho pulmonary dysplasia (BPD) and Retinopathy of prematurity (ROP). Detailed neurodevelopmental assessment was done at 6 and 9 month of postnatal age, using Bayley Scales of Infant Development Third edition, in the Institute of Pediatric Neurodisorder and Autism (IPNA).
In this study, composite score >70 was taken as ‘normal’ and <70 as ‘delayed’. Dependent variable was neurodevelopmental outcome and independent variables were PRBC transfusion, gestational age, birth weight, fetal growth at birth, multiple gestation, hemoglobin status, hospital stay, number of trans-fusion and comorbidities. The cognitive, receptive language, expressive language, gross motor, fine motor developmental scores of preterm infants were computed and then categorized and analyzed. Categorical data were analyzed by using Chi square test and continuous data were analyzed by indepen-dent sample t test. Correlation between transfusion and neurodevelopmental delay were evaluated using a logistic regression model adjusted for gestational age, birth weight and hemoglobinstatus. Data were analyzed using the statistical package for social sciences (SPSS) version 25. P value < 0.05 is considered as statistically significant.
2.1 Inclusion criteria
- • Admitted preterm (<37 weeks), Low birth weight (<2500 gm) neonates in the Department of Neonatology, BSMMU, Dhaka, Bangladesh.
2.2 Exclusión criteria
- Major congenital malformation (e.g.- omphalocele, gastroschisis, tracheoesoph-ageal fistula, anorectal malformation, gross hydronephrosis with posterior urethalvulve, encephalocele, meningomyelocele etc.)
- Newborn with severe cardiorespiratory compromise.
- Patients with severe perinatal asphyxia.
2.3 Data analysis
After collection, data were entered into a personal computer then edited, analyzed, plotted and were presented in graphs and tables; categorical scale was analyzed by using Chi square test and continuous scale was analyzed by t test. Data was analyzed using the statistical package for social sciences (SPSS) version 25. P value <0.05 was considered as level of significance.
2.4 Logistic regression analysis
Correlation between transfusion and neuro-developmental outcome was evaluated using a logistic regression model adjusted for GA, BW and Hb status. To adjust outcome multiple logistic regression analysis was performed, using variables found to be significant in previous similar studies.
3. Results
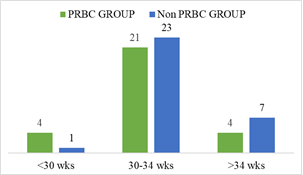
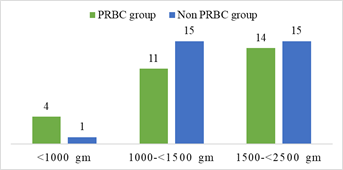
Demographic characteristics of the studied infants are presented in Table 1. Total 60 preterm low birth weight patients were included in the study. Among them 29 (48.3%) patients who got PRBC and 31 (51.7%) patients who did not get PRBC. Mean gestational age was 32.75 weeks and mean birth weight was 1503.33 g. Mean Apgar score at 1 minute and 5 minute 6.91 and 8.35 respectively. Gender distribution reflected male 32 (53.3%) and female 28 (46.7%); LUCS were 48 (80%) and NVD were 12 (20%) in number. According to place of delivery, 52 (86.7%) were (BSMMU) inborn delivery and out born patients 8 (13.3%). Most of them were AGA 40 (66.7%), SGA 20 (33.3%) in number. Regarding multiple gestation 12 (20%) and single gestation 48 (80%) in number in the study population (Table-1). In gestational age <30 weeks were 4 (13.8%) and 1 (3.2%); 30-34 weeks were 21 (72.4 %) and 23 (74.2%); >34 weeks were 4 (13.8%) and 7 (22.6%) in both PRBC group and non PRBC group respectively (Figure-2). Birth weight <1000 g were 4 (13.8%), within 1000- <1500 gm 11 (37.9%) and 1500- <2500 gm were 14 (48.3%) in PRBC group. Birth weight <1000 g were 1 (3.2%), within 1000- <1500 gm 15 (48.4%) and1500- <2500 gm were 15 (48.4%) in non PRBC group respectively (figure-3). In PRBC group 15 (52%) were male and 14 (48%) were female; in non PRBC group 1 7 (55%) and 14 (45%) were male and female respectively (Figure 4).
Baseline and clinical characteristics of the PRBC group and non PRBC group are presented in Table 2. Among 60 preterm low birth weight patients 29 got packed red blood cell and 31 did not get packed red blood cell. Between PRBC group and non PRBC group LUCS were 23 (79.3%) and 25 (80.6%) respectively and NVD were 6 (20.7%) and 6 (19.4%) respectively (table-4.2). According to place of delivery, 23 (79.3%) and 29 (93.5%) were (BSMMU) inborn delivery in PRBC group and non PRBC group respectively. Outborn patients 6 (20.7%) and 2 (6.5%) were in PRBC group and non PRBC group respectively. Gender distribution reflected male 15 (51.7%) and female 14 (48.3%) in PRBC group, 17 (54.8%) male and 14 (45.2%) female in non PRBC group. Birth weight <1000 g were 13.8%, within 1000- <1500 gm 37.9% and 1500- <2500 gm were 48.3% in PRBC group respectively. Birth weight <1000 g were 3.2%, within 1000- <1500 gm 48.4% and 1500- <2500 gm were 48.4% in non PRBC group respectively. In gestational age <30 weeks were 13.8% and 3.2%; 30- 34 weeks were 72.4 % and 74.2%; >34 weeks were 13.8% and 22.6% in both PRBC group and non PRBC group respectively. GA ranges from 27- 36 weeks in PRBC group; 29-36 weeks in non PRBC group. BW ranges from 790- 2400 gm in PRBC group; 930- 2385 gm in non PRBC group. Most of them were AGA 72.4% and SGA 27.6% in PRBC group respectively. In non PRBC group AGA 61.3% and SGA 38.7% respectively. Regarding multiple gestation 10.3% and 29%; single gestation 89.7% and 71% in PRBC and non PRBC group respectively. There are no significant difference in both group regarding gestational age, birth weight, fetal growth, Apgar score and initial hemoglobin level. Hospital stay was more in PRBC group and it is statistically significant (p= 0.001) (Table-2).
Table 3 is showing the comparison of maternal factors between PRBC and non PRBC group of neonates. None of the maternal factors such as pregnancy induced hypertension (PIH), gestational diabetes mellitus (GDM), antepartum hemorrhage (APH), maternal premature rupture of membrane (PROM) >18 hours, intrapartum infection, were found to be significant between two groups. Sepsis presented as 20.7%, 25.8%; TTN presented as 6.9%, 9.7%; prematurity presented as 34.5%, 38.7%; neonatal jaundice 3.4%, 12.9% in PRBC and non PRBC group respectively. RDS presented as 34.5%, 12.9%; in PRBC and non PRBC group respectively and it is statistically significant (p value-0.048) (Table 4). In PRBC group, 34.5% patients had comorbidity and it is statistically significant. Among them BPD 3.4%; NEC 6.9%; ROP 13.8%; both BPD and ROP had 6.9% patients; both NEC and ROP had 3.5% patients. In non PRBC group, most have no comorbidities, 96.8% patients. Only 3.2% patient had ROP (Table 5). Patients received PRBC for bloodletting 48.2%and for lower hemoglobin level 51.8% (Table 6). Table 7 shows at 6 and 9 month of age most patients neurodevelopmental outcome of PRBC group is delayed. It is not statistically significant. 8 and 1 infant have cognitive delay at 9 month of age in PRBC and non PRBC group respectively. It is statistically significant (p value-0.010).
|
Characteristics |
Findings |
|
Gestational age (weeks), Mean ± SD |
32.75 ± 1.94 |
|
Birth weight (g), Mean ± SD |
1503.33 ± 390.09 |
|
Sex, n (%) |
|
|
Male |
32 (53.3) |
|
Female |
28 (46.7) |
|
Mode of delivery, n (%) |
|
|
LUCS |
48 (80) |
|
NVD |
12 (20) |
|
Place of birth, n (%) |
|
|
Inborn |
52 (86.7) |
|
Out born |
8 (13.3) |
|
Fetal growth at birth, n (%) |
|
|
AGA |
40 (66.7) |
|
SGA |
20 (33.3) |
|
Apgar score, Mean ± SD |
|
|
1 minute |
6.91 ± 0.68 |
|
5 minute |
8.35 ± 0.62 |
|
Multiple gestation, n (%) |
|
|
Single |
48 (80) |
|
Multiple |
12 (20) |
|
PRBC status, n (%) |
|
|
PRBC group |
29 (48.3) |
|
Non PRBC group |
31 (51.7) |
SD: Standard Deviation, LUCS: Lower Uterine Caesarean section, NVD: Normal Vaginal Delivery, AGA: Appropriate for gestational age, IUGR: Intrauterine growth restriction., SGA: Small for gestational age, PRBC group: those who got packed red blood cell, Non PRBC group: those who did not get packed red blood cell.
Table 1: Demographic characteristics of enrolled study population (N= 60).
|
Parameter |
PRBC group |
Non PRBC group |
P value |
|
n=29 |
n=31 |
||
|
Number of patients, n (%) |
29 (48.3) |
31 (51.7) |
|
|
Mode of Delivery, n (%) |
|||
|
LUCS |
23 (79.3) |
25 (80.6) |
0.897NS |
|
NVD |
6 (20.7) |
6 (19.4) |
|
|
Place of delivery, n (%) |
|||
|
BSMMU |
23 (79.3) |
29 (93.5) |
0.105NS |
|
Outborn |
6 (20.7) |
2 (6.5) |
|
|
Sex, n (%) |
|||
|
Male |
15 (51.7) |
17 (54.8) |
0.809NS |
|
Female |
14 (48.3) |
14 (45.2) |
|
|
Birth weight (gm), n (%) |
|||
|
(<1000gm) |
4 (13.8) |
1 (3.2) |
|
|
(1000-<1500gm) |
11 (37.9) |
15 (48.4) |
0.303NS |
|
(1500-<2500 gm) |
14 (48.3) |
15 (48.4) |
|
|
Gestational age (weeks), n (%) |
|||
|
<30 weeks |
4 (13.8) |
1 (3.2) |
|
|
30-34 weeks |
21 (72.4) |
23 (74.2) |
0.266NS |
|
>34 weeks |
4 (13.8) |
7 (22.6) |
|
|
Gestational age (weeks) Mean ± SD |
32.21 ± 2.19 |
33.26 ± 1.53 |
|
|
Birth weight (gm) |
1457 ± 429 |
1545 ± 351 |
|
|
Mean ± SD |
|||
|
Fetal growth at birth, n (%) |
|||
|
AGA |
21 (72.4) |
19 (61.3) |
0.361NS |
|
SGA |
8 (27.6) |
12 (38.7) |
|
|
Multiple Gestation, n (%) |
|||
|
Single |
26 (89.7) |
22 (71.0) |
0.071NS |
|
Multiple |
3 (10.3) |
9 (29.0) |
|
|
Apgar score, Mean ± SD |
|||
|
1 minute |
6.88 ± 0.680 |
6.93 ± 0.69 |
0.931NS |
|
5 minute |
8.29 ± 0.624 |
8.40 ± 0.62 |
0.848NS |
|
Initial hemoglobin (gm/dl), |
17.88 ± 3.099 |
18.36 ± 2.80 |
0.366NS |
|
Mean ± SD |
|||
|
Hospital stay (days), |
24.34 ± 11.875 |
10.23 ± 5.80 |
0.001S |
|
Mean ± SD |
|||
Chi-square test for categorical data, Independent sample t test for continuous data. P < 0.05 were considered as significant. NS- not significant, S- significant, SD: Standard Deviation, PRBC group- Infants who got packed red blood cell, non PRBC group- Infants who did not get packed red blood cell.
Table 2: Baseline and clinical characteristics of PRBC and non PRBC group (N=60).
|
Parameter |
PRBC group |
Non PRBC group |
P value |
|
n = 29(%) |
n = 31(%) |
||
|
PIH |
|||
|
Yes |
7 (58.3) |
5 (41.7) |
0.438NS |
|
No |
22 (45.8) |
26 (54.2) |
|
|
GDM |
|||
|
Yes |
3 (30) |
7 (70) |
0.204NS |
|
No |
26 (52) |
24 (48) |
|
|
APH |
|||
|
Yes |
1 (33.3) |
2 (66.7) |
1.00NS |
|
No |
28 (49.1) |
29 (50.9) |
|
|
PROM >18 hours |
|||
|
Yes |
3 (37.5) |
5 (62.5) |
0.512NS |
|
No |
26 (50) |
26 (50) |
|
|
Intrapartum Infection |
|||
|
Yes |
3 (50) |
3 (50) |
1.00NS |
|
No |
26 (48.1) |
28 (51.9) |
|
|
PIH and GDM |
|||
|
Yes |
1 (50) |
1 (50) |
1.00NS |
|
No |
28 (48.3) |
30 (51.7) |
|
|
GDM and APH |
|||
|
Yes |
3 (100) |
0 (0) |
0.107NS |
|
No |
26 (45.6) |
31 (54.4) |
|
|
GDM and Intrapartum Infection |
|||
|
Yes |
2 (100) |
0 (0) |
0.229NS |
|
No |
27 (46.6) |
31 (53.4) |
|
|
PIH and Intrapartum Infection |
|||
|
Yes |
0 (0) |
2 (100) |
0.492NS |
|
No |
29 (50) |
29 (50) |
|
Chi-square test for categorical data. P < 0.05 were considered as significant. NS- not significant, S-significant, PRBC group- Infants who received packed red blood cell, non PRBC group- Infants who did not receive packed red blood cell.
Table 3: Maternal factors between PRBC and non PRBC group of neonates (N=60).
|
Initial diagnosis |
PRBC group n=29(%) |
Non PRBC group n=31(%) |
p value |
|
|
Sepsis |
6 (20.7) |
8 (25.8) |
0.640NS |
|
|
Respiratory distress syndrome |
10 (34.5) |
4 (12.9) |
0.048S |
|
|
Transient tachypnea of newborn |
2 (6.9) |
3 (9.7) |
0.697NS |
|
|
Prematurity |
10 (34.5) |
12 (38.7) |
0.734NS |
|
|
Neonatal Jaundice |
1 (3.4) |
4 (12.9) |
0.297NS |
|
Chi-square test for categorical data, P < 0.05 were considered as significant. NS- not significant, S-significant.
Table 4: Initial diagnosis of the newborn, (N= 60).
|
PRBC status |
P value |
|||
|
PRBC group n=29 (%) |
Non PRBC group n=31 (%) |
|||
|
Comorbidities of pts |
No comorbidities |
19 (65.5) |
30 (96.8) |
0.002S |
|
Having comorbidities |
10 (34.5) |
1 (3.2) |
||
|
BPD |
1 (3.4) |
0 |
|
|
|
NEC |
2 (6.9) |
0 |
||
|
ROP |
4 (13.8) |
1 (3.2) |
||
|
BPD & ROP |
2 (6.9) |
0 |
||
|
NEC & ROP |
1 (3.5) |
0 |
||
Chi-square test for categorical data, P < 0.05 were considered as significant. NS- not significant, S- significant. BPD- Bronchopulmonary dysplasia; NEC- Necrotizing enterocolitis; ROP- Retinopathy of prematurity.
Table 5: Comorbidities among the neonates, (N= 60).
|
Indication |
PRBC group (n =29) |
Percentage (%) |
|
Bloodletting |
14 |
48.2 |
|
Low hemoglobin level |
15 |
51.8 |
Table 6: Indication of PRBC transfusion (n=29).
|
PRBC group n= 29 (%) |
Non PRBC group n=31(%) |
P value |
|
|
Cognition at 6 months |
|||
|
Delayed |
17 (60.7) |
14 (50) |
0.420NS |
|
Not delayed |
11 (39.3) |
14 (50) |
|
|
Cognition at 9 months |
|||
|
Delayed |
8 (40) |
1 (5.3) |
0.010S |
|
Not delayed |
12 (60) |
18 (94.7) |
|
|
Language at 6 months |
|||
|
Delayed |
3 (10.7) |
4 (14.3) |
0.686NS |
|
Not delayed |
25 (89.3) |
24 (85.7) |
|
|
Language at 9 months |
|||
|
Delayed |
2 (10) |
1 (5.3) |
0.579NS |
|
Not delayed |
18 (90) |
18 (94.7) |
|
|
Motor at 6 months |
|||
|
Delayed |
21 (75) |
18 (64.3) |
0.383NS |
|
Not delayed |
7 (25) |
10 (35.7) |
|
|
Motor at 9 months |
|||
|
Delayed |
9 (45) |
8 (42.1) |
0.855NS |
|
Not delayed |
11 (55) |
11 (57.9) |
|
Chi-square test for categorical data, P < 0.05 were considered as significant. NS- not significant, S- significant, composite score <70= delayed, >70= Normal.
Table 7: Neurodevelopmental outcome in PRBC and non PRBC group (N= 60).
4. Discussion
In this prospective observational study, 33 preterm low birth weight infants in the packed red blood cell group (PRBC group). Out of them, at 6 and 9 months of age followed up 28 (84.84%) and 20 (60.60%) infants respectively. Among the 35 preterm low birth weight non packed red blood cell group (Non PRBC group), at 6 and 9 month of age followed up 28 (80.00%) and 19 (54.28%) infants respectively. Total 60 preterm low birth weight neonates were included in this study. Mean gestational age was 32 weeks and mean birth weight was 1503 g. Santos et al. [28] found in their study, mean gestational age 29 weeks, mean birth weight 1046 g.29 (48.3%) patients who received PRBC and 31 (51.7%) patients who did not receive PRBC. Between PRBC group and non PRBC group LUCS were 79.3% and 80.6% respectively and NVD were 20.7% and 19.4% respectively. Gender distribution reflected male 51.7% and female 48.3% in PRBC group, 54.8% male and 45.2% female in non PRBC group. Birth weight <1000 g were 13.8%, within 1000- <1500 gm 37.9% and 1500-<2500 gm were 48.3% in PRBC group. Birth weight <1000 g were 3.2%, within 1000-<1500 gm 48.4% and 1500-<2500 gm were 48.4% in non PRBC group. In gestational age <30 weeks were 13.8% and 3.2%; 30- 34 weeks were 72.4 % and 74.2%; >34 weeks were 13.8% and 22.6% in both PRBC group and non PRBC group respectively.
In this study, we found there is no significant difference in respect of GA, BW in PRBC and non PRBC group. Keir et al. [29] showed that Infants who did not receive RBC transfusion/s had higher birth weight (p<0.01) and higher gestational age at the time of birth (p<0.01) as compared with those who received transfusion/s. There is no significant difference in both group between Apgar score and initial hemoglobin level. Mean hemoglobin level in PRBC group 17.88 g/dl. Wang et al. [30] showed in their study- average initial hemoglobin level was 14.8 g/dL. Sepsis presented as 20.7%, 25.8%; RDS presented as 34.5%, 12.9% and it is statistically significant; TTN presented as 6.9%, 9.7%; prematurity presented as 34.5%, 38.7% in PRBC and non PRBC group respectively. In this study, RBC transfusion in the PT LBW infants was associated with increased risk of neonatal morbidity. Hospital stay was more in PRBC group and it is statistically significant. According to Chirico et al. [31] short-term outcomes showed no difference in death or major morbidity with restrictive or liberal transfusion regimens. Although detrimental effects of RBC transfusion have been reported in critically ill patients of various age groups, most of these studies were based on observational studies and failed to provide any cellular or molecular evidence of causality. Histological studies have suggested perturbation of microcirculatory regulation by RBC transfusion is involved, hence compromising tissue oxygenation.
In preterm infants, evidence accumulated on the association between RBC transfusion and adverse outcomes in this population. The most serious outcomes are death and the development of BPD, NEC, IVH, and ROP. Most previous prospective studies compared outcomes in mostly LBW infants receiving care under a liberal (keeping ahigher hematocrit level) versus a restrictive (keeping a lower hematocrit level) rule of transfusion. Zhang et al. [32] demonstrated an association between PRBC transfusion and an increased incidence of BPD in preterm infants. The incidence of BPD was higher with earlier PRBC transfusions (within 3 weeks of life). Greater numbers of PRBC transfusions also increased the incidence of BPD and its severity. In our study, those patients who develop BPD got multiple PRBC transfusion and mostly got transfusion more than 7 days of age. None of the patients developed BPD in non PRBC group. In this prospective observational study, 15.38 % patients died during hospital stay. Wang et al. [30] showed in their study, In-hospital mortality rate was 18%. Santos et al. [33] found that Intra-hospital death occurred in 27.8% patients. The association between the risk of dying and RBC transfusions in the neonatal setting must be interpreted in relation to the consistency, strength, and biological plausibility. Further studies are needed to better define this association.
In the meantime, clinicians should strongly consider risks and benefits for morbidity and mortality of low birth weight preterm infants. The comparison of 1st and 2nd follow up of cognitive, language, motor scaled score which were low in PRBC group and it was only significant for cognition at 9 month of age. The comparison of 1st and 2nd follow up of cognitive, language, motor composite score which were low in PRBC group and it was only significant for cognition at 9 month of age. Whyte et al. [14] in the PINT Outcome study, there was no statistically significant difference in the primary outcome as death or cognitive delay, in the restrictive group and liberal group. Wang et al. [30] showed, there is no significant difference of neurodevelopmental outcome between infants who got transfusion less than 7 days of age with those who does not got transfusion within 7 days of age. The authors addressed and excluded other possible confounding effects for neurodevelopment such as hemoglobin level on growth, or abnormal iron status from different transfusion practice. They thought there was weak evidence of benefit from higher hemoglobin threshold for blood transfusion (to keep a higher hemoglobin level) with clinical and statistical significance, which could not be dismissed as accidental. However, they advocated caution in interpretation of these results and called for additional investigation of the effects of transfusion in ELBW infants. Chirico, [34] found that neurodevelopmental long-term outcomes seemed more favorable in the liberal group at first evaluation, especially for boys, and significantly better in the restrictive group at a later clinical and brain RMN investigation, especially for girls.
In our study, composite score of motor at 6 month age is significantly lower in multiple transfusion groups. Whyte et al. [14] showed there were no statistical significant differences between infants treated with high or low transfusion thresholds in the composite primary outcome, death or neurodevelopmental impairment. In our study, we found cognition at 9 month of age significantly delayed. Most of the newborn had neurodevelopmental outcome delayed in PRBC group and it was not statistically significant.
5. Conclusion
All the neurodevelopmental domain cognition, motor and language score at 6 and 9 month of age are found lower mostly in RBC transfusion group. Motor at 6 month of age is significantly delayed in multiple transfusion group. Cognition score at 9 month of age is found significantly delayed in RBC transfusion group. Comorbidities and hospital stay found more in red blood cell transfusion group.
References
- Strauss RG, Simon TL, Snyder EL, et al. Blood component transfusions for infants. Rossi's Principles of Transfusion Medicine. 4th ed. United States of America: Blackwell Publishing Ltd. (2009): 470-481.
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and world wide estimates of preterm birth rates in the year 2010 with time trends since1990 for selected countries: a systematic analysis and implications. Lancet 379 (2012): 2162-2172.
- Howson C, Kinney M, Lawn J. World Health Organization, March of Dimes, PMNCH, Save the Children:Born too soon: the global action report on preterm birth. In The global action report on preterm birth, WHO. Geneva (2012).
- Oestergaard MZ, Inoue M, Yoshida S, et al. Neonatal Mortality Levels for 193 Countries in 2009 with Trends since 1990: a Systematic Analysis of Progress, Projections,and Priorities. PLoSMed 8 (2011).
- Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF: Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trendssince 2000. Lancet, 379 (2012): 2151-2161.
- Beck S, Wojdyla D, Say L, et al. Van Look PF: The worldwide incidence of preterm birth: asystematic review of maternal mortality and morbidity. Bulletin of the world health organization 88 (2010): 1-80.
- World Health Organization. Coverage of maternity care. A listing of available information. Geneva: World Health Organization (1997).
- Lawn JE, Gravett MG, Nunes TM, et al. Global report on preterm birth and stillbirth (1 of 7) definitions, description of the burden and opportunities to improve data. British Medical Centre Pregnancy Childbirth, 10 (2010).
- Lawn JE, Cousens S. Zupan, J. 4 million neonatal deaths: when? Where? Why?. Lancet 365 (2005): 891-900.
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371 (2008): 261-269.
- Madan A, Kumar R, Adams MM, et al. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. Journal of Perinatology 25 (2005): 21-25.
- Lin JC, Strauss RG, Kulhavy JC, et al. Phlebotomy over draw in the neonatal intensive care nursery. Pediatrics 106 (2000).
- Strauss RG, Simon TL, Snyder EL, et al. Blood component transfusions for infants. Rossi's Principles of Transfusion Medicine. 4th ed. United States of America: Blackwell Publishing Ltd (2009): 470-481.
- Whyte RK, Kirpalani H, Asztalos EV, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics 123 (2009): 207-213.
- Strauss RG. Transfusion therapy in neonates. American Journal of Disease & Childhood 145 (1991): 904-911.
- New HV, Stanworth SJ, Engelfriet CP, et al. Neonatal transfusions. Vox Sang 96 (2009): 62-85.
- Ho J, Sibbald W J, Chin Y I. Effects of storage on efficacy of red cell transfusion: when is it not safe?. Critical Care of Medicine 31 (2003): 687-697.
- Keir A K, McPhee A J, Andersen C C, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatric Research 73 (2013): 75-79.
- Chenell Donadee, Nicolaas J H Raat, Tamir Kanias, et al. Nitric oxides cavenging by red blood cell microparticles and cell free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124, (2011): 465-476.
- Tinmouth A. Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion 46 (2006): 2014-2027.
- Somani A, Steiner ME, Hebbel RP. The dynamic regulation of microcirculatory conduit function: features relevant to transfusion medicine. Transfusion and Apherresis Science 43 (2010).
- La Gamma EF, Blau J. Transfusion-related acute gut injury: feeding, flora, flow, and barrier defense. Seminars in Perinatology 36 (2012): 294-305.
- Nopoulos Peg C, Conrad Amy L, Bell Edward F, et al. Long-term Outcome of Brain Structure in Premature Infants: Effects of Liberal vs Restricted Red Blood Cell Transfusion. Archieve of Pediatrics and Adolescent Medicine 165 (2011): 443-450.
- Eicher C, Seitz G, Bevot A, et al. The ‘Effects of transfusion thresholds on neurocognitive outcome of extremely low birth-weight infants (ETTNO)’study: back-ground, aims, and study protocol. Neona-tology 101 (2012): 301-305.
- Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity?. Medical Hypotheses, 66 (2006): 355-364.
- Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Ameri-can Journal of Perinatology, 23 (2006): 451-458.
- Fortes Filho JB, Eckert GU, Procianoy L, et al. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye 23 (2009): 25-30.
- Dos Santos AM, Guinsburg R, de Almeida MF, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. Journal of Pediatrics 159 (2011): 371-376.
- Keir A K, McPhee A J, Andersen C C, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatric Research 73 (2013): 75-79.
- Wang M, Audi G, Kondev F G, et al. The AME2016 atomic mass evaluation. Chin. Phys. 41 (2017):
- Chirico F, Sirmon D G, Sciascia S, et al. Resource orchestration in family firms: Investigating how entrepreneurial orienta-tion, generational involvement, and partici-pative strategy affect performance. Strategic Entrepreneurship Journal, 5 (2011): 307-326.
- Kena G, Aud S, Johnson F, et al. The condition of education 2014. NCES (2014): 2014-2083
- Alkire S, Roche J M, Santos M E, et al. Multidimensional poverty index 2011: brief methodological note (2011).
- Nordqvist M, Sharma P, Chirico F. Family firm heterogeneity and governance: A configuration approach. Journal of Small Business Management 52 (2014): 192-209.