Presumed Ocular Tuberculosis: a Retrospective Case Review
Article Information
Jacob D. Grodsky, John Lee, Niloofar Piri*
Department of Ophthalmology, School of Medicine, Saint Louis University, USA
*Corresponding author: Niloofar Piri, Department of Ophthalmology, School of Medicine, Saint Louis University, 1225 S. Grand Blvd, Garden Level, Saint Louis, MO
Received: 07 June 2022; Accepted: 15 June 2022; Published: 29 June 2022
Citation: Jacob D. Grodsky, John Lee, Niloofar Piri. Presumed Ocular Tuberculosis: a Retrospective Case Review. Journal of Ophthalmology and Research 5 (2022): 108-116.
Share at FacebookAbstract
Despite the common misconception that tuberculosis (TB) is an uncommon disease in developed countries such as the United States; cases have become increasingly more prevalent. While 80% of patients diagnosed with TB have pulmonary findings, one common extrapulmonary presentation of TB is ocular involvement. In this retrospective case series, we present the clinical findings in nine cases of presumed ocular TB. All nine of these patients presented with an inflammatory component of their ocular TB manifestation. All patients who completed systemic TB treatment achieved quiescence of their ocular inflammation without flare up. Most of these patients had undiagnosed TB at their time of presentation, thus, highlighting the importance of further studies leading to more precise diagnostic guidelines and protocols. This will help ensure uniformity in care so that patients will be diagnosed and treated in a timely manner in order to prevent irreversible visual damage secondary to intraocular inflammation. Over the ten year time period included in this study, one-third of the patients were diagnosed over the past one year, suggesting potentially rising TB numbers in the US.
Keywords
Tuberculosis; ocular tuberculosis; inflammation; systemic treatment; extrapulmonary tuberculosis
Tuberculosis articles; ocular tuberculosis articles; inflammation articles; systemic treatment articles; extrapulmonary tuberculosis articles
Article Details
1. Intruduction
Tuberculosis (TB) is one of the most common infectious etiologies of morbidity and mortality around the world. Caused by the bacteria Mycobacterium tuberculosis, this disease is thought to have the largest impact primarily in developing countries, affecting an estimated one-third of the world’s population [1–3]. Under-reporting and lack of proper diagnosis have been cited as the major hindrances preventing proper treatment of TB. Unlike the conventional belief that TB is uncommon in the United States (US), the incidence and prevalence is increasing. This has been speculated to be partially attributed the increasing number of immigrant populations from endemic countries who pose a risk to the US population.
While commonly thought to primarily affect the pulmonary system, TB can, in fact, present in nearly any organ system [1]. It is estimated that nearly 80% of patients diagnosed with TB have pulmonary findings, while the remaining 20% have extrapulmonary findings [4]. One such organ of possible TB infiltration is the eyes. Ocular TB is thought to be caused by direct invasion of the TB bacilli or an immunogenic reaction secondary to an extraocular infection. The reported incidence of ocular TB varies depending on many factors such as patient population and diagnostic criteria. Due to the various medical specialties involved with making such a diagnosis, as well as the likely temporal gap between TB diagnosis and ocular disease diagnosis, we hypothesize that the true prevalence of ocular TB is higher than suspected. This study reports the clinical findings, diagnosis, and treatment of nine patients with presumed ocular tuberculosis at a major academic institution in the US.
2. Methods
This retrospective chart review involved investigating the charts of patients seen at Saint Louis University Hospital as well as the associated outpatient physician’s offices, SLUCare. This study and protocol were reviewed and approved by the Saint Louis University Institutional Review Board (Assurance No: FWA00005304). This study was performed in adherence with the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996.
A request was submitted to the SLU Information Technology Services department to obtain the medical record numbers of patients with tuberculosis with encounters at Saint Louis University Hospital and SLUCare and billed for service dates between January 1, 2011, and March 1, 2021. A comprehensive list of ICD-9 and ICD-10 codes for tuberculosis was provided, and IDX (GE Healthcare, Chicago, Illinois) software was utilized to identify patients seen at our institution within this timeframe who had these diagnosis codes listed as the first, second, third, or fourth diagnosis in their chart.
The initial search yielded a list of 426 patients with a history of tuberculosis. The charts of these patients were all investigated, and 351 patients were excluded due to a lack of a documented appointment with the Department of Ophthalmology in their chart. We extensively reviewed the charts of the remaining patients who were established with the Department to determine the reason for appointment, exam findings, and treatments. Patients presenting for routine eye exams or follow ups without acute ocular complaints, as well as those lost to follow up or without adequate available information regarding disease progression, were excluded. Similarly, the remaining patient charts were examined for information regarding type of tuberculosis, treatment, as well as chronological association with ocular complaints. Those whose charts lacked complete information regarding TB diagnosis and treatment course, or whose TB diagnosis and ocular disease were deemed to be unrelated, were excluded. This ultimately resulted in an additional 66 charts excluded from the study. There were nine unique patients included in this study.
Utilizing our institution’s electronic medical record (Epic Systems Corporation, Verona, Wisconsin) as well as scanned copies of charts from visits prior to its implementation, we reviewed the charts of these nine patients to obtain information from visits not only in ophthalmology but also with other specialties such as primary care, pulmonology, and infectious disease. The data collected included patient characteristics such as date of birth, sex, race, and history of immunosuppression; information regarding TB such as the date the diagnosis was first mentioned in the chart, type of TB, systemic manifestations, and treatment course; and information regarding ocular complaints such as the date of the first ophthalmology/optometry encounter after TB diagnosis, reason for the encounter, eye exam findings, diagnosis, treatment course, vision prior to and after treatment, and overall course and outcome.
Our primary objective was to identify patients with a history of tuberculosis and assess whether they had any ocular manifestations of this disease. Our secondary objective was to focus on the subset of patients with presumed ocular tuberculosis and to collect data regarding the clinical presentation and course throughout treatment to further understand this disease and identify patterns in ocular manifestations.
3. Results
As mentioned above in detail, nine patients were ultimately deemed to have presumed findings of ocular TB and were included in this study.
3.1 Patient characteristics
Age at the time of presentation with an ocular complaint ranged from 18-60 for patients included in this study (Table 1).
Table 1: Patient Demographics
Seven patients (77.8%) had tuberculosis diagnosed by a positive QuantiFERON-TB Gold (QFG) blood test. This includes one patient who also had a documented positive purified protein derivative (PPD) skin test with 15 mm induration as well as one patient with miliary disease who had a positive QFG blood test in addition to a urine culture positive for acid-fast bacillus (AFB) as well as cultures of chest wall, psoas, gluteal, and sacroiliac abscesses positive for AFB (Table 2). One patient (11.1%) had only a positive PPD skin test as their diagnostic test; however, induration measurement was unavailable in the medical chart. One patient (11.1%) had a positive sputum culture of Mycobacterium tuberculosis.
Cases 1, 5, 7, and 9 had other systemic tuberculosis involvement in addition to their ocular presentation, as demonstrated in Table 2.
Table 2: Patient Characteristics—mechanism of tuberculosis diagnosis, systemic treatment used, systemic and ophthalmic involvement, presence of immunosuppression history, and final outcome of ocular manifestations after treatment
Consistent with generally accepted standards of care, tuberculosis treatment of these patients slightly varied. Four patients (44.4%) were treated with the standard drug regimen of rifampin, isoniazid, pyrazinamide, ethambutol, and vitamin B6 (RIPE). Two patients (22.2%) were treated with isoniazid and vitamin B6 for 9 months, one patient (11.1%) received rifampin for 4 months, exact treatment regimen for one patient was unavailable as the patient received treatment as a teenager about 60 years prior to presentation, and one patient (11.1%) has refused treatment multiple times. One patient (Case 4) had an unusual treatment course. This patient was initially started on isoniazid and vitamin B6 for a planned standard 9-month treatment. Treatment was stopped after three months due to the development of a new visual field defect. The patient was then later started on rifabutin, however, after 6 weeks developed malaise, nausea, and transaminitis. Given the lack of complete TB treatment and the patient’s increased risk for TB reactivation given her concurrent use of oral prednisone for sequential papillitis, the patient was later restarted on isoniazid and vitamin B6 for a 9-month treatment course.
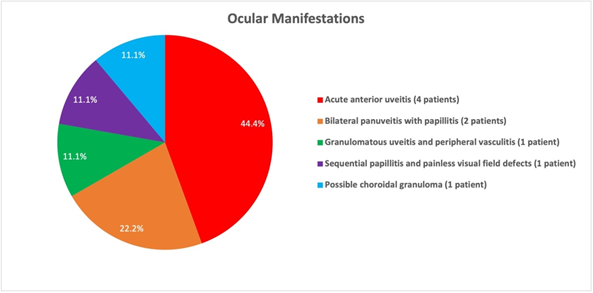
All of the identified patients (100%) had an inflammatory component of their ocular tuberculosis manifestation (Figure 1). Four patients (44.4%) presented with acute anterior uveitis. Two patients (22.2%) presented with bilateral panuveitis with papillitis. One patient (11.1%) developed sequential papillitis and painless visual field defects. One patient (11.1%) presented with granulomatous uveitis and peripheral vasculitis. One patient, case 9, had a particularly aggressive course of TB resulting in dissemination and Pott’s disease. In addition to the extensive tuberculoid lesions found throughout this patient’s skeletal and genitourinary system, this patient developed a chorioretinal lesion temporal to the fovea believed to be a choroidal granuloma. Given the time correlation between the progression of TB and the systemic lesions with the development of this presumed choroidal granuloma, it was assumed that this lesion was also due to TB.
Eight patients (88.9%) had improvement in ocular inflammation to quiescence without flare-up after completing systemic TB treatment. Case 9 has remained quiescent with a residual granuloma without active inflammation. One patient (11.1%), case 8, refused treatment for TB at initial diagnosis. After multiple episodes of recurrent anterior uveitis while remaining on topical corticosteroids, this patient had another positive QFG test, was referred to infectious disease, but continued to refuse systemic TB treatment. None of these patients required systemic corticosteroids to be added to their regimen after being started on TB treatment, and inflammation was noted to solely respond to systemic anti-TB medication.
As previously mentioned, our initial search covered a period of 122 months, or just over ten years. Within this timeframe, we identified nine patients who fit the inclusion criteria of this study and had a course consistent with presumed ocular TB. Interestingly, of these nine patients, three of them, or one-third, presented within the past year, potentially indicating a more recent increase in disease prevalence.
4. Discussion
Ocular tuberculosis is a specific systemic presentation of mycobacterial infection generally caused by direct invasion of the TB bacilli or an immunogenic reaction secondary to an extraocular infection. Tuberculosis has a proclivity for causing caseating granulomatous inflammation primarily in the lungs, but it can also affect the gamut of organs in the human body.
Ocular TB can present clinically in a myriad of ways both extraocularly and intraocularly. These presentations are both nonspecific and mercurial. Primary ocular TB describes focal disease, while secondary ocular TB arises with hematogenous dissemination or contiguous spread from a local insult. Primary TB infections predominantly affect the external aspects of the eye including the conjunctiva and cornea and commonly present as keratitis, scleritis, ulceration, or phlyctenulosis. Intraocular TB manifestations are mainly secondary to systemic seeding, primarily from a pulmonary focus. While studies indicate that choroiditis is the most common intraocular presentation of TB, anterior uveitis, retinal vasculitis, vitritis, and papillitis can also be found in patients with ocular TB [5–9]. In our sample of nine patients, each of these manifestations presented in at least one patient. Thus, it is imperative for physicians to consider ocular TB in their differential diagnosis, especially given that ocular TB can present in a nonspecific manner similar to more common conditions that lead to ocular inflammation.
TB is often recognized in medicine to be “the great imitator” and can therefore present with diverse clinical pictures. Given its ability to often masquerade as an ocular inflammatory reaction without obvious tuberculoid correlation, as well as the fact that testing for ocular TB is generally limited to invasive, expensive, and often less available definitive diagnostic tests such as polymerase chain reaction (PCR) with ocular fluid samples, early diagnosis of ocular TB is exceptionally difficult. Therefore, diagnosis of ocular TB is often made presumptively in concert with tuberculin skin tests and interferon gamma release assays after other possible etiologies have been excluded [10–15]. Favorable response to anti-tuberculosis therapy (ATT) may also indirectly serve as evidence further elucidating the potential diagnosis.
The nine cases included in this study presented with a variety of ocular involvement including anterior uveitis, panuveitis, optic nerve edema, sequential papillitis, granulomatous uveitis, peripheral vasculitis, and possible choroidal granuloma. The predominant clinical presentation in our group of patients was anterior uveitis (44%) rather than posterior uveitis, which has been the most common presentation in prior reports [15, 16]. Of note, the two patients who had a history of immunosuppression (cases 1 and 2) both experienced bilateral anterior uveitis. Additionally, extraocular TB manifestations were only seen in 44% of patients, and a known history of TB exposure was only obtained in 22% of our patients. This presents critical evidence that patient reported history of TB exposure or presence of symptomatic systemic TB disease is unreliable criteria for the diagnosis of ocular TB.
This is an exceptionally critical finding of this study, as it emphasizes the exceedingly low proportion of patients with a known history of TB exposure and manifestations of ocular TB. This highlights the general lack of presumption of diagnosis amongst both our patient population as well as amongst physicians and accentuates the critical nature of obtaining TB testing even in patients with no reported history of exposure or symptomatic systemic manifestations. Those patients without known exposure and without extraocular manifestations are most at risk for being misdiagnosed and therefore being left untreated for TB. This, unfortunately, can cause patients to endure chronic ocular inflammation and ultimately increase the risk of vision loss.
Given that TB is often overlooked in the differential diagnosis of patients with ocular inflammation, we suspect that the prevalence is actually much higher than reported. Similarly, it is possible that this prevalence is currently on the rise. In our study population of nine patients identified over the past ten years, three of these patients presented within the past year.
Within our study of nine patients, we were able to identify potential, but unconfirmed, mechanisms of TB exposure in three of our cases. The patient in Case 1 was born in a high-risk country and currently works as a postal carrier. The patient in Case 3 works as a manager at a local pizza restaurant. The patient in Case 9 presented shortly after arriving in the United States as a refugee from a high-risk country. Laboratory confirmation of ocular TB is often limited due to the invasive, expensive, and logistic difficulty with obtaining ocular samples for PCR testing. In each of the cases presented here, the diagnosis of ocular TB was a presumptive diagnosis based on chronological correlation of clinical presentation and response to both topical ocular medications and systemic anti-tuberculoid treatment without flare up during the follow up period. Accordingly, a subset of patients who had both ocular inflammatory disease and positive TB testing but lacked either a chronological or clinical response correlation between the two diseases were excluded from this study.
A concern that this report raises is the duration of time between most patients’ presentation with ocular disease and the investigation into TB as the potential cause. Barring the cases where a positive TB test was taken prior to eye manifestations, this time ranged from 3 days to 13 years from first reported ocular manifestation to testing for TB. In these cases, there was significant clinical improvement of ocular disease after the administration of ATT. In our study group, a favorable response to complete ATT was noted in 89% of patients. One patient (case 8) has refused systemic anti-TB treatment, cancelled all infectious disease appointments, and continues to experience recurrent anterior uveitis despite topical corticosteroid use. Given the relative efficacy and cost efficiency of TB treatment, it is crucial to establish the diagnosis and initiate treatment early in these patients with ocular TB with the objective of providing optimal chances of preserving sight [5, 17, 18]. In addition, proper diagnosis of ocular TB is crucial to avoid the use of systemic corticosteroids, which would likely aggravate the underlying TB infection and could put the patient at potential risk of permanent visual damage.
While this is a relatively large study compared to current literature on ocular TB, a limitation of this study is the fairly small sample size of nine patients as well as the retrospective nature of the study. In addition, the lack of PCR testing or confirmed microbiologic diagnosis of ocular TB, as well as the various systemic TB treatments used amongst the cases, are limitations of this series. Lastly, there are many inherent limitations with the use of electronic medical records (EMR) as well as the fact that it was implemented at our institution during our study period. Some patient information was found on scanned paper documents from prior to the EMR implementation, however, there were likely patients that fit our inclusion criteria and were missed in our search due to the difficulty in obtaining this data via a search through a large amount of paper charts. The use of the EMR, especially as more integration occurs across hospital systems and specialties, will likely assist in identifying more patients with ocular TB in the future. As younger patients continue to have more and more of their medical history in one central EMR, rather than partially on paper and partially electronic, data will be easier to follow and disease correlations easier to detect. This will allow for better evaluation of a rare cause of intraocular inflammation in the future. Finally, future prospective studies with more definitive diagnostic criteria for ocular TB including PCR and sequencing for Mycobacteria tuberculosis from samples of intraocular fluid will be of value and advance the understanding of disease prevalence, manifestations, and ultimately treatment, as well as help establish a standard treatment protocol for patients with ocular tuberculosis.
5. Conclusion:
The reported incidence of ocular TB in the US is likely underestimated and often disregarded in the differential diagnosis of patients with intraocular inflammation without a clear known history of TB. This leads to inconsistent and inadequate management, exposing patients to increased chances of inflammatory recurrence and vision loss. Thus, it is important to address this issue with studies leading to more precise diagnostic guidelines and protocols that can ensure uniformity in care. This would facilitate patients being treated in a timely and effective manner to ultimately prevent irreversible visual damage secondary to intraocular inflammation. Given an increased number of immigrant populations, incidence of ocular TB may potentially be on the rise, and ophthalmologists must be aware and perform appropriate testing in intraocular uveitis cases.
Declaration of Interest Statement:
The authors report no conflict of interest.
Disclosure statement:
There were no sources of funding or financial support for this project.
Data availability statement:
The data that support the findings of this study are available from the corresponding author, NP, upon reasonable request.
Funding:
There were no sources of funding or financial support for this project.
Acknowledgements:
None
References
- Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The Diagnosis and Treatment of Tuberculosis. Dtsch Aerzteblatt Online 116 (2019): 729-735.
- Schlossberg D, Maher D RM. The global epidemic of tuberculosis: a World Health Organization perspective. In: Tuberculosis and Nontuberculous Mycobacterial Infections, WB Saunders Company; (1999): 104-115.
- Dye C, Scheele S, >Dolin P, Pathania V, Raviglione MC. Global Burden of Tuberculosis. JAMA 282 (1999): 677.
- Albert DM, Raven ML. Ocular Tuberculosis. Schlossberg D, ed. Microbiol Spectr 4 (2016): 103-107.
- Raviglione MC. Tuberculosis. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education (2018).
- Ni C, Papale JJ, Robinson NL, Wu BF. Uveal tuberculosis. Int Ophthalmol Clin 22 (1982): 103-124.
- Helm CJ, Holland GN. Ocular tuberculosis. Surv Ophthalmol 38 (1993): 229-256.
- Regillo CD, Shields CL, Shields JA, Eagle RC, Lehr J. Ocular tuberculosis. J Am Med Assoc 266 (1991): 1490.
- Bouza E, Merino P, Muñoz P, Sanchez-Carrillo C, Yánez J, Cortés C. Ocular tuberculosis a prospective study in a general hospital. Medicine (Baltimore) 76 (1997).
- Llorenç V, González-Martin J, Keller J, et al. Indirect supportive evidence for diagnosis of tuberculosis-related uveitis: from the tuberculin skin test to the new interferon gamma release assays. Acta Ophthalmol 91 (2013): e99-e107.
- Denkinger CM, Dheda K, Pai M. Guidelines on interferon-γ release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect 17 (2011): 806-814.
- Kardos M, Kimball AB. Time for a change? Updated guidelines using interferon gamma release assays for detection of latent tuberculosis infection in the office setting. J Am Acad Dermatol 66 (2012): 148-152.
- Elangovan S, Govindarajan S, Mayilvakanam L, Gunasekaran N. Clinical Profile and Treatment Response of Patients with Ocular Inflammation due to Presumed Ocular Tuberculosis: A Retrospective Study. Turkish J Ophthalmol 49 (2019): 188-193.
- Sheu S-J, Shyu J-S, Chen L-M, Chen Y-Y, Chirn S-C, Wang J-S. Ocular manifestations of tuberculosis. Ophthalmology 108 (2001): 1580-1585.
- Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol 52 (2007): 561-587.
- Basu S, Monira S, Modi R, et al. Degree, duration, and causes of visual impairment in eyes affected with ocular tuberculosis. J Ophthalmic Inflamm Infect 4 (2014): 3.
- Saini JS, Mukherjee AK, Nadkarni N. Primary tuberculosis of the retina. Br J Ophthalmol 70 (1986): 533-535.
- Zumla A, Mwaba P, Squire SB, Grange JM. The tuberculosis pandemic which way now? J Infect 38 (1999): 74-79.