Prevalence and Prognosis of Familial Hypercholesterolemia in Acute Coronary Syndromes Patients
Article Information
Alberto Corderoa,b*, Rosa Mª, Lidónc, Almudena Castrod, Lorenzo Fácilae, Julio Nuñez f,b, José Mostazag, José Ramón González-Juanateyh,b
aCardiology Department, Hospital Universitario de San Juan, Alicante, Spain
bCentro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Spain
cCardiology Department, Hospital Valle Hebrón, Barcelona, Spain
dCardiology Department, Hospital La Paz, Madrid, Spain
eCardiology Department, Hospital General Universitario, Valencia, Spain
fCardiology Department, Hospital Clínico Universitario, Valencia, Spain
gInternal Medicine Department, Hospital Universitario La Paz, Madrid, Spain
hCardiology Department, Complejo Hospital Universitario de Santiago, Santiago de Compostela, Spain
*Corresponding Author: Alberto Cordero, Cardiology Department, Hospital Universitario de San Juan, Alicante, Spain
Received: 03 September 2020; Accepted: 14 September 2020; Published: 18 September 2020
Citation: Alberto Cordero, Rosa M, Lidón, Almudena Castro, Lorenzo Fácila, Julio Nuñez, José Mostaza, José Ramón González-Juanatey. Prevalence and Prognosis of Familial Hypercholesterolemia in Acute Coronary Syndromes Patients. Cardiology and Cardiovascular Medicine 4 (2020): 528-539.
Share at FacebookAbstract
Background: Familiar hypercholesterolemia (FH) is genetic disease that leads to increased serum low-density lipoprotein cholesterol (LDLc) and premature acute coronary syndromes (ACS). The objective of our study was describing the prevalence and prognosis of FH, assessed by Dutch Lipid Clinic Network (DLCN) criteria, in patients with ACS.
Methods: We designed a multicentre, observational, prospective and nationwide registry of ACS patients admitted in 30 hospitals. The DLCN criteria were analysed in all patients and they were classified in:unlikely-FH (0-2 criteria), possible-FH (3-5 criteria), probable-FH (6-7 criteria) or definite-FH (>7 criteria). Premature ACS was defined if age at admission was <55 in men or <65.
Results: we included 868 patients, 72.3% males and 20.6% diabetics. Unlikely-FH accounted for 84.2% of the cohort; the prevalence of possible FH was 14.5%, probable-FH 1.1% and only 1 patient had definite-FH. The prevalence of possible or probable FH increased up to 21% in patients with premature ACS. Patients with possible/probable FH had lower mean age but more peripheral arterial disease; also, had been diagnosed more often of hypercholesterolemia and had higher LDLc and statins treatments before admission. A progressive increase in the incidence of major cardiovascular events (MACE) was observed by each increase in one DLCN criteria (OR= 1.23 95% CI 1.08-1.39) or, globally, in patients with possible/probable FH (OR: 2.31 95% CI 1.35-3.96).
Conclusions: 15.8% of patients with ACS have possible/probable FH and the prevalence increases up to 24% in patients with premature ACS. Patients with possible/probable FH have higher risk of in-hospital MACE.
Keywords
Familial hypercholesterolemia; LDLc
Familial hypercholesterolemia articles, LDLc articles
Article Details
Introduction
Familial hypercholesterolemia (FH) is a genetic condition that leads to increased serum low-density lipoprotein cholesterol (LDLc) and premature onset of coronary heart disease [1,2]. FH has an autosomal co-dominant transmission and several genetic mutations have been clearly identified [3,4] although the diagnosis by clinical criteria has an excellent correlation with genetic test [3]. The prevalence of FH has been estimated in 1/300-500 persons although recent reports have found that prevalence of probable FH can be as high as 8.3% in patients with established coronary heart disease [5,6] and even higher in patients with acute coronary syndromes (ACS) [5-8].
Most available evidence related to prevalence of FH in ACS is based on retrospective analyses of databases [5,7,8]. The objective of our study was describing the prevalence and clinical features of FH, assessed prospectively by clinical criteria, in a cohort of patients admitted for ACS.
Methods
We designed a multicentre, observational, prospective and nationwide registry of patients admitted for an ACS in 30 hospitals from Spain. The Dutch Lipid Clinic Network (DLCN) criteria were analysed in all patients and they were classified in: unlikely-FH (0-2 criteria), possible-FH (3-5 criteria), probable-FH (6-7 criteria) or definite-FH (>7 criteria) [9]. ACS was defined by presence of typical clinical symptoms of chest pain and electrocardiographic changes indicative of myocardial ischemia/lesion and/or elevation of serum markers of myocardial damage [10]. ACS was classified as ST-elevation myocardial infarction (STEMI) and non-ST elevation ACS according to the electrocardiographic findings. Mortality risk was assessed by the GRACE score [10] and patients were categorized, according to current recommendations, into low (<108), intermediate (109-139) or high risk (>140). In-hospital prognosis was assessed by the incidence of any major cardiovascular event (MACE), that included all-cause mortality, heart failure, stroke, un-planned revascularization and major bleeding.
Risk factors, clinical antecedents, treatments, complementary test and main diagnosis at discharge were collected from all patients by trained medical staff. Glomerular filtration rate was estimated from serum creatinine values with the CKD-EPI equation. For the antecedent of previous coronary heart disease patients needed to have a clinical diagnosis of myocardial infarction, stable or unstable angina or angina-driven coronary revascularization. Previous heart failure was codified if patients had at least one hospitalization with such main diagnosis at discharge-medical report as well as those with typical signs and symptoms of heart failure that had a compatible imagine diagnosis (X-ray or echocardiogram). According to their equivalencies and the 2013 ACCC/AHA guidelines [11], intensive statin treatment was considered atorvastatin 40-80 mg/day or rosuvastatin 20 mg/day; atorvastatin 20-40 mg/day, simvastatin 20-40 mg/day were considered moderate intensity regimens; atorvastatin 10 mg/day, simvastatin 10 mg/day and pravastatin 10-40 mg/day were categorized as low intensity lipid-lowering strategy. In-hospital outcome was assessed by the incidence of any major cardiovascular event (MACE), including death, heart failure, stroke or major bleeding.
The study was promoted the working groups of Preventive Cardiology and Cardiac Rehabilitation as well as Coronary Heart Disease and Acute Cardiac Care of Sociedad Española de Cardiología (Spanish Society of Cardiology) and was approved and endorsed by its Research Agency (Agencia de Investigación de la Sociedad Española de Cardiología). The study protocol and the informed consent were validated by the Ethics Committee of Hospital La Paz.
Statistical analyses
Quantitative variables are presented as mean (SD) and differences were assessed by ANOVA test. Qualitative variables are presented as percentages and differences were analysed by t-Student and Chi-square tests. Statistical difference was accepted at p<0.05. The relationship between the DLCN criteria and age or MACE was assessed by logistic binary regression, adjusted by age, gender, previous cardiovascular disease, biochemical determinations, medical treatments and revascularization; results are presented in histogram with the predicted MACE incidence. The accuracy of the model was tested by its calibration assessed by the Hosmer-Lemeshow test, and its discrimination capacity, evaluated with the area under the curve (AUC) of the probability predicted by the model. All analyses were performed using STATA 14.3 (StataCorp. 2009. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
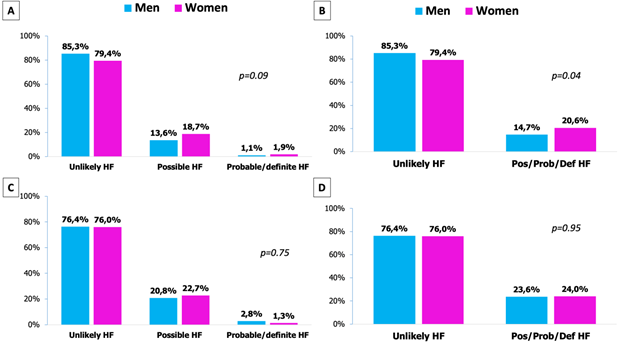
As shown in Table 1, 84.2% were unlikely to have FH, 14,5% had possible FH and 1.3% had probable or definite FH. Less than half of the cohort had previous diagnosis of dyslipidaemia but this was higher in all categories of FH; curiously, 27.3% of patients with probable or definite FH had no previous diagnosis of dislipemia. A non-significant trend to higher female gender was observed in the categories of possible and probable/definite FH; when these last two categories were analysed together, women had higher prevalence of any category of FH (Figure 1A and 1B). One third of the patients were classified as premature ACS and it was statistical and exponentially higher in all categories of FH (Figure 1C and 1D). The rate of angiography and revascularization were high; patients with possible or probable/definite FH underwent angiography more frequently but no differences were found regarding the number of vessels with significant lesions.
|
|
Total |
Unlikely FH |
Possible FH |
Probable/definite FH |
p |
|
N |
868 |
731 (84.2%) |
126 (14.5%) |
11 (1.3%) |
|
|
Age |
61.2 (11.2) |
61.9 (11.3) |
57.8 (10.3) |
54.7 (11.1) |
0.01* |
|
Females |
17.8% |
16.8% |
23.0% |
27.3% |
0.18 |
|
Body mass index |
28.4 (4.4) |
28.3 (4.5) |
28.8 (4.3) |
29.3 (4.9) |
0.88 |
|
Diabetes |
20.6% |
21.3% |
16.7% |
18.2% |
0.48 |
|
Hypertension |
48.0% |
47.6% |
50.0% |
54.6% |
0.80 |
|
Current smokers |
27.3% |
27.8% |
25.0% |
0.0% |
0.91 |
|
Dyslipidemia |
47.2% |
43.8% |
65.1% |
72.7% |
<0.01 |
|
Previous heart failure |
2.3% |
2.1% |
4.0% |
0.0% |
0.04 |
|
Previous coronary heart disease |
11.1% |
10.7% |
13.5% |
9.1% |
0.63 |
|
Peripheral arterial disease |
5.0% |
4.4% |
5.6% |
36.4% |
<0.01 |
|
Previous stroke |
4.5% |
4.7% |
3.2% |
9.1% |
0.58 |
|
COPD |
4.4% |
4.9% |
1.6% |
0.0% |
0.02 |
|
On-treatment with statin |
27.9% |
26.4% |
35.7% |
36.4% |
0.08 |
|
On-treatment with ezetimibe |
2.3% |
2.5% |
1.6% |
0.0% |
0.73 |
|
STEMI |
52.6% |
51.3% |
59.5% |
63.6% |
0.18 |
|
Premature ACS |
33.5% |
30.4% |
49.21% |
63.6% |
<0.01 |
|
GRACE score |
118.3 (26.0) |
119.8 (25.8) |
110.9 (26.2) |
112.3 (21.7) |
0.01* |
|
GRACE score >140 |
21.8% |
23.5% |
14.3% |
0.0% |
0.06 |
|
LVEF (%) |
0.55 (0.11) |
0.55 (0.10) |
0.52 (0.13) |
0.49 (0.13) |
0.06 |
|
Angiography |
89.8% |
88.5% |
96.8% |
90.9% |
0.02 |
|
Angioplasty |
82.4% |
81.4% |
88.1% |
81.8% |
0.19 |
|
Number of vessels with lesions |
1.5 (0.9) |
1.4 (0.9) |
1.6 (0.9) |
1.6 (1.0) |
0.07 |
|
Number of vessels treated |
1.1 (0.7) |
1.1 (0.7) |
1.2 (0.7) |
1.2 (0.7) |
0.59 |
|
Total cholesterol (mg/dl) |
183.8 (43.4) |
174.3 (36.5) |
227.9 (47.9) |
225.8 (37.4) |
<0.01* |
|
LDL cholesterol (mg/dl) |
114.2 (39.1) |
105.2 (31.0) |
161.5 (44.1) |
150.6 (31.1) |
<0.01* |
|
HDL cholesterol (mg/dl) |
42.6 (12.1) |
42.5 (12.3) |
42.9 (11.9) |
45.3 (7.9) |
0.73 |
|
Triglycerides (mg/dl) |
149.6 (115.7) |
148.8 (122.7) |
154.2 (73.6) |
141.5 (58.3) |
0.87 |
|
Creatinine (mg/dl) |
0.9 (0.6) |
0.9 (0.6) |
0.9 (0.3) |
0.8 (0.2) |
0.35 |
|
GFR ml/min/1.72m2 |
87.1 (20.5) |
86.1 (20.5) |
92.2 (19.9) |
94.2 (20.2) |
0.01* |
|
GFR <60 ml/min/1.72m2 |
10.1% |
10.6% |
7.6% |
10.0% |
0.62 |
|
Hemoglobin (g/dl) |
14.2 (1.7) |
14.1 (1.7) |
14.4 (1.6) |
14.5 (1.9) |
0.33 |
|
HbA1c (%) |
6.4 (3.3) |
6.4 (3.2) |
6.5 (3.5) |
5.9 (0.7) |
0.87 |
COPD: chronic obstructive pulmonary disease; FH: familial hypercholesterolemia; GFR: glomerular filtration rate;HDL: high-density lipoprotein; LDL: low-density lipoprotein; LVEF: left ventricle ejection fraction; STEMI: ST-elevation myocardial infarction; p: for the comparison between unlikely HF and the rest
Table 1: Clinical characteristics of the patients according to the DCNL criteria
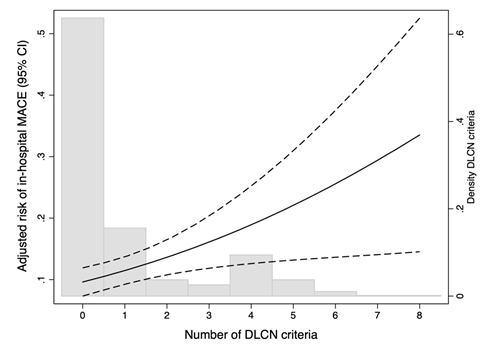
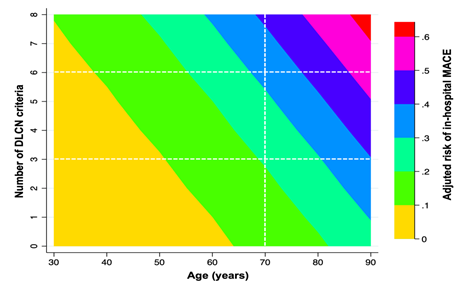
The incidence was in-hospital MACE was 10.6% (95% CI 8.6-12.7). The incidence was significantly higher (p=0.003) in each category of FH: unlikely 9.4%; possible FH 15.1%; probable/definite FH 36.4%. As shown in Figure 2, the risk of in-hospital MACE increased as the number of DLCN criteria did at any age. A progressive increase in the incidence of MACE was observed by each increase in one DLCN criteria (OR= 1.23 95% CI 1.08-1.39; p=0.002) and it was highest in patients with probable/definite FH (OR: 2.31 95% CI 1.35-3.96; p=0.001). To minimize the effect of age categorized in the DLCN criteria we also assessed the incidence of in-hospital MACE according to age, as a continuous variable. The higher presence of DLCN criteria the higher risk of MACE was observed at any age (Figure 3). Models were accurately calibrated (p=0.42) and had an excellent discriminatory capacity (AUC 0.75 95% CI 0.71-0.79).
The use of guideline-recommended clinical guidelines was very high (Table 2). Small but statistically significant differences were observed between groups regarding some medical treatments. Overall use of statins was 89.0% and most patients that did not receive statins had unlikely FH since all patients with any category of FH were discharged on statins. Similarly, the use of ezetimibe was also two-fold higher in any category of FH as compared to patients with unlikely FH.
|
|
Total |
Unlikely FH |
Possible FH |
Probable/definite FH |
p |
|
Aspirin |
87.4% |
97.6% |
97.6% |
100.0% |
0.082 |
|
Clopidogrel |
24.1% |
24.0% |
26.2% |
9.1% |
0.002 |
|
Ticagrelor |
40.2% |
40.8% |
36.5% |
45.5% |
0.001 |
|
Prasugrel |
21.3% |
19.8% |
28.6% |
27.3% |
0.001 |
|
Betablockers |
77.7% |
76.1% |
86.5% |
81.8% |
0.004 |
|
ACEI |
61.4% |
59.5% |
69.8% |
90.9% |
<0.001 |
|
ARB |
11.5% |
11.5% |
11.9% |
0.0% |
0.620 |
|
Statins |
89.0% |
87.4% |
98.4% |
100.0% |
0.004 |
|
High-dose statin |
86.6% |
84.8% |
96.8% |
90.9% |
0.001 |
|
Ezetimibe |
7.1% |
5.6% |
14.3% |
18.2% |
<0.001 |
|
Fibrates |
2.9% |
2.6% |
4.8% |
0.0% |
0.002 |
|
Oral hipoglucemiants |
17.9% |
18.1% |
16.7% |
18.2% |
0.003 |
|
Insulin |
7.3% |
7.4% |
6.4% |
9.1% |
0.003 |
|
Calcium channel blockers |
7.3% |
7.23% |
7.9% |
0.0% |
0.003 |
|
Diuretics |
15.4% |
15.3% |
15.9% |
18.2% |
0.004 |
ACEI: angiotensin-receptor enzyme-inhibitors; ARB: angiotensin receptor blocker
Table 2: Medical treatments recommended at the time of hospital discharge.
Discussion
Main results of our study reflect that 16% of patients admitted for an ACS have possible FH although roughly 2% have probable or definite FH. The prevalence of FH is higher in women or patients with premature ACS. Moreover, despite the largely lower mean age, patients with ≥3 criteria of FH have higher in-hospital MACE. This was a prospective and multicentre study specifically designed to assess the actual prevalence of FH in ACS patients and since clinical features of our population are quite similar to previous reports [5-8,12,13] we believe that our results might representative of daily clinical practice.
Low-density lipoprotein cholesterol (LDLc) levels correlate strongly and linearly with coronary heart disease incidence [14]. Serum LDLc levels are the result of the interaction between lifestyle habits and personal metabolism, mainly genetically determined [15]. FH is one of the leading causes of abnormally elevated LDLc and premature CHD [16] and, therefore, its identification in patients with ACS seems crucial because it can lead to intensive counselling and more close follow-up by specific clinical units [7,17]. Our results highlight that the overall prevalence of definitive or probable FH was low; nonetheless, 16% of patients had possible or probable FH and it reached more that 25% in the subset of premature ACS patients.
The prevalence of FH has not been widely described in ACS cohorts and, even less, assessed prospectively by current clinical criteria [12]. Clinical profile of possible FH patients was marked by much lower mean age and higher prevalence of dyslipidaemia and statin treatment before hospital admission. Premature ACS has been clearly related to smoking, diabetes, drugs abuse but also, genetic disorders. Hypercholesterolemia [18], and specifically FH, have been outlined as one its leading causes of premature ACS [17,19]. Cardiovascular disease is a relevant cause of population loss of potential years of life lost [20] and premature ACS represents a clinical challenge since these patients have longer life-long probability of recurrent events [21]. Therefore, premature ACS patients should be considered primary targets for primary and secondary prevention strategies.
One of the most relevant issues related to FH is that it usually under diagnosed or unknown by the patients [1,15]. Our results highlight that one third of the patients with possible FH had not been previously diagnosed of hypercholesterolemia despite having a mean LDLc of 161.5 (44.1) mg/dl. The population screening for cardiovascular risk factors has demonstrated to be efficient in terms of primary prevention of cardiovascular disease [15,22] and, especially, detecting patients with high levels of LDLc. The identification of FH in the setting of ACS has not been accurately investigated and since all patients should achieve the treatment target for very high-risk patients, currently recommended >50% reduction from baseline levels and target LDLc <55 mg by the ESC guidelines [15], the diagnosis of FH might seem less determinant. In contrast, the experience of specialized lipid units has provided the evidence that the accurate identification and diagnosis of FH increases patients´ adherence [16] to medication and, also, expands the early diagnosis of relatives [23]. Moreover, our results clearly highlight that the presence of clinical criteria of FH increase the probability of a MACE within the ACS hospitalization what might reflect that the clustering of FH criteria correlate with the atherosclerotic burden. The multi-territory imaging analysis have outlined that the development and progression of atherosclerosis is triggered even with non-elevated LDLc levels [24] and the effect is time-dependent18. Since patients with FH have elevated LDLc levels since they are born, they have higher burden of cardiac and non-cardiac atherosclerosis what might explain that the presence of DLCN criteria correlate with prognosis. Moreover, the risk matrix highlights that for the same given age the higher presence of DLCN criteria increased the incidence of MACE.
FH patients deserve the strongest medical treatment, especially intensive lipid-lowering strategies, but also post-ACS guideline recommended treatments [15]. Almost all patients with possible FH in our cohort received statins at the time of hospital discharged and more than 3 quarters at the highest doses. The overall statin use rate in our cohort was very high, as expected and recommended by clinical guidelines [15]. Statins were the only medical therapy that was statistically more used in patients with possible FH what agrees with previous reports [7]. Since the diagnosis of FH was assessed retrospectively, not within hospitalization, our data might reflect that clinicians were conscious of the relevant role of lipid-lowering treatment in ACS patients at younger age and elevated LDLc levels. Intensive LDLc lowering, especially with statins, has contributed to large improvements in prognosis of post-ACS patients [25,26]. Intensive LDLc control is main target for prevention in FH patients [17] and the risk of cardiovascular events remains high despite statin treatment [27,28]. Combination of lipid-lowering drugs seems to be the most attractive lipid-lowering strategy for FH patients [29] as well as post-ACS patients [26]. The safety and effectiveness of ezetimibe have also been demonstrated by the IMPROVE-IT trial [26] and it was the only drug available for statin combination at the time of inclusion period. The proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors are currently available and could be a very useful lipid-lowering strategy for patients with possible or probable FH [30]. Recent estimations have outlined that around 20% of patients with established cardiovascular disease would be candidate for PSCK-o inhibitors treatment despite optimal lipid-lowering treatment. We hope that the one-year follow-up of our study would provide reliable information related to LDLc control as well as actual prognosis in these patients.
Our study has several limitations that deserve consideration. There may be many unmeasured confounders or details about physician or patient decision-making that were not captured in our study protocol. Second, the study was based on observational, non-randomized data, and thus, associations between various treatments and outcomes may be confounded by unmeasured variables. Nonetheless, ssince clinical features and event rates were similar to other reports [5-8,12,13,16,23] we believe that this limitation should not impair the relevancy of our results.
Conclusions
Possible FH is quite prevalent in patients admitted for ACS, especially when presenting at early ages. Current smoking, previous cardiovascular disease, elevated LDLc and younger age are the most eminent features, because no differences in the extent of coronary lesions or other features were observed. The number of DLCN criteria correlated with the incidence of in-hospital MACE. Our results highlight the large prevalence of possible FH in ACS patients and should increase the awareness on clinicians of this genetic condition that leads to premature CHD and should encourage them to treat all risk factors aggressively, as well as to actively search for related potential FH patients among family members. The accurate identification of FH during the hospital stay might be critical for specific post-discharge referral to specific units, long-term maintenance of high-dose statins, and identification of family members.
Conflicts of interest
Authors declare that there are no conflicts of interest related to the results of this study.
Acknowledgements
This study received a non-restricted grant of AMGEN.
Investigators received the support from Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV).
References
- Mata P, Alonso R, Perez-Jimenez F. Deteccion de la hipercolesterolemia familiar- un modelo de medicina preventiva. Rev Esp Cardiol 67 (2014): 685-8.
- de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of Familial Hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 133 (2016): 1067-72.
- Civeira F, Ros E, Jarauta E, Plana N, Zambon D,et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol 102 (2008): 1187-93, 1193 e1.
- Gidding SS, Ann Champagne M, de Ferranti SD, Defesche J, Ito MK, et al, Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young CoC, Stroke Nursing CoFG, Translational B, Council on L, Cardiometabolic H. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation 132 (2015): 2167-92.
- De Backer G, Besseling J, Chapman J, Hovingh GK, Kastelein JJ, et al. Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis 241 (2015): 169-175.
- Faggiano P, Pirillo A, Griffo R, Ambrosetti M, Pedretti R, et al. Prevalence and management of familial hypercholesterolemia in patients with coronary artery disease: The heredity survey. International Journal of Cardiology 252 (2018): 193-198.
- Nanchen D, Gencer B, Auer R, Raber L, Stefanini GG, et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J 36 (2015): 2438-45.
- Rallidis LS, Triantafyllis AS, Tsirebolos G, Katsaras D, Rallidi M, Moutsatsou P, Lekakis J. Prevalence of heterozygous familial hypercholesterolaemia and its impact on long-term prognosis in patients with very early ST-segment elevation myocardial infarction in the era of statins. Atherosclerosis 249 (2016): 17-21.
- Damgaard D, Larsen ML, Nissen PH, Jensen JM, Jensen HK, Soerensen VR, Jensen LG, Faergeman O. The relationship of molecular genetic to clinical diagnosis of familial hypercholesterolemia in a Danish population. Atherosclerosis 180 (2005): 155-60.
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39 (2017): 119-177.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63 (2014): 2889-934.
- Dorsch MF, Lawrance RA, Durham NP, Hall AS. Familial hypercholesterolaemia is underdiagnosed after AMI. BMJ 322 (2001): 111.
- Gager GM, Jilma B, Winter M-P, Hengstenberg C, Lang IM, et al. Ticagrelor and prasugrel are independent predictors of improved long-term survival in ACS patients. European Journal of Clinical Investigation 2020: e13304.
- Ference BA, Bhatt DL, Catapano AL, Packard CJ, Graham I, et al. Association of Genetic Variants Related to Combined Exposure to Lower Low-Density Lipoproteins and Lower Systolic Blood Pressure With Lifetime Risk of Cardiovascular Disease. JAMA 322 (2019): 1381-1391.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). European Heart Journal 41 (2020): 111-188.
- Perez de Isla L, Alonso R, Watts GF, Mata N, Saltijeral Cerezo A, et al. Attainment of LDL-Cholesterol Treatment Goals in Patients With Familial Hypercholesterolemia: 5-Year SAFEHEART Registry Follow-Up. J Am Coll Cardiol 67 (2016): 1278-85.
- Civeira F, International Panel on Management of Familial H. Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis 173 (2004): 55-68.
- Navar-Boggan AM, Peterson ED, D'Agostino RB, Sr., Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 131 (2015): 451-8.
- Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 36 (2015): 2425-37.
- Bucholz EM, Normand SL, Wang Y, Ma S, Lin H, Krumholz HM. Life Expectancy and Years of Potential Life Lost After Acute Myocardial Infarction by Sex and Race: A Cohort-Based Study of Medicare Beneficiaries. J Am Coll Cardiol 66 (2015): 645-55.
- Pérez de Isla L, Arroyo-Olivares R, Alonso R, Muñiz-Grijalvo O, Díaz-Díaz JL, et al. Incidencia de eventos cardiovasculares y cambios en el riesgo estimado y en el tratamiento de la hipercolesterolemia familiar: registro SAFEHEART. Revista Española de Cardiología 2020.
- Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, et al, European Society of C. European Society of Cardiology: Cardiovascular Disease Statistics 2019. European Heart Journal 41 (2020): 12-85.
- Mulder JWCM, Galema-Boers AMH, de Jong-Verweij LM, Hazelzet JA, Roeters van Lennep JE. The development and first results of a health-related outcomes set in familial hypercholesterolemia (FH) patients: Knowledge is health. Atherosclerosis 293 (2020): 11-17.
- López-Melgar B, Fernández-Friera L, Oliva B, García-Ruiz JM, Sánchez-Cabo F, et al. Short-Term Progression of Multiterritorial Subclinical Atherosclerosis. Journal of the American College of Cardiology 75 (2020): 1617.
- Puri R, Nissen SE, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, St John J, Wolski K, Uno K, Kataoka Y, Nicholls SJ. Factors underlying regression of coronary atheroma with potent statin therapy. Eur Heart J 34 (2013): 1818-25.
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, et al. Investigators I-I. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 372 (2015): 2387-97.
- Mohrschladt MF, Westendorp RG, Gevers Leuven JA, Smelt AH. Cardiovascular disease and mortality in statin-treated patients with familial hypercholesterolemia. Atherosclerosis 172 (2004): 329-35.
- Barkas F, Elisaf M, Milionis H. Statins decrease the risk of stroke in individuals with heterozygous familial hypercholesterolemia: A systematic review and meta-analysis. Atherosclerosis 243 (2015): 60-64.
- Catapano AL, Farnier M, Foody JM, Toth PP, Tomassini JE, Brudi P, Tershakovec AM. Combination therapy in dyslipidemia: where are we now? Atherosclerosis 237 (2014): 319-35.
- Toth PP, Sattar N, Blom DJ, Martin SS, Jones SR, Monsalvo ML, Elliott M, Davis M, Somaratne R, Preiss D. Effect of Evolocumab on Lipoprotein Particles. Am J Cardiol 121 (2018): 308-314.