Renal Cystic Nephroma, a Diagnostic Challenge and Dilemma since 19th Century, A Literature Review
Article Information
Darouichi M1*, Till JP2, Constanthin PE2
1Institute medical and Chirurgical Champel, Geneva, Switzerland
2University Hospital of Geneva, Switzerland
*Corresponding Author: Darouichi M, Institute medical and Chirurgical Champel, Geneva, Switzerland
Received: 03 March 2019; Accepted: 18 March 2019; Published: 21 March 2019
Citation: Darouichi M. Renal Cystic Nephroma, a Diagnostic Challenge and Dilemma since 19th century, A Literature Review. Archives of Clinical and Biomedical Research 3 (2019): 012-020.
Share at FacebookAbstract
Kidney cystic nephroma is a rare tumor with a prevalence of less than 1% and predominant in adult women. This benign entity still poses real preoperative diagnostic problems when comparing to cystic renal cell carcinoma. Indeed, despite advances in medical imaging, there still is a lack of specificity of radiological signs and surgical exploration is necessary for diagnosis. Here, we report an interesting case in a 53-year-old woman. Sonography, abdominal CT scan and MRI suspected a cystic renal malignant tumor, leading to a right radical nephrectomy. The diagnosis of cystic nephroma was finally made by histological and immunohistochemically staining. We will discuss this case that precisely illustrates the diagnostic dilemma regarding this disease. We will then discuss the Physio-pathological, immunohistochemical and radiological aspects of this rare entity of uncertain etiology with a review of the literature.
Keywords
Cystic nephroma; Imaging; Histopathology; Immunohistochemically
Article Details
1. Case Report
A 52 year old woman, consulted for suspicion of right renal colic. She had never presented any such episode. During the clinical examination, the patient, who was afebrile, reported pain on percussion of the right renal lodge. Renal ultrasonography was performed and showed a cystic mass measuring 10 cm × 9 cm with multiple cystic masses in the center, right superior polar (Figure 1). An abdominal tomography (CT) was performed (Figure 2a-d), showing a complex multicystic right renal mass of 13 × 14 cm of diameter with contrast enhancement of the septa. There was a thickening of the anterior perirenal fascia without lymphadenopathy or venous involvement. MRI in T1-weighted image showed a well-defined mass with hypo-intense and patchy, slightly hyperintense in right kidney. T2-weighted image showed the mass with marked hyperintense and patchy hypo intense septa (Figure 3). Serum electrolytes, complete blood count and renal function were within normal ranges and the cytobacteriological examination of the urine was sterile. In front of this suspicion of renal cell carcinoma, the indication of a surgical exploration was posed. Radical nephrectomy was performed with a diagnostic of cystic tumor of uncertain origin.
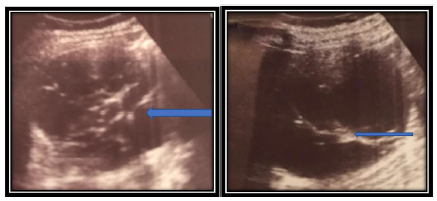
Figure 1: Ultrasound revealed a globular right kidney with contours distorted by a multi-locular cystic lesion with septas (arrows). There was no evidence of a solid mass or calcification. The polycystic mass, with a heterogeneous content, had multiple anechoic boxes and was well circumscribed by the surrounding renal parenchyma.
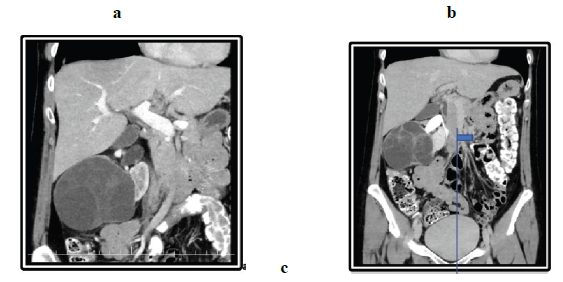
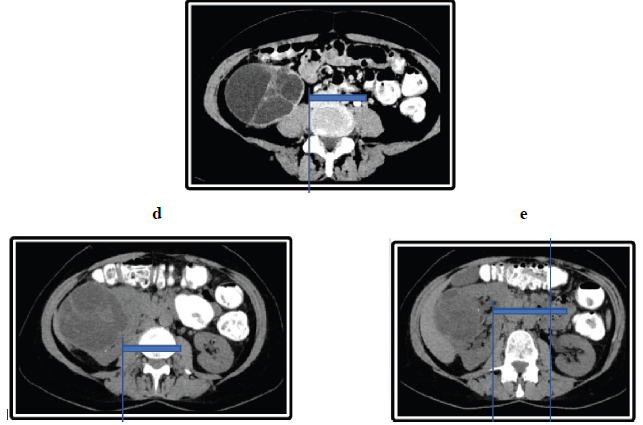
Figure 2: Abdominal CT-Scan, (a, b, c) Axial and coronal CT image shows a large, low-attenuation mass multiple thin septations from the kidney. The mass extends partially into the pelvis (small arrow). The normal renal parenchyma is seen, and there is a thin claw of renal parenchyma extending around a part of the (arrowheads). Axial unenhanced imaging shows exophytic renal mass in the middle third and an anterior inferior portion of the right kidney with (arrow). (d, e-Unenhanced axial).
Figure 2: Abdominal CT-Scan, (a, b, c) Axial and coronal CT image shows a large, low-attenuation mass multiple thin septations from the kidney. The mass extends partially into the pelvis (small arrow). The normal renal parenchyma is seen, and there is a thin claw of renal parenchyma extending around a part of the (arrowheads). Axial unenhanced imaging shows exophytic renal mass in the middle third and an anterior inferior portion of the right kidney with (arrow). (d, e-Unenhanced axial).
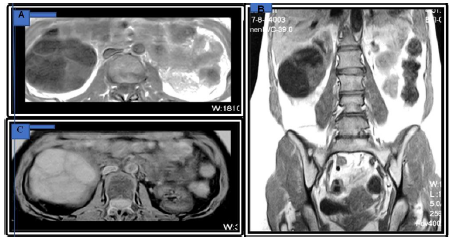
Figure 3: MRI-Abdominal Magnetic resonance imaging findings of cystic nephroma. (A) Transverse and Coronal; (B) T1-weighted image show a well-defined mass with hypo intense and patchy slight hyperintense (arrow) in right kidney; (C) Transverse T2-weighted image shows the mass with mark hyperintense and patchy hypo intense septa (arrow).
The patient suffered no complications postoperatively and was discharged home 10 days post nephrectomy. Definitive pathological diagnosis is benign cystic nephroma 17 cm long axis, excised in full. The definitive pathological diagnosis was benign cystic nephroma of 17 cm of the long axis, excised in totality.
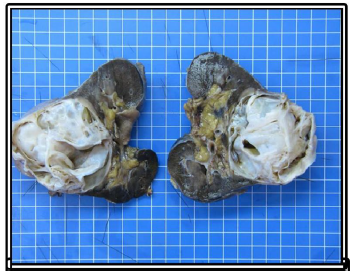
Macroscopy of the operative kidney, measuring 20 × 16 × 13 cm and weighting 1682 g. At the cuts, a voluminous multilocular mass measuring 17 × 12 × 12 cm with cysts ranging from 0.5 cm to 4.5 cm. The tumor is well delineated relatively to the non-tumoral renal parenchyma, which is physiological in appearance with a cortex at 1 cm. It arrives at 0.2 cm from the renal capsule, which presents focal fat tissue without evidence of infiltration of the tumor. At the hilum, the arterial, venous and ureteral section slices are identified with a ureter of 4 cm long and 0.3 cm in diameter. No lymph nodes were identified.
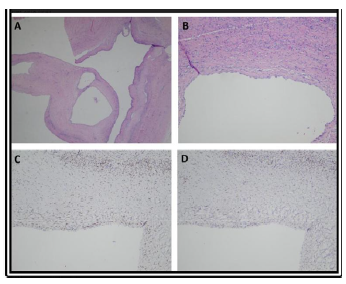
Microscopy of the lesion is well defined by a fibrous capsule, consisting of multiple cystic formations of variable sizes bordered by a monostratified, flat to cubic epithelium. The lining epithelial cells have the appearance of nails to upholster with a low to moderately abundant epsilon cytoplasm and granular oval nuclei associated with a nucleolus. No mitosis observed. The cysts are subtended by cellular fibrous septa, vascularized and sometimes edematous, without cell proliferation or immature cells.
On immunohistochemically examination, the epithelial cells are positive for cytokeratin’s 7 and 19; stromal cells are positive for estrogen receptors, progesterone and CD10. In the septa there are some bundles of smooth muscle cells positive for desmin and smooth muscle actin
2. Discussion
Cystic nephroma, also called multilocular cystic nephroma, is a rare, benign cystic tumor of the kidney. It represents less than 1% of all renal tumors and is predominant in adult women [1]. To our knowledge, less than 200 cases are described in the international literature under a variety of terminology of at least 20-25 Anglo-Saxon synonyms [2]. Cystic nephroma is defined by the presence of associated epithelial and stromal elements without solid nodules and is composed of multiple cysts that are not communicating with the collecting system, but are separated by fibrous septa, well encapsulated and non-infiltrating [3]. Since the first description in 1892 by Edmunds under the equivocal term of cystadenoma or cystic adenoma [4] and the first publication in 1951 (5) by Powell, many Anglo-Saxon terminologies were attributed to this renal cystic neoplasm with different classifications. Despite several studies on cystic renal tumors, which account for 10% of all renal neoplasia, including the largest series of 187 cases by Castillo in 1991, no consensus is yet established on an adequate terminology of this histological variety with a cystic character of the kidney [6]. However, histopathological results by sex and age have classified cystic kidney tumors into six histological types:
- Intrinsic multilocular cystic cancer (33%),
- Necrotic cancer pseudocyst (31%),
- Intrinsic unilocular cystic cancer (6%),
- Cancer developed on the wall of a cyst at (6%),
- Cystic multilocular nephrosis (21% of renal cystic lesions and 2% of kidney cancers) sometimes degenerating (3%) [7].
In addition, these studies concluded that there are two forms of cystic nephroma: the infantile, congenital form affecting children younger than two years old, considered a distinct entity with a biological behavior totally different from the benign nephroma acquired form in the adult, presenting predominantly in the female and often unilaterally [8]. Therefore, in adults, the bilateralism and malignant transformation of benign cystic nephroma are exceptional, but have still been described in the literature [9]. If its classification is confusing and controversial due to the existence of multiple synonyms, its exact pathogenesis is even more so, being neither clear nor certain until today. However, several etiological theories are advanced. One theory is that the cystic nephroma is due to a malformation that increases by accumulation of fluid and cystic dilatation of individual cubicles, giving it the appearance of a tumor cyst [10]. Others are inclined to classify this entity among mixed epithelial-stromal tumors of the kidney, particularly when the type of ovarian stroma with fibrous elements is present in the tumor. This latter hypothesis is consistent with the latest WHO classification of renal tumors [11], knowing that its pathogenesis is based on the influence of female hormones, especially in patients taking estrogenic contraceptives. The presence of estrogen-progestin receptors in the stroma of its tumors seem to confirm and reinforce the latter hypothesis [12].
Adding to these challenges of pathogenesis and classification, the clinical presentation is often nonspecific or even misleading. Presence of renal colic pain, pyelonephritis, hematuria, urinary tract infection and arterial hypertension in adults are reported in the literature. The clinical presentation can sometimes be with acute abdominal pain due to spontaneous rupture of the cyst [13]. Despite modern and efficient imaging, there is no preoperative radiological diagnosis of renal cystic nephroma. The fortuitous discovery of a cystic kidney mass during a routine examination for nonspecific symptomatology is often a source of uncertainty and the dilemma between benign and malignant lesions. Standard abdomen radiographs have no contribution and can exceptionally show a retroperitoneal mass effect, rarely with calcifications [14]. Ultrasound findings show a unilateral rounded, avascular cystic mass with an average diameter of approximately 10 cm. The tumor is composed with of anechoic, multi-cystic or multilocular lesions delineated by hyperechoic septa without solid mass. Doppler is suggested as useful in the differentiation of benign and malignant multilocular cystic lesions [15].
Abdominal computed tomography (CT Scan) with and without intravenous contrast remains the test of choice to guide preoperative diagnosis. Cystic neprhoma on CT Scan usually appears as a well-circumscribed, multi-cystic mass, of hypo-attenuated content without contrast excretion into the cystic location. The contours are smooth without extension or infiltration of adjacent structures. The capsule and its septa can be enhanced with IV contrast administration [16] thus often making it constitute a challenge to differentiate it from the Bosniak III cyst masses. Calcifications are rarely visible in the septa or peripheral capsule, as in our case. MRI is rarely indicated, the cystic component giving a hypo-intense signal in T1-weighted and hyper-intense sequences in T2. The septa and the capsule are hypo-intense in T1 and T2 because of their fibrous component [17]. Cystic nephroma appears avascular, hypo- or hyper-vascular in digitized angiography without diagnostic specificity [18]. It has no interest in the characterization of these masses, for which only an indication of partial surgery currently justifies its realization. Given a nonspecific clinical presentation and the low specificity of imaging, the diagnosis constitutes a challenge, thus the use of the term dilemma as our title. Some authors listed certain criteria for the diagnosis of this entity. In 1951, Powell and Boggs [19] defined diagnostic criteria for cystic nephroma as:
- Multilocular solitary cystic kidney lesion,
- Without communication or relation with the basin,
- Possessing a cubic or endothelial epithelial mucosa,
- No nephrogenic tissue in the septa and,
- renal healthy parenchyma on the rest of the organ.
In 1956, Boggs and Kimmelstiel [20] also included the presence of immature tissue in the septa. These criteria defined by Boggs and Kimmelstiel allow the differentiation of cystic nephroma from polycystic disease, polycystic kidney, single renal cysts and cystic renal cell carcinoma.
In 1989, Joshi and Beckwith [21] postulated the diagnosis criteria as, (1) The entire lesion being composed of cysts of various sizes and by septa that separate the cysts; (2) Cystic lesion being clearly distinct from the normal renal parenchyma; (3) The only solid component of the cysts being the septa; (4) Cysts being lined by cuboidal or hobnail cell epithelium; (5) The septa being composed of fibrous tissues, possibly including well-differentiated renal tubules. Differential diagnosis is essential between benign cystic nephroma and malignant cystic renal masses because the prognosis changes completely, especially with cystic, partially differentiated nephroblastoma, cystic clear cell carcinoma and multilocular cystic renal cell carcinoma. Also, the mixed epithelial and stromal tumor (MEST) of the kidney is a rare benign entity that has a great clinical, radiological and pathological similarity to the cystic nephroma, described in 1998 by Michal and Syrucek. Previously known as leiomyomatous renal hamartoma, multilocular cyst with ovarian stroma, cystic hamartoma of the renal pelvis, or adult mesoblastic nephromy [22].
Finally, the diagnosis is established by histopathological examination and almost exclusively on immunohistochemistry. The cystic components of cystic nephroma are of variable size and are coated with flat, cubic or nailed epithelial cells, and septa are thin with variable cellularity. The presence of atypical epithelial cells bordering the cysts and the nuclear characteristics of the clear cells in the septa is falsely insipid and the positivity for vimentin and EMA does not distinguish cystic nephroma from renal cystic carcinoma. The stromal positivity to estrogen or progesterone receptors may be useful, however, since a substantial proportion of cases are ER / PR-negative, the absence of staining does not exclude the diagnosis of malignancy [23]. The role of immunohistochemistry in the differential diagnosis, which includes multicystic renal cell carcinoma, is also unknown [24]. A systematic review of the literature on cystic nephroma case reports and different series publications from 1892 to the present has provided a comprehensive description of epidemiological characteristics, clinical symptoms, imaging data, and anatomopathological features (Table 1).
|
US |
Multiple anechoic spaces traversed by thin septa No solid elements A beak of claw of normal parenchyma around the periphery of a well-defined mass Displacement of the renal collecting system |
|
CT |
A sharply circumscribed, multi-septated renal mass Attenuation value of the cyst contents equal to or slightly higher than that of water Septations enhance following contrast material administration, but contrast material does not accumulate within individual loculi Well-defined margins Muticystic architecture Herniation into the renal tumor capsule |
|
MRI |
Low signal intensity of the tumor capsule |
|
ANGIO |
Multilocular cystic renal tumors usually appear hypo-vascular |
|
MACRO |
Unilateral and solitary lesion-sharp interface with normal renal parenchyma Multilocular cysts of varying sizes with thin septa and no solid elements Cysts are non-communicating with each other or the renal pelvis Cysts contain serous fluid |
|
MICRO |
Cysts lined by tubular epithelium which may be columnar or flat, characteristically hobnail in appearance Fibroblastic stroma between cysts Smooth and skeletal muscle tissue and undifferentiated metanephric blastoma may be present. |
Table 1: Radiological and pathological features of cyclic nephroma [25].
Surgical treatment of total or partial nephrectomy according to the size and location of the lesion is curative of this benign entity in adults. However, tumor recurrences have been observed, but regeneration or local recurrence of partial nephrectomy remains exceptional [26].
3. Conclusion
The relevance of this presentation once again sums up the challenge and dilemma in cystic nephroma’s preoperative clinical and radiological diagnosis since these tumors are classified as category III. Their treatment is still surgical. It should be included in the differential diagnosis and considered in middle-aged women with a history of exogenous estrogen with unilateral, solitary cystic kidney tumor. The diagnosis is established solely by immunohistopathological examination of the tumor. Finally, it is an entity still scientifically debated and researched since 1982 until today.
Acknowledgment
We thank Aida Darouichi and Dr Paul Constanthin for professional advice and support during this study.
References
- Wahal SP, Mardi K. Multilocular cystic renal cell carcinoma: a rare entity with review of literature. J Lab Physicians 6 (2014): 50-52.
- Baldauf MC, Shulz DM. Multilocular cyst of the kidney.Report of three cases with review of the literature. Am J Clin Pathol 65 (1976): 93-102.
- Billiet I, Van Poppel H, Baert L. Actual approach to cystic nephroma. Eur Urol 14 (1988): 280-286.
- Edmunds W. Cystic adenoma of the kidney. Trans Pathol Soc Lond 43 (1892): 89-90.
- Powell T, Shakman R, Johnson HD. Multilocular cysts of the kidney. Br J Urol 23 (1951): 142-152.
- Castillo OA, Boyle ET Jr, Kramer SA. Multilocular cysts of kidney. A study of 29 patients and review of literature. Urology 37 (1991): 156-162.
- Antic T, Perry KT, Harrison K, et al. Mixed epithelial and stromal tumor of the kidney and cystic nephroma share overlapping features: reappraisal of 15 lesions. Arch Pathol Lab Med 130 (2006): 80-17.
- Hélénon O, André M, Correas JM, et al. Cystic nephroma and cystic partially differentiated nephroblastoma: Two entities or one? Adv Anat Pathol 1 (1994): 99-102.
- Cheng EY, Cohn RA, Palmer LS, et al. A rare case of bilateral multilocular renal cysts. J Urol 157 (1997): 1861-1862.
- Michal M, Hes O, Bisceglia M, et al. Mixed epithelial and stromal tumors of the kidney. A report of 22 cases. Virchows Arch 445 (2004): 359-367.
- World Health Organization Classification of Tumours: Pathology-iarc (2004).
- Jevremovic D, Lager DJ, Lewin M. Cystic nephroma (multilocular cyst) and mixed epithelial and stromal tumor of the kidney: A spectrum of the same entity? Ann Diagn Pathol 10 (2006): 77-82.
- Dell’Atti L. An unusual presentation of cystic nephroma in an adult man. Rare Tumors 7 (2015): 5860.
- Silver IM, Boag AH, Soboleski DA. Best cases from the AFIP: multilocular cystic renal tumor-cystic nephroma. Radio Graphics 28 (2008): 1221-1225.
- Hirai T, Ohishi H, Yamada R, et al. Usefulness of colour Doppler flow imaging in differential diagnosis of multilocular cystic lesions of the kidney. J Ultrasound Med 14 (1995): 771-776.
- Del Riego Martín M, Landeras Alvaro R, López Rasines G, et al. Computerized axial tomography (CAT) and echography (US) of multilocular cystic nephroma [in Spanish]. Arch Esp Urol 49 (1996): 619-621.
- Kettritz U, Semelka RC, Siegelman ES, et al. Multilocular cystic nephroma: MR imaging appearance with current techniques, including gadolinium enhancement. J Magn Reson Imaging 6 (1996): 145-148.
- Hsiao HL, Wu WJ, Chang MY, et al. Unusual case of multilocular cystic nephroma treated with nephron sparing technique: A case report. Kaohsiung J Med Sci 22 (2006): 515-518.
- Powell T, Shackman R, Johnson HD. Multilocular cysts of the kidney. Br J Urol 23 (1951): 142-152.
- Boggs LK and Kimmelstiel P. Benign multilocular cystic nephroma: report of two cases of so-called multilocular cyst of the kidney. J Urol 76 (1956): 530-541.
- Joshi VV, Beckwith JB. Multilocular cyst of the kidney and cystic, partially differentiated nephroblastoma. Terminology and criteria for diagnosis. Cancer 64 (1989): 466-479.
- Michal M, Syrucek M. Benign mixed epithelial and stromal tumor of the kidney. Pathol Res Pract 194 (1998): 445-448.
- Stamatiou K, Polizois K, Kollaitis G, et al. Cystic nephroma: a case report and review of the literature. Cases J ; 1 (2008): 267.
- Sanjay Mukhopadhyay, Alfredo Luis Valente, and Gustavo de la Roza. Cystic Nephroma: A Histologic and Immunohistochemical Study of 10 Cases. Archives of Pathology and Laboratory Medicine 128 (2004): 1404-1411.
- Christopher Wilkinson, Victor Palit, Mallikarjun Bardapure. Adult multilocular cystic nephroma: Report of six cases with clinical, radio-pathologic correlation and review of literature Urol Ann 5 (2013): 13-17.
- Luithle T, Szavay P, Furtwängler R, et al. Treatment of cystic nephroma and cystic partially differentiated nephroblastoma--a report from the SIOP/GPOH study group. J Urol 177 (2007): 294-296.