Prediction of outcome of Patients Undergoing Whipple’s Pancreatoduodenectomy before Operation: A Prospective Validation of a Novel Risk Scoring System
Article Information
Samrat Ray1*, Subhashish Das1, Vivek Mangla1, Amitabh Yadav1, Parul Chugh2, Naimish N Mehta1, Samiran Nundy1
1Department of Surgical Gastroenterology and Liver Transplantation, Sir Ganga Ram Hospital, New Delhi, India
2Department of Biostatistics and Research, Sir Ganga Ram Hospital, New Delhi, India
*Corresponding Author: Samrat Ray, DNB (GI Surgery), Surgical Gastroenterology and Liver Transplantation, Sir Ganga Ram Hospital, New Delhi, India
Received: 12 April 2021; Accepted: 19 April 2021; Published: 30 April 2021
Citation: Citation: Samrat Ray, Subhashish Das, Vivek Mangla, Amitabh Yadav, Parul Chugh, Naimish N Mehta, Samiran Nundy. Prediction of outcome of Patients Undergoing Whipple’s Pancreatoduodenectomy before Operation: A Prospective Validation of a Novel Risk Scoring System. Journal of Surgery and Research 4 (2021): 229-240.
Share at FacebookAbstract
Background: Despite the high morbidity following Whipple’s pancreatoduodenectomy operations, there is still a lack of an objective pre-operative tool, based only on clinical and biochemical parameters to predict the outcome following the procedure that might be implemented.
Materials and Methods: Using a multivariate regression model, the significant predictors of post-operative outcome were identified in a set of retrospective database of patients (2006-2017), and a risk score developed by binary logistic regression method. This was validated in a set of prospective patients (2017-2020). The model’s predictive accuracy and discriminative ability were assessed using the receiver operating characteristics (ROC) analysis.
Results: On multivariate analysis in the retrospective cohort (n=442), the significant predictors of post-operative outcome were identified as peak bilirubin levels, pre-operative stenting and nature of the disease (Benign/Malignant). A risk score was derived and validated on the prospective cohort (n=182). The mean risk for an unfavourable outcome was 24% for a score of /=15. This was further tested on the validation cohort for individual risk scores (AUC=0.793). There was no significant difference between observed and expected risk of major complications (p=0.31).
Conclusion: The risk score showed a fair accuracy in predicting post-operative morbidity in the prospective cohort. Therefore, we propose this to be used as a quick aid to predict the operative outcome in patients posted for pancreatoduodenectomy on an outpatient basis using simple pre-operative clinical and laboratory variables.
Keywords
Novel, Risk scoring, Pre-operative, Validation
Article Details
1. Introduction
The mortality associated with Whipple’s pancreatoduodenectomy (WPD) has seen a considerable decline over the last 5-6 decades dropping to less than 5% from around 20% [1]. However, despite significant advancements in surgical expertise and post-operative critical care management, the morbidity associated with this procedure remains considerably high; between 30% to 50% [2]. Most of the literature about risk prediction models in patients undergoing WPD have focussed on parameters such as intra-operative blood loss, gland texture or pancreatic duct diameter [3-6]. These parameters are mostly surrogate markers for predicting the risk of development of a pancreatic fistula and its resultant sequelae. However, there has been a lack of a simple risk scoring tool using basic pre-operative demographic, clinical and laboratory parameters that could be used on an outpatient basis to predict the outcome in patients undergoing this procedure. We aimed therefore to develop a simple risk scoring model using the basic demographic, clinical and laboratory variables on an outpatient basis and validate the same on a heterogenous cohort of patients undergoing WPD.
2. Materials and Methods
The study was performed in a single unit of Surgical Gastroenterology at a high-volume tertiary care centre. All patients having undergone Whipple’s pancreatoduodenectomy (WPD) from January 2006 to December 2016 were included in the retrospective cohort. Patients who had undergone WPD elsewhere and were subsequently referred to our centre for post-operative complications; those with metastatic disease and those having undergone WPD as a part of multi-visceral resection were excluded from the study population. Also, the patients with borderline resectable cancer having received neo-adjuvant therapy were excluded from the study population. The data was collected by the principal investigator (SR) using our current electronic database and compiled on an excel sheet.
The demographic variables we studied included age, gender, body mass index (BMI) and pre-operative performance status (WHO-PS) and the clinical variables included co-morbidities (using the Charlson’s co-morbidity index), nature of the disease (benign or malignant), presence or absence of cholangitis (defined according to the revised Tokyo guidelines) at the time of index presentation, location of the lesion (ampulla/head of pancreas/duodenum/distal bile duct) and the presence or absence of pre-operative biliary stenting (endoscopic or percutaneous) [7,8]. Benign diseases included Chronic pancreatitis with head mass (with low/equivocal CA 19-9 levels), vascular malformations, trauma, groove pancreatitis, low-risk IPMN/cystic neoplasms. Nature of the disease and location of the lesion were determined by the pre-operative cross-sectional imaging with/without endoscopy guided tissue diagnosis. History of multiple stent exchanges at the time of presentation did not add up more points in the score. The laboratory variables included haemoglobin, bilirubin, creatinine, albumin (uncorrected), AST (aspartate aminotransferase) levels and prothrombin time-International normalized ratio/INR (Uncorrected; without vit K therapy). These variables were determined at the time of index presentation to the outpatient department (in a worked-up referred patient) or in the subsequent visit (in a patient presenting to the healthcare system for the first time). In already admitted patients who were in-house referrals from other departments, the worst values during the hospital stay were considered.
As per the protocol of the unit, all patients underwent a classical WPD (Kausch-Whipple) by the open technique. Reconstruction was performed by either the isolated loop (Machado’s) or by a single loop pancreato-jejunostomy(PJ) either by the dunking or duct-mucosa technique according to the operating surgeon’s choice. A feeding jejunostomy was done in all patients [9]. Although certain operative variables such as blood loss, transfusion requirements, morphology of the pancreatic duct and gland texture were included in the patient database. These were not included in the data analysed. Based on the post-operative outcome, the retrospective cohort was divided into 2 groups (see discussion for details):
- Favourable prognosis: Clavien-Dindo (CD) grade</= II and Length of stay (LOS) </=10 (days).
- Unfavourable prognosis: Clavien-Dindo (CD) grade III or above (including mortality) and/or Length of stay (LOS) >10 (days).
The patients were followed up for a period of 90 days after operation. Peri-operative mortality was defined as occurring during the course of the index hospital admission or within 90 days of follow up.
2.1 Statistical analysis
The retrospective cohort was divided into 2 groups based on the outcome variables. Univariate analysis using Chi-square test, Fisher exact t test and Mann-Whitney U test were performed on all the pre-operative demographic, clinical and laboratory variables, wherever applicable. A p-value<0.05 was set to be significant. Based on this, variables found significant on univariate analysis were entered into a Binary logistic regression model (multivariate analysis). Using the Framingham risk scoring model, the variables found significant on the Binary logistic regression were used and a scoring system developed [10]. Briefly, using this technique, weighted beta coefficients were calculated for each significant variable. For each variable, a reference level was set. Based on the reference value and the weighted coefficients for each variable, the distance (in regression units) between the individual category from its base (reference) value was computed and risk scores calculated. The risk scoring was performed for all consecutive patients undergoing WPD (fulfilling the inclusion criteria) between January 2017 and December 2019 by the co-investigator (SD) and entered on an excel sheet. The patients in the validation cohort were divided into 2 groups based on the outcome variables (CD grading and LOS) by the principal investigator (SR). The risk score calculated by the co-investigator was validated in the prospective cohort by the biostatistician (PG) following a “triple blinding” strategy. Validation was performed using the C-statistics. This was done by running a Receiver operator characteristic (ROC) curve on the individual scores and calculating the area under the curve (AUC). A P value<0.05 was set as statistically significant. The sensitivity, specificity and predictive values of the scoring system were also calculated.
3. Results
There was a total of 442 patients in the retrospective cohort and 182 patients in the prospective validation cohort. The mean age in the retrospective cohort was 53.5 years (Range: 13-80). There were 315 males and 127 females (M:F 2.5:1). Their mean Body Mass Index (BMI) was 20.7 kg/m2. All patients in the cohort had a performance status (PS) </= 2 with the majority of the patients having a Charlson’s co-morbidity index of 4 and above (n=263;59.5%). The most common medical co-morbidity in the cohort was diabetes mellitus (n=67; 15.1%). Of the total patient population in the cohort, 407 patients underwent WPD for malignancy (92%) and 35 patients for benign causes (8%). The most common malignant indication for surgery was carcinoma of the head of the pancreas (HOP) (n=225; 55.3%). Among the benign indications of surgery, the most common was a chronic pancreatitis associated head mass (n=15; 42.8%). Pre-operative biliary stenting was done in 112 patients (25%) with the majority undergoing endoscopic stent placement (110; 98.2%), only 2 required a percutaneous procedure. The most common indication for stenting was cholangitis, followed by hyperbilirubinaemia (a bilirubin level above 10 mg/dl according to our departmental protocol). 81 patients in the retrospective cohort had moderate-severe cholangitis (n=81; 18.3%). The mean haemoglobin level of the cohort was 11.3 gm/dl and the mean bilirubin level was 5 mg/dl (Range: 0.3-31) (Table 1 and 2).
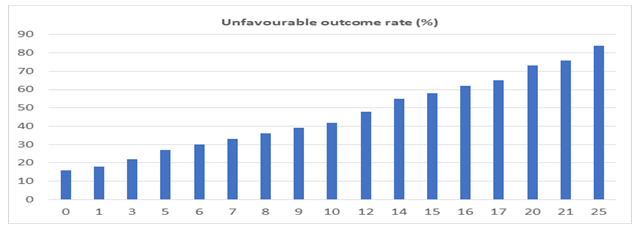
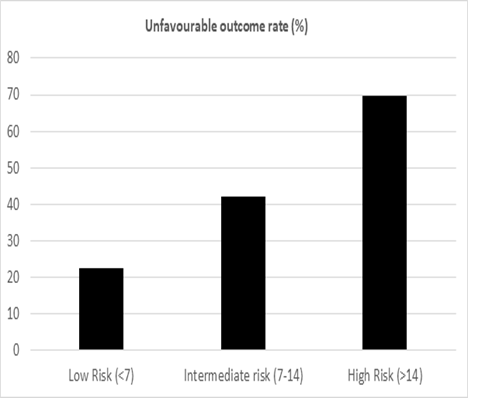
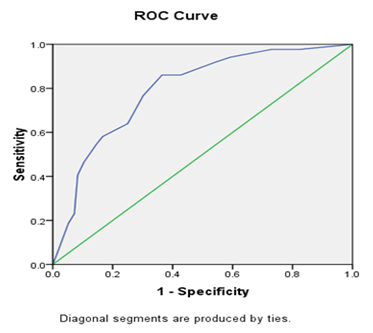
72 (16.2%) patients had major complications (defined as CD grades III and IV). Major re-intervention was required in 10 patients (2.2%); the most common indication being early extraluminal bleeding, which was seen in 4 out of 10 patients. The average post-operative length of stay (LOS) was 12.6 days (Range: 7-42 days) and the overall in-hospital/90-day mortality was 3.8%. On univariate analysis, the malignant nature of the disease, location of the lesion in the ampulla, presence of pre-operative stenting and presence of cholangitis were found to be associated with an unfavourable outcome (CD>II and/or LOS>10 days) (Table 1). Among the laboratory parameters, a higher serum bilirubin and SGOT levels and lower serum albumin level were found to be associated with a worse outcome (p<0.05) (Table 2). Multivariate binary logistic regression revealed serum bilirubin levels, status of pre-operative stenting and nature of the disease (Benign/Malignant) to be associated significantly with the outcome. Using the Framingham risk scoring model, a novel risk score was developed using the above three variables, ranging from 0-25 (Table 3). The predictive performance of the scoring system was expressed as individual possible values (Figure 1) and as score clusters (low risk: <7; intermediate risk: 7-14 and high risk: >14) (Figure 2). The discriminative ability of the score was assessed on the validation cohort (n=182). The ROC curve is shown in Figure 3. The Area under the curve (AUC) was 0.793, with a 95% confidence interval of 0.728-0.859; p=0.0001. The scores were found to have a 67.9% sensitivity and 83.6% specificity with an accuracy of 74.2%, when applied to the validation cohort. The Positive predictive value (PPV) was 67.9% and the negative predictive value (NPV) was 83.6%.
|
Variables |
Total (%) n=442 |
Favourable (%) n=253 |
Unfavourable (%) n=189 |
P Value (Univariate) |
|
PS |
0.89 |
|||
|
</=2 |
442 (100) |
253 (100) |
189 (100) |
|
|
>2 |
0 |
0 |
0 |
|
|
CCI |
0.67 |
|||
|
</=3 |
179 (40.5) |
98 (38.7) |
81 (42.8) |
|
|
>3 |
263 (59.5) |
155 (61.3) |
108 (57.1) |
|
|
Nature of disease |
0.03 |
|||
|
Benign |
35 (7.9) |
12 (4.7) |
23 (12.2) |
|
|
Malignant |
407 (92.1) |
241 (95.2) |
166 (87.8) |
|
|
Location |
0.04 |
|||
|
Ampulla |
17 (3.8) |
4 (1.6) |
13 (6.8) |
|
|
Distal CBD |
89 (20.1) |
55 (21.7) |
34 (18) |
|
|
Head of Pancreas |
275 (62.2) |
161 (63.6) |
114 (60.3) |
|
|
Duodenum |
61 (13.8) |
33 (13.1) |
28 (14.8) |
|
|
Stented |
0.01 |
|||
|
Yes |
110 (24.9) |
32 (12.6) |
78 (41.3) |
|
|
No |
332 (75.1) |
221 (87.3) |
111 (58.7) |
|
|
Cholangitis |
0s.01 |
|||
|
Present |
81 (18.3) |
22 (8.6) |
59 (31.2) |
|
|
Absent |
361 (81.7) |
231 (91.3) |
130 (68.7) |
|
Table 1: Demographic and Clinical characteristics of the retrospective cohort (compared).
|
Variable |
Total n=442 |
Favourable n=253 |
Unfavourable n=189 |
P value (Univariate) |
|
Mean age |
53.5 |
53.7 |
53.2 |
0.66 |
|
Mean BMI |
20.7 |
20.6 |
21 |
0.67 |
|
Mean Hb |
11.3 |
11.4 |
11.3 |
0.89 |
|
Mean Bilirubin |
5 |
3.5 |
7.1 |
0.02 |
|
Mean SGOT |
75.2 |
63.6 |
90.9 |
0.03 |
|
Mean Creatinine |
1.3 |
1.5 |
0.9 |
0.11 |
|
Mean INR |
1.1 |
1.1 |
1.2 |
0.88 |
|
Mean Albumin |
3.1 |
3.1 |
1.8 |
0.04 |
Table 2: Clinical and laboratory variables in the retrospective cohort (compared).
|
Area |
Std. Errora |
Asymptotic Sig.b |
Asymptotic 95% Confidence Interval |
|
|
Lower Bound |
Upper Bound |
|||
|
0.793 |
0.033 |
0 |
0.728 |
0.859 |
Test Result Variable(s): Scores
Figure 3: Prediction of risk of unfavourable outcome: ROC curve
|
Variables |
Categories |
Beta (β) |
P value |
Risk score |
|
Bilirubin |
< 2 |
0.066 |
<0.001 |
0 |
|
2-5 |
1 |
|||
|
5-10 |
3 |
|||
|
10-20 |
7 |
|||
|
>20 |
11 |
|||
|
Stenting |
Unstented |
1.143 |
<0.001 |
0 |
|
Stented |
9 |
|||
|
Nature of disease |
Benign |
0.661 |
0.001 |
0 |
|
Malignant |
5 |
|||
|
Total |
25 |
Table 3: Pre-operative risk score (Based on multivariate analysis).
4. Discussion
A Whipple’s PD is a challenging operation, owing to the complex anatomy of the pancreatico-duodenal region and the nature of the malignancy that merits this procedure recent literature has shown a declining trend of mortality associated with this operation in most high-volume centres to less than 4% [11]. However, the long duration of the operation (usually 7-9 hrs), the presence of multiple reconstructions, the intra-operative transfusion requirements etc have all been attributed to be associated with a persistently high morbidity in WPD [12]. In a developing country like India, factors such as impaired nutritional status and prolonged lag time associated with detection of malignancy and intervention might be other contributing factors towards a high morbidity associated with WPD.
Most of the scoring systems developed in the past have focussed on identifying ‘high-risk’ factors on imaging or intra-operative events. These have been well known to be associated with determining the risk of complications after WPD, such as post-operative pancreatic fistulae (POPF) or post-pancreatectomy haemorrhage (PPH). However, there has been a lack of a scoring modality solely based on the clinical, demographic and laboratory parameters of the patient that could be implemented at the index outpatient visit by the surgeon to stratify the patient into a “high-risk” vs a “low-risk” group. One of the earliest reports on an objective prediction of outcome after complex general/vascular operations comes from Gawande et al in who devised a score using factors such as blood loss, mean heart rate etc to predict the outcome after major surgery; called a Surgical Apgar Score (SAS) [13]. This was further validated on 189 patients undergoing pancreatic operations by Aoyama et al in 2016, who found a significant association of a score between 0-4 patients with overall morbidity after surgery (p=0.046) [14]. In addition, they also reported a significant association of Body Mass Index (BMI) with morbidity (p=0.0013). Braga et al, in 2011 performed a similar analysis on a cohort of 700 patients undergoing PD [15]. They devised a score using pancreatic duct diameter, parenchymal texture, operative blood loss and the ASA status. The score showed a good accuracy on the validation cohort (AUC 0.79; p<0.03). This was perhaps the earliest attempt at devising a pre-operative risk scoring to predict major complications after PD. Uzunoglu et al in 2014 proposed a novel scoring using pre-operative variables called the PREPARE score, to predict the risk of major complications following pancreatic surgeries [16]. The score focused only on the pre-operative clinical and laboratory variables and was performed on a heterogenous cohort of patients across multiple centres. In 2016, Wiltberger et al performed a retrospective analysis over a 21-year period to identify the factors that could enable risk stratification of patients undergoing PD [17]. They reported ASA status, BMI and cardiac and pulmonary co-morbidities to be independently associated with a worse outcome; and developed a scoring system using these variables.
The present study has been carried out on similar principles as the previous reported studies. Using the retrospective database, the pre-operative factors independently associated with a worse outcome after WPD were identified. Elevated bilirubin levels, presence of pre-operative stenting and malignant nature of the disease were found to be associated with a worse outcome. Therefore, on an outpatient basis, using these variables the surgeon may calculate the risk and stratify the patient into a low-, intermediate- and high-risk group and predict the chances of an unfavourable outcome in objective terms (as percentage). The scoring system is however, to be used solely for the purpose of a prediction of outcome; at the time of pre-operative counselling. The scores are not meant to affect the management plan of the patient. For example, a patient of distal cholangiocarcinoma with a pre-operative bilirubin level of 15 mg/dl would have a stenting done (as per the institutional protocol). According to the scoring system, he/she would fall in the high-risk category, which would imply an unfavourable prognosis. However, this would not have any implication on the management. As per the institution protocol, this patient would undergo surgery after waiting for a period of at least four weeks (for the inflammation to settle and bilirubin levels to come down below 5 mg/dl). Division of the cohort into favourable and unfavourable outcomes was based on the studies that have shown a positive correlation of a higher CD grading (major complications) with higher length of post-operative stay and thereby bearing a worse clinical and financial outcome for the patient [18, 19].
Most of the previous scoring systems as described above were limited by the difficulty of application on an outpatient basis. The PREPARE score was a promising attempt by the authors. However, this was limited by the lack of standardization of operative and diagnostic protocols among the patients, since it was a multicentre study. The study by Wiltberger et al was limited by its retrospective design. Another potential area of limitation could be a lack of an objective evaluation of co-morbidities in the study population. The present study is one of the earliest attempts at devising an exclusive pre-operative scoring system in patients undergoing WPD from the Indian sub-continent. The strength of the study design lies in the triple-blinding strategy implemented by the investigators and the data analyst and the validation of the score on a prospective cohort of patients over a 3-year time period. Another strength of this study could be the homogeneity of the study population by keeping it restricted to patients undergoing the operation in a single unit of the department by surgeons following a uniform protocol (under the leadership of Prof SN/Author no.7), thereby eliminating the possibility of any major variable outcome due to varied levels of technical expertise and acumen. There have been certain other studies in the recent times aiming to assess the post-operative outcomes in patients undergoing Whipple’s PD. However, these have mostly focussed on the intra-operative or post-operative clinical parameters. Yu et al. devised a modified early warning score (MEWS) incorporating post-operative cardiopulmonary parameters and reported an accuracy rate of 90% [20]. Another multi-institutional study by Mungroop et al devised a fistula risk score (FRS) using pre-operative and intra-operative variables and found a good predictability of the score in minimally invasive PD (MIPD) [21]. This was an attempt to revise the existing fistula risk score (an important marker of outcome in Whipple’s PD) in a cohort of minimally invasive PD. However, the same needs to be validated further.
However, the present scoring would not be applicable to predict the risk of individual common complications after WPD. This is a limitation of the study and is attributed to the lack of a subgroup analysis of the cohort by stratifying them into individual categories of complications. Another limitation is the potential overlap present between the scoring variables and other clinical factors affecting them. For example, in a patient with very high pre-operative bilirubin level (>10 mg/dl), stenting would have been already performed at the time of presentation (if referred from some other centre) or would be eventually performed before the surgery. This would imply an unfavourable outcome anyway (additive effect of the variables), irrespective of the scoring points. Also, presence of stenting could be associated with complications such as cholangitis (Confounder), which would in turn portend a worse prognosis even if the score comes in the range of low or intermediate risk. Therefore, the scoring might find its applicability more in patients with lower range of serum bilirubin levels pre-operatively with no cholangitis. Since, nature of the disease (in the present scoring) is determined mostly by the aid of some pre-operative imaging, this could be eventually affected by the final histopathology. For example, a benign head mass of the pancreas with chronic pancreatitis (with low pre-operative CA 19-9) could eventually turn out to be malignant, thereby increasing the beta error of the score. Therefore, this scoring can be used exclusively as a rough guide for a pre-operative prediction of outcome in patients undergoing PD. The points mentioned above would be the potential lacunae of this score and therefore, these would need to be explained to the patients at the time of pre-operative counselling session. Perhaps, the presence of these lacunae contributes to a relatively low sensitivity of the score, when validated on the prospective cohort of population (see results). The score also needs to be validated further on a more heterogenous set of patients at centres of different level of expertise and surgical protocol before being implemented into routine practice.
To conclude, this novel pre-operative risk scoring could be a good objective way of risk-stratification of patients undergoing WPD and can be a useful tool to guide them on the probable risk of an unfavourable outcome on an outpatient basis, which in turn would prepare the patient for a major surgery from psychological and financial stand-points. However, this needs further validation on more varied set of patient population before gaining an applicability in routine clinical practice.
Funding statement
There are no funders for this project
Conflict of Interest
Authors declare no conflicts of interest for this article
Ethical Approval
Not required
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 61 (2011): 133-134.
- DeOliveira ML,Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 244 (2006): 931-937.
- Winter JM, Cameron JL, Campbell KA. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10 (2006): 1199-1210.
- Gaujoux S, Cortes A, Couvelard A. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 148 (2010): 15-23.
- Tamijmarane A, Bhati CS, Mirza DF. Application of Portsmouth modification of physiological and operative severity scoring system for enumeration of morbidity and mortality (P-POSSUM) in pancreatic surgery. World J Surg Oncol 6 (2008): 39.
- Wellner UF, Kayser G, Lapshyn H. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB 12 (2010): 696-702.
- Charlson ME, Pompei P, Ales KL. A new method of classifying prognostic comorbidity in longitudinal studies: Development and Validation. J Chronic Dis 40 (1987): 373-383.
- Kiriyama S, Kozaka K, Takada T. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholangitis. J Hepatobiliary Pancreat Sci 25 (2018): 17-30.
- Machado MC, da Cunha JE, Bacchella T, et al. A modified technique for the reconstruction of the alimentary tract after pancreatoduodenectomy. Surg Gynecol Obstet 143 (1976):271- 272.
- Sullivan LM, Massaro JM, D'Agostmo RB. Presentation of multivariate data for clinical use: the framingham study risk score functions. Stat. Med 23 (2004):1631- 1660.
- Relles DM, Burkhart RA, Pucci MJ. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg 18 (2014): 279-285.
- Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Annals of surgical oncology 18 (2011): 2126-2135.
- Gawande AA, Kwaan MR, Regenbogen SE, et al. An Apgar score for surgery. J Am Coll Surg. 204 (2007): 201-208.
- Aoyama T, Katayama Y, Murakawa M. Risk assessment of pancreatic surgery by surgical Apgar score and body mass index. Int Surg 101 (2016): 263-269.
- Braga M, Capretti G, Pecorelli N. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 254 (2011): 702-708.
- Uzunoglu F, Reeh M, Vettorazzi E. Pre-operative pancreatic resection (PREPARE) score: A prospective multicentre-based morbidity risk score. Ann Surg 260 (2014): 857-864.
- Wiltberger G, Muhl B, Benzing C. Preoperative risk stratification for major complications following pancreaticoduodenectomy: Identification of high risk patients. Int J Surg 67 (2016): 33-39.
- Ray S, Mehta NN, Mangla V. A comparison between the comprehensive complication index and the Clavien-Dindo grading as a measure of post-operative outcome in patients undergoing gastrointestinal surgery- a prospective study. J Surg Res 244 (2019): 417-424.
- Clavien PA, Vetter D, Staiger R, et al. The comprehensive complication index (CCI): added value and clinical perspectives 3 years down the line. Ann Surg 265 (2017): 1045- 1050.
- Yu M, Huang B, Liu P. Detection of deteriorating patients after Whipple surgery by a modified early warning score (MEWS). Ann Transl Med 7 (2019): 574-580.
- Mungroop TH, Klompmaker S, Wellner UF. Updated alternative fistula risk score (ua-FRS) to include minimally invasive pancreatoduodenectomy. Pan-European validation. Ann Surg (2019).