Proposed Mechanisms of Heart Failure Developing in SARS-CoV-2 Infected Patients
Article Information
Iwan Cahyo Santosa Putra1, Joshua Henrina1, Hoo Felicia Hadi Gunawan1, Irvan Cahyadi1, Leonardo Paskah Suciadi2*
1Research Assistant, Siloam Heart Institute, Siloam Hospitals Kebon Jeruk, Indonesia
2Cardiologist, Siloam Heart Institute, Siloam Hospitals Kebon Jeruk, Indonesia
*Corresponding author: Leonardo Paskah Suciadi, Siloam Heart Institute, Siloam Hospitals Kebon Jeruk, Indonesia
Received: 23 April 2020; Accepted: 02 May 2020; Published: 13 May 2020
Citation: Iwan Cahyo Santosa Putra, Joshua Henrina, Hoo Felicia Hadi Gunawan, Irvan Cahyadi, Leonardo Paskah Suciadi. Proposed Mechanisms of Heart Failure Developing in SARS-CoV-2 Infected Patients. Cardiology and Cardiovascular Medicine 4 (2020): 176-190.
Share at FacebookAbstract
Coronavirus Disease of 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). On March 12, 2020, the World Health Organization (WHO) announced the pandemic state of COVID-19. Later, this disease has already known to affect multiple organs, including the heart. Therefore, higher morbidity and mortality occurred in elderly and patients with combined comorbidities. Based on the source of insults of heart failure risk, it can be classified into extrinsic and intrinsic risk. Extrinsic risks are anything that is related other than the virus itself, particularly treatments that are needed to deal with COVID-19 but subsequently affect the cardiovascular system. In contrast, the intrinsic risks are anything related to SARS-CoV-2 infection and its impact on the cardiovascular system. Furthermore, the pathophysiology of heart failure pertaining to cardiac output can be divided into three components, which are preload, myocardial contractility, and afterload. These components do not exclusively affect the left ventricle function but also the right ventricle. In addition, the risks of heart failure due to SARS-CoV2 infection exist both in the short and long term, based on their time onset. In this literature review, we propose the pathophysiology of the alteration of each of these components, which ultimately ends in heart failure.
Keywords
COVID-19; SARS-CoV-2; Heart failure; Preload; Contractility; Afterload
COVID-19 articles, SARS-CoV-2 articles, Heart failure articles, Preload articles, Contractility articles, Afterload articles
Article Details
Abbreviations
COVID-19 : Coronavirus Disease of 2019
SARS-CoV-2 : Severe acute respiratory syndrome coronavirus-2
WHO : World Health Organization
RV : Right ventricle
PAL : Alveolar pressure
PPL : Pleural pressure
PTP : Transpulmonary pressure
ITP : Intrathoracic pressure
mPAP : Mean pulmonary arterial pressure
LAPm : Mean left atrial pressure
PVR : Pulmonary vascular resistance
PTM : Transmural pressure
PEEP : Positive end-expiratory pressure
RAP : Right atrial pressure
ARDS : Acute respiratory distress syndrome
hsTnI : High-sensitivity cardiac troponin I
NT-proBNP : N-Terminal pro B-type Natriuretic Peptide
CK-MB : Creatine kinase myocardial band
AKI : Acute kidney injury
ACE2 : Angiotensin converting enzyme-2
EMB : Endomyocardial biopsies
CRP : C-reactive protein
NF-kB : Nuclear factor kB
AT1R : Angiotensin II type I receptor
CSS : Cytokine storm syndrome
BNIP3 : BCL2 adenovirus E1B 19 kDa protein-interacting protein 3
HIF-1 : Hypoxia-inducible factor-1
MI : Myocardial infarction
1. Introduction
The World Health Organisation (WHO) has announced the pandemic of Coronavirus Disease of 2019 (COVID-19) on March 12, 2020 [1]. It is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Based on the symptomatology of COVID-19, it is divided into several clinical manifestations according to its continuum, specifically the initial phase, acceleration phase, and recovery phase. In the acceleration phase, there are damages to multiple target organs (heart, lungs, gastrointestinal) and cytokine storm [2]. Notably, the cardiac involvement in SARS-CoV-2 infection can have multiple forms, such as cardiac injury, arrhythmia, or hemodynamic instability, all of which can subsequently lead to heart failure [3]. In addition, based on single-center case series and observational cohort study, cardiovascular diseases in COVID-19 patients are rather prevalent, 8% [4] and 15% [5], respectively.
The underlying pathogenesis of heart failure is the defects in one or more components pertaining to cardiac output, which are preload, afterload, and myocardial contractility. Meanwhile, there are myriad etiologies for these defects, such as myocardial injury, coronary artery disease, cardiomyopathies, and valvular heart disease [6]. In the setting of SARS-CoV-2 infection, the known mechanism of heart failure is thought to be caused by myocarditis [7]. However, the comprehensive pathogenesis is still unknown. Therefore, we would like to propose the pathogenesis of heart failure from the source of the insults, which are extrinsic and intrinsic insults and based on the preload, contractility, and afterload point of view.
2.Preload
2.1. Extrinsic risk of heart failure in SARS-CoV2 infected patients
A substantial number of patients with severe and critical SARS-CoV2 infection need to be intubated due to impending or ongoing respiratory failure [8]. The lung function of these patients is replaced transiently with the use of mechanical ventilation. While it is meant to be life-saving, there are cardiac consequences of mechanical ventilation usage, particularly to the right ventricle (RV) [9]. To clearly understand these impacts, we must understand the essential components of heart and lung physiology in the setting of mechanical ventilation.
These components consist of lung and thorax intracavitary components; alveolar, pleural, transpulmonary, and intrathoracic pressure (PAL, PPL,, PTP, and ITP).9 Transpulmonary pressure (PTP) is the product of alveolar minus pleural pressure [10]. Meanwhile, ITP is also equal to pleural pressure, and its pressure is exerted to all of the intrathoracic organs because they are encased within the rigid chest wall [11–13]. There are also the blood flow components to the lung, which are mean pulmonary arterial pressure (mPAP) and mean left atrial pressure (LAPm) as the driving pressure and pulmonary vascular resistance (PVR) [14]. Another component to account is the transmural pressure (PTM) of the blood vessel, which is the outward pressure exerted by the blood [9].
To simplify, when a patient is on mechanical ventilation with positive end-expiratory pressure (PEEP), the PPL, which physiologically is negative, becomes positive—note that ITP is equal to the PPL [15]. Therefore, the PTM of thoracic blood vessels, vena cava, and thoracic aorta are diminished by ITP. Also, ITP is transmitted to the right atrium through the pericardium and increases the right atrial pressure (RAP) [16,17]. Consequently, both aforementioned factors reduce the venous return to the right atrium and right ventricle, hence the reduced preload.
3. Contractility
3.1. Extrinsic risk of heart failure in SARS-CoV2 infected patients
As for now, no proven effective therapies for this virus currently exist. Knowledge regarding SARS-CoV-2 virology that is expanding rapidly provides a significant number of potential drug targets. The data and/or recommendations are antimalarial drugs, anti-viral, anti-inflammatory drugs, and other miscellaneous treatments/drugs [18].
Chloroquine and hydroxychloroquine seem to be able to block viral entry into cells by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification. These agents, through attenuation of cytokine production and inhibition of autophagy and lysosomal activity in host cells, appear to also have immunomodulatory effects [18].
Chinese experts reported chloroquine was successfully used to treat a series of more than 100 COVID-19 cases resulting in improved radiologic findings, enhanced viral clearance, and reduced disease progression [19]. However, the clinical trial design and outcomes data have not yet been presented, preventing validation [18]. They recommended chloroquine phosphate tablets, at a dose of 500 mg twice per day for 10 days, for patients diagnosed as mild, moderate and severe cases of SARS-CoV-2 pneumonia, provided that there were no contraindications to the drug [19].
Gautret et. al, in an open-label nonrandomized French study of 36 patients (20 in the hydroxychloroquine group and 16 in the control group) reported improved virologic clearance with hydroxychloroquine, 200 mg, every 8 hours compared with control patients receiving standard supportive care. Depending on their clinical presentation, the authors also reported an addition of azithromycin to the treatment, which was found to be significantly more superior for virus elimination [20].
Chloroquine or hydroxychloroquine-related cardiac disorder is a rare but severe adverse event, which can lead to death. A systematic review by Chatre et.al, regarding cardiac complication of chloroquine and hydroxychloroquine found conduction disorders were the main side effect reported, affecting 85% of patients. Other non-specific adverse cardiac events included ventricular hypertrophy (22%), hypokinesia (9.4%), heart failure (26.8%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%). For 78 patients reported to have been withdrawn from treatment, some recovered normal heart function (44.9%), while for others progression was unfavorable, resulting in irreversible damage (12.9%) or death (30.8%) [21].
Blignaut et.al, studied ex vivo chloroquine treatment on heart function and glucose uptake, mitochondrial function, and in vivo treatment on heart function. The mechanism suggested for this cardiotoxicity is in short and long term. Short-term blockage of the K+ channels and accumulation of Ca2+ can result in decreased heart contractility function, tachycardia and prolongation of the QT interval [22]. In the long term, accumulation of metabolic products and autophagy suppression can result in mitochondrial dysfunction [22].
Proper mitochondrial function is necessary in tissues and organs that are of high energy demand, including the heart. Mitochondrial impairment is usually also found in cardiovascular disease. Enhanced production of reactive oxygen species, depletion of cell ATP pool, extensive cell damage, and apoptosis of cardiomyocytes would happen due to uncoupling of the electron transport chain in dysfunctional mitochondria. Mitophagy is a process, during which cells clear themselves from dysfunctional and damaged mitochondria using an autophagic mechanism. Deregulation of this process in the failing heart, accumulation of dysfunctional mitochondria worsens things even more [23]. This dysfunction plays an important role in cardiovascular homeostasis and insulin signalling in myocardial metabolism and leads to signi?cant cardiac manifestations including cardiomyopathy with concentric hypertrophy and conduction disorders, and subsequent heart failure [22,24].
It is recommended that routine electrocardiography is done to rule out the development of QT interval prolongation or bradycardia and patient interviews to seek the appearance of visual and/or mental disturbance/deterioration [18]. Administration of other drugs known to prolong the QT interval such as quinolones, ondansetron, various anti-arrhythmic agents (especially Amiodarone), antidepressant, as well as antipsychotic drugs should also been taken into precaution [21].
Pneumonia in itself has various side effects on the cardiovascular system [25]. SARS-CoV-2 also has been thought to have the ability to cause direct myocardial injury and dysfunctions [26–29]. The use of chloroquine and hydroxychloroquine in this setting needs to be reconsidered, especially in combination with azithromycin, an agent which also prolongs the QT interval, and also in patients with cardiovascular comorbidities [18,30].
3.2. Intrinsic risk of heart failure in SARS-CoV2 infected patients
The pathophysiology of heart failure may result from impaired contractility which can be caused by myriad cardiac insults such as myocardial injury, cardiomyopathies, or valvular heart disease [31,32]. In the setting of SARS-CoV-2 infection, myocardial injury plays a key role as an etiology of heart failure, shown by the increased of several cardiac biomarkers, such as high high-sensitivity cardiac troponin I (hsTnI), n-terminal pro b-type natriuretic peptide (NT-proBNP), creatine kinase myocardial band (CK-MB), and myoglobin [33–35]. In three descriptive studies of patients hospitalized with laboratory-confirmed COVID-19, the incidence rates of myocardial injury are 7%, 10%, 20%, and 28%, respectively [5,36–38]. Meanwhile, the in-hospital mortality rate of COVID-19 patients with myocardial injury are 51% and 60% [37,38]. In contrast the in-hospital mortality rate of patients without myocardial injury are 4,5% and 9% [37,38].
The epidemiological profile of COVID-19 patients who are at increased risk of myocardial injury are the older men with higher prevalence of comorbidities than those who are younger and without comorbidities [37,38]. Also, myocardial injury is associated with more severe SARS-CoV2 infection, manifested with higher incidences of ARDS, acute kidney injury (AKI), electrolyte disturbances, hypoproteinemia and coagulation disorders [39].
The mechanism of acute myocardial injury caused by SARS-CoV-2 infection might be related to angiotensin converting enzyme-2 (ACE2). Angiotensin converting enzyme-2 is ubiquitously expressed in human organs, such the heart, blood vessels, kidneys, and intestines [40]. As of today, there are no evidence that shows SARS-CoV-2 could directly infect cardiomyocytes, but there are two case reports of fulminant myocarditis in a patient with a positive COVID-19 based on sputum testing from Wuhan and Italy. However, endomyocardial biopsies (EMB) are not performed [26,27,41].
In mice infected with human strain of SARS-CoV1, viral entry via ACE2 causes down regulation of myocardial ACE2 levels and induces myocardial inflammation and damage [42]. The SARS-CoV appears to directly infect the myocardial cells. In a retrospective case series, SARS-CoV viral RNA was detected in 35% of autopsied hearts [43]. Derived from recent study, in comparison to SARS-CoV 1, the affinity of SARS-CoV-2 binding to ACE2 is 10-fold to 20-fold higher [44]. Thus, we suggest that SARS-CoV-2 may cause a direct viral infection to cardiomyocytes and vascular endothelium through its binding with ACE-2. SARS-CoV-2 infection also downregulates ACE-2, whereas this enzyme is responsible for converting angiotensin II to angiotensin (1-7), a cardiac tissue protector. This agent possesses anti-inflammatory, anti-fibrosis, anti-oxidant, and vasodilative properties [45,46]. Therefore, reduced ACE2- related signalling pathways also plays a role in myocardial injury.
Furthermore, when myocardial injury presents in COVID-19 patients, they also demonstrate severe systemic inflammation, reflected in greater leukocyte counts, higher levels of C-reactive protein (CRP), and procalcitonin [37,38]. Based on several studies, angiotensin II is the responsible agent that cause proinflammatory effects in the setting of SARS-CoV 2 infection [47,48]. Angiotensin II mediated by angiotensin II type I receptor (AT1R) will activate transcription factors, primarily the nuclear factor kB (NF-kB) and activating protein-1 (AP-1). Both increase the adhesion and accumulation of monocytes and neutrophils to endothelial and mesangial cells, which release proinflammatory cytokines (interleukin-1, interleukin-6 and interleukin-12) and chemokines (interleukin-8, monocyte chemoattractant protein-1, and IP-10). All of these culminates in cytokine storm syndrome (CSS) and precipitates myocardial cells apoptosis and necrosis [33,47–49].
Acute inflammatory responses can also lead to ischemia in the presence of preexisting cardiovascular diseases. This is explained by the exacerbation of inflammatory activity within coronary atherosclerotic plaques during systemic inflammatory response, making them prone to rupture [50,51]. Furthermore, systemic inflammation also causes endothelial dysfunction, increases the procoagulant activity of the blood and endothelin-1 levels, promotes smooth muscle cell proliferation, platelet aggregation and fibrin deposition in atherosclerotic plaques, which can contribute to the formation of an occlusive thrombus over a ruptured coronary plaque [51-57]. Consequently, this sequence will result in type 1 myocardial infarction (MI).
Another mechanism responsible for heart failure incidence in SARS-CoV2 infected patients is type 2 myocardial infarction due to demand ischemia caused by respiratory dysfunction and hypoxia [31,32,58,59]. Hypoxia can induce intracellular acidosis through anaerobic metabolism which results in lactic acid accumulation [60]. This state activates transcription and translation of the mRNA and protein of death-promoting BCL2 adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) gene, respectively, and mediated by hypoxia-inducible factor-1 (HIF-1) site. Furthermore, acidosis activates BNIP3 by promoting membrane translocation and stabilizing it. This results in myocardial cell death [60,61].
In conclusion, intrinsic myocardial injury in COVID-19 patients can occur via hyperinflammatory syndrome, direct viral infection, ACE2 downregulation, and type 1 or type 2 MI.
4. Afterload
4.1. Extrinsic risk of heart failure in SARS-CoV2 infected patients
For brief explanations regarding heart and lung interaction in the setting of mechanical ventilation, see above. Mechanical ventilation also affects the right ventricular afterload. With the positive PPL, the transmural pulmonary artery reflected as mPAP will be reduced, and concurrently the pulmonary vascular resistance also increases. These result in increased right ventricular afterload [15].
In contrast with the left ventricle, which is a pressure generator with higher contractility reserves, the right ventricle is a flow generator with low contractility reserves due to highly compliant pulmonary vessels at low pressure [62,63]. These explain the sensitivity of the right ventricular afterload to cyclic tidal inflation and may result in right ventricular failure, especially in the setting of pre-existing right ventricular dysfunction or exaggerated pulmonary vascular constriction due to acute respiratory distress syndrome (ARDS) [15,64–66], which is not uncommon in COVID-19 [8,67].
4.2 Intrinsic risk of heart failure in SARS-CoV2 infected patients
SARS-CoV-2 infection can cause severe lung injury leading to ARDS, which is present in 37% of COVID-19 patients who are hospitalized [69]. SARS-CoV-2 is thought to cause downregulation of ACE-2 in the lungs. This can increase vascular permeability and inflammation in the lungs, thereby reducing lung function [70]. In one case report of Chinese COVID-19 patients, pathological examination of lung tissue found pneumococcal desquamation and hyaline membrane formation indicating the occurrence of ARDS [71].
The severe pulmonary injury also can cause pulmonary vascular changes in the form of vascular remodelling, pulmonary vascular endothelial dysfunction, and increased vascular tone [72]. These conditions occur as a result of immune dysregulation that causes proinflammatory cytokines (interleukin-6 and tumor necrosis factor - alpha) and growth factors (platelet-derived growth factors, epidermal growth factors, or vascular endothelial growth factors) that cause endothelial dysfunction, endothelial cell proliferation, and vascular smooth muscle [73,74].Pulmonary vascular remodelling can precipitate pulmonary hypertension characterized by mean pulmonary arterial pressure of equals to or more than to 25 mmHg in resting conditions [75].
Ultimately, pulmonary hypertension due to pulmonary vascular remodelling increased the RV afterload, and this leads to an increase in RV wall stress. Based on Laplace’s law, the primary compensation of myocardial stress is through myocardial hypertrophy [76]. Whereby an increased RV wall stress causes an increase in oxygen demand which in the end affects the RV contractility [77]. This vicious cycle finally stops with right ventricular heart failure ensue [78].
5. Future Implications SARS-CoV2 Infected Patients
While there is strong evidence to support the acute cardiovascular complications, including cardiac injury caused by COVID-19, conversely, there is no evidence to support the long term cardiovascular complications caused by this virus. However, a metabolomic study conducted by Wu et al., revealed patients that have recovered from SARS-CoV infection from the SARS 2009 outbreak, are still experiencing abnormality in their lipid metabolism after 12 years [79]. Furthermore, based on clinical questionnaires and examination, these patients in comparison with the control group, had experienced various diseases, including cardiovascular disease [79].
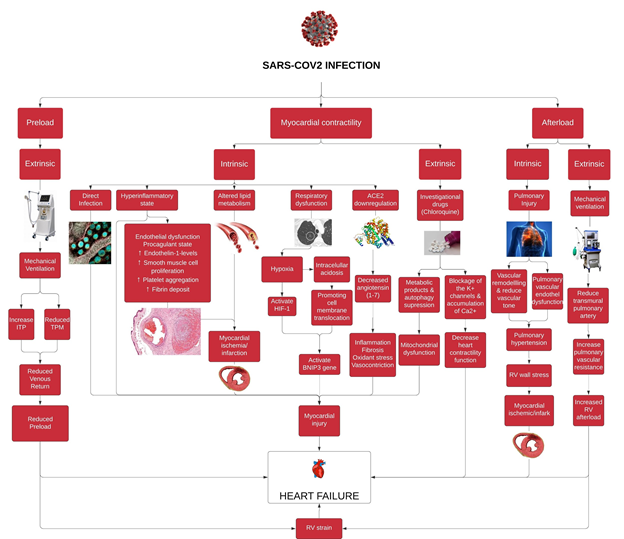
Also, based on a matched cohort study from two population-based, multicenter, observational cohorts (Atherosclerosis Risk in Communities Study; ARICS and Cardiovascular Health Study; CHS) consisted of 3813 subjects (1271 and 2542 cases participant, respectively), there is a significant increased short and long term risk of cardiovascular disease, with the highest risk in the first year after pneumonia hospitalization [80] (Figure 1).
Figure 1: This is a schematic diagram of proposed pathophysiology of heart failure in SARS-CoV2 Infected Patients. SARS-CoV-2: Severe acute respiratory syndrome - coronavirus 2, ITP: Intrathoracic pressure, TPM: Transpulmonary pressure, HIF-1: Hypoxia inducible factor-1; BNIP3: BCL2 and adenovirus E1B 19-kDa interacting protein 3; ACE2: Angiotensin converting enzyme 2, RV: Right ventricle
6. Conclusion
The SARS-CoV2 infection is a global public health concern as it becomes a pandemic. Despite the respiratory system being the major organ disrupted by this infection, currently it is also considered that this virus affects multiple organs including the heart. Heart failure is one of the commonest sequences of this disease, however little is known regarding its pathogenesis. As we have proposed in the aforementioned paragraphs, the risk of heart failure in COVID-19 patients can be distinguished from the source of insults as extrinsic or intrinsic. Both of which can cause significant alterations in preload, myocardial contractility, and afterload components, then subsequently leading to cardiac output disturbance.
Based on the preload component, mechanical ventilation appears to reduce the preload, particularly the right ventricle. Meanwhile, in the myocardial contractility component, several extrinsic insults are caused by the usage of investigational drugs. In addition, intrinsic insults consisted of hyperinflammatory state, direct infection, ACE2 downregulation, hypoxemia, altered lipid metabolism. Finally, extrinsic factors such as mechanical ventilation and intrinsic factors including pulmonary hypertension due to acute lung injury and ARDS can increase afterload, specifically the right ventricle.
7. Acknowledgment
None
8. Funding
None
9. Conflict of interest
The authors declare that there is no conflict of interest
10. Authors contribution
Iwan Cahyo Santosa Putra, Joshua Henrina, Hoo Felicia Hadi Gunawan, and Irvan Cahyadi contributed to conception and design, literature research, and drafting the manuscript. Iwan Cahyo Santosa Putra also provides revision of the manuscript. Leonardo Paskah Suciadi contributed in revision of the manuscript, supervision, and final approval of the manuscript.
References
- WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2020 Apr 13]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis 2020: 7.
- ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic [Internet]. [cited 2020 Apr 23]. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl 395 (2020): 1054–62.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323 (2020): 1061-9.
- Reddi B a. J, Shanmugam N, Fletcher N. Heart failure—pathophysiology and inpatient management. BJA Educ 17 (2017): 151–60.
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020
- Grübler MR, Wigger O, Berger D, Blöchlinger S. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly 2017.
- Rahn H, Otis AB, Chadwick LE, Fenn WO. The pressure-volume diagram of the thorax and lung. Am J Physiol-Leg Content 146 (1946): 161–78.
- Holt J. P., Rhode E. A., Kines Helga, Ruth Hawkins. Pericardial and Ventricular Pressure. Circ Res 8 (1960): 1171–81.
- Tyberg JV, Smith ER. Ventricular diastole and the role of the pericardium. Herz 15 (1990): 354–61.
- Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJAM, et al. Mechanical Ventilation–Induced Intrathoracic Pressure Distribution and Heart-Lung Interactions. Crit Care Med 42 (2014): 1983–90.
- Naeije R. Pulmonary vascular resistance: A meaningless variable? Intensive Care Med 29 (2003): 526–9.
- Vieillard-Baron A, Loubieres Y, Schmitt J-M, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 87 (1999): 1644–50.
- Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part I: basic concepts. Intensive Care Med 35 (2009): 45–54.
- Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part II: practical implications in critical care. Intensive Care Med 35 (2009): 198–205.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA [Internet]. 2020 [cited 2020 Apr 19]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2764727
- Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chin J Tuberc Respir Dis 43 (2020): 185–8.
- Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 20 (2020): 105949.
- Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers Y-M. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018;41:919–31.
- Blignaut M, Espach Y, van Vuuren M, Dhanabalan K, Huisamen B. Revisiting the Cardiotoxic Effect of Chloroquine. Cardiovasc Drugs Ther 33 (2019): 1–11.
- Chistiakov DA, Shkurat TP, Melnichenko AA, Grechko AV, Orekhov AN. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med 50 (2018): 121–7.
- Yogasundaram H, Hung W, Paterson ID, Sergi C, Oudit GY. Chloroquine-induced cardiomyopathy: a reversible cause of heart failure. ESC Heart Fail 5 (2018): 372–5.
- Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. The Lancet 381 (2013): 496–505.
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020.
- Zeng JH, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, et al. First Case of COVID-19 Infection with Fulminant Myocarditis Complication: Case Report and Insights [Internet]. LIFE SCIENCES; 2020 Mar [cited 2020 Apr 18]. Available from: https://www.preprints.org/manuscript/202003.0180/v1
- Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J 2020.
- Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020.
- Geng Y-J, Wei Z-Y, Qian H-Y, Huang J, Lodato R, Castriotta RJ. Pathophysiological Characteristics and Therapeutic Approaches for Pulmonary Injury and Cardiovascular Complications of Coronavirus Disease 2019. Cardiovasc Pathol. 2020: 107228.
- Douglas P Zipes, Peter Libby, Robert O Bonow, Douglas L Mann, Gordon F Tomaselli. Braunwald’s Heart Disease A Textbook of Cardiovascular Medicine. Eleventh. Elsevier 2019.
- Leonard S. Lilly. Pathophysiology of Heart Disease A Collaborative Project of Medical Students and Faculty. fifth. Wolters Kluwer 2011.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl 395 (2020): 497–506.
- Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 48 (2020): E008.
- Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. Journal of Medical Virology 2020.
- Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation 2020.
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020.
- Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiology
- Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol 2020.
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020.
- Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020.
- Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39 (2009): 618–25.
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289 (2003): 2801–9.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367 (2020): 1260–3.
- Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 169 (2013): 477–92.
- Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 107 (2016): 154–62.
- Wong CK, Lam CWK, Wu AKL, Ip WK, Lee NLS, Chan IHS, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136 (2004): 95–103.
- Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol 115 (2020): 31.
- Wang X, Khaidakov M, Ding Z, Mitra S, Lu J, Liu S, et al. Cross-talk between inflammation and angiotensin II: studies based on direct transfection of cardiomyocytes with AT1R and AT2R cDNA. Exp Biol Med Maywood NJ 237 (2012):1394–401.
- Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J 34 (2007): 11–8.
- Haidari M, Wyde PR, Litovsky S, Vela D, Ali M, Casscells SW, et al. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis 208 (2010): 90–6.
- Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet Lond Engl 381 (2013): 496–505.
- Birck MM, Pesonen E, Odermarsky M, Hansen AK, Persson K, Frikke-Schmidt H, Heegaard PM, Liuba P. Infection-induced coronary dysfunction and systemic inflammation in piglets are dampened in hypercholesterolemic milieu. American Journal of Physiology-Heart and Circulatory Physiology 300 (2011): H1595-601.
- Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107 (2003): 762–8.
- Modica A, Karlsson F, Mooe T. Platelet aggregation and aspirin non-responsiveness increase when an acute coronary syndrome is complicated by an infection. J Thromb Haemost JTH 5 (2007): 507–11.
- Schuetz P, Christ-Crain M, Zimmerli W, Mueller B. Repeated measurements of endothelin-1 precursor peptides predict the outcome in community-acquired pneumonia. Intensive Care Med 37 (2011): 970–80.
- Fan J, Unoki H, Iwasa S, Watanabe T. Role of endothelin-1 in atherosclerosis. Ann N Y Acad Sci 902 (2000): 84–93.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 Apr 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK554776/
- Räsänen J, Nikki P, Heikkilä J. Acute myocardial infarction complicated by respiratory failure. The effects of mechanical ventilation. Chest 85 (1984): 21–8.
- Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci 99 (2002): 12825–30.
- Graham RM, Frazier DP, Thompson JW, Haliko S, Li H, Wasserlauf BJ, et al. A unique pathway of cardiac myocyte death caused by hypoxia–acidosis. J Exp Biol 207 (2004): 3189–200.
- Magder S. The left heart can only be as good as the right heart: determinants of function and dysfunction of the right ventricle. Crit Care Resusc J Australas Acad Crit Care Med 9 (2007): 344–51.
- Pinsky MR, Desmet J-M, Vincent JL. Effect of Positive End-expiratory Pressure on Right Ventricular Function in Humans. Am Rev Respir Dis 146 (1992): 681–7.
- Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 42 (2016): 739–49.
- Berger D, Bloechlinger S, Takala J, Sinderby C, Brander L. Heart–lung interactions during neurally adjusted ventilatory assist. Crit Care 18 (2014): 499.
- Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 42 (2016): 862–70.
- Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020: 200490.
- Benson H, Akbarian M, Adler LN, Abelmann WH. Hemodynamic effects of pneumonia: I. Normal and hypodynamic responses. The Journal of clinical investigation 49 (1970): 791-8.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020.
- Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin 2020.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8 (2020): 420–2.
- Price LC, McAuley DF, Marino PS, Finney SJ, Griffiths MJ, Wort SJ. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol-Lung Cell Mol Physiol 302 (2012): L803–15.
- Rabinovitch Marlene, Guignabert Christophe, Humbert Marc, Nicolls Mark R. Inflammation and Immunity in the Pathogenesis of Pulmonary Arterial Hypertension. Circ Res 115 (2014): 165–75.
- Huertas Alice, Perros Frédéric, Tu Ly, Cohen-Kaminsky Sylvia, Montani David, Dorfmüller Peter, et al. Immune Dysregulation and Endothelial Dysfunction in Pulmonary Arterial Hypertension. Circulation 129 (2014): 1332–40.
- Ryan JJ, Thenappan T, Luo N, Ha T, Patel AR, Rich S, et al. The WHO Classification of Pulmonary Hypertension: A Case-Based Imaging Compendium: Pulm Circ 2 (2012): 107-21.
- Westerhof BE, Saouti N, van der Laarse WJ, Westerhof N, Vonk Noordegraaf A. Treatment strategies for the right heart in pulmonary hypertension. Cardiovasc Res 113 (2017): 1465–73.
- Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res 113 (2017): 1474–85.
- Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol 73 (2019): 1463–82.
- Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci Rep 7 (2017): 1-2.
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C-CH, et al. Association Between Hospitalization for Pneumonia and Subsequent Risk of Cardiovascular Disease. JAMA 313 (2015): 264–74.