Polyclonal Anti-D Antibodies Significantly Reduce the Rate of Miscarriages in Rh(D) positive Women with Recurrent Pregnancy loss
Article Information
Frauke RINGEL1, Falk LEWANDOFSKI2, Holger KIESEWETTER3, Jalid SEHOULI4, Berthold HOPPE5, Alina KIESEWETTER6, Reinhard HANNEN7, Christian Friedrich STOLL8, Sylvia MAAS9, Abdulgabar SALAMA10,*
1 Dr. Frauke RINGEL, Ph.D., Berlin, Germany, Medicover, Hemostaseologicum Mitte
2 Mr. Falk LEWANDOFSKI, Berlin, Germany, Medicover, Hemostaseologicum Mitte
3 Prof. Holger KIESEWETTER, MD, Berlin, Germany, Medicover, Hemostaseologicum Mitte
4 Prof. Jalid SEHOULI, MD, Berlin, Germany, Charite, Universitaetsmedizin Berlin, Klinik fuer Gynaekologie mit Zentrum für onkologische Chirurgie, Campus Virchow-Klinikum
5 Dr. Berthold HOPPE, MD, Berlin, Germany, Medicover, Hemostaseologicum Mitte, and Institute of Laboratory Medicine, BG Klinikum Unfallkrankenhaus Berlin, and MSH Medical School Hamburg, Germany
6 Ms. Alina KIESEWETTER, Berlin, Germany, Medicover, Hemostaseologicum Mitte
7 Dr. Reinhard HANNEN, MD, Berlin, Germany, CERES Kinderwunschzentrum Berlin
8 Dr. Christian Friedrich STOLL, MD, Berlin, Germany, CERES Kinderwunschzentrum Berlin
9 Dr. Sylvia MAAS, MD, Potsdam, Germany, Frauenärzte Maas
10 Prof. Abdulgabar SALAMA, MD, Berlin, Germany, Medicover, Hemostaseologicum Mitte, and Charite, Universitaetsmedizin Berlin, Klinik fuer Gynaekologie mit Zentrum für onkologische Chirurgie, Campus Virchow-Klinikum
*Corresponding Author: Prof. Dr. Abdulgabar Salama, MD, Klinik für Gynäkologie mit Zentrum für onkologische Chirurgie, Charité Universitaetsmedizin Berlin, Campus Virchow-Klinikum, Germany.
Received: 07 February 2023; Accepted: 13 February 2023; Published: 15 March 2023
Citation:
Frauke RINGEL, Falk LEWANDOFSKI, Holger KIESEWETTER, Jalid SEHOULI, Berthold HOPPE, Alina KIESEWETTER, Reinhard HANNEN, Christian Friedrich STOLL, Sylvia MAAS, Abdulgabar SALAMA. Polyclonal Anti-D Antibodies Significantly Reduce the Rate of Miscarriages in Rh(D) positive Women with Recurrent Pregnancy loss. Obstetrics and Gynecology Research. 6 (2023): 107-114
Share at FacebookAbstract
Background:
Macrophages play a key role in all environmental conditions surrounding pregnancy. Coating of autologous red blood cells (RBCs) with polyclonal antibodies to Rh(D) antigen may result in an immunomodulation and improved outcome in Rh(D) positive women with recurrent pregnancy loss (RPL).
Methods:
A total of 60 Rh(D) positive women (age 23 to 45 years) with a history of RPL and ineffective treatment with low molecular weight heparin (LMWH) and/or aspirin were included in this retrospective study. In addition to this treatment, Anti-D (300 μg) was given subcutaneously to each woman either prior to pregnancy and/or two times within 12 weeks of gestation.
Results:
Treatment with Anti-D in non-responders to heparin/aspirin resulted in successful pregnancies in 67% of all cases. The remaining women had only aborts (23%) or did not become pregnant (10%). None of the treated women has developed anemia due to this treatment or any other significant adverse reaction. The rate of successful pregnancies does not appear to be influenced by the administration of: Anti-D prior to pregnancy, age, thrombophilia or previous alive births.
Conclusion:
The improved outcome following the administration of Anti-D in women with RPL might be explained by immune modulations induced by different immune reactions including polarization of decidual macrophages. The results obtained in this study clearly indicate that Anti-D is safe and highly effective in treatment of Rh(D) positive women with RPL. However, further studies are required to support our results and to find out the optimal dose and timing of Anti-D administration
Keywords
Abort, Anti-D; Aspirin; Low Molecular Weight Heparin; New Treatment; Recurrent Pregnancy Loss
Abort articles Abort Research articles Abort review articles Abort PubMed articles Abort PubMed Central articles Abort 2023 articles Abort 2024 articles Abort Scopus articles Abort impact factor journals Abort Scopus journals Abort PubMed journals Abort medical journals Abort free journals Abort best journals Abort top journals Abort free medical journals Abort famous journals Abort Google Scholar indexed journals Anti-D articles Anti-D Research articles Anti-D review articles Anti-D PubMed articles Anti-D PubMed Central articles Anti-D 2023 articles Anti-D 2024 articles Anti-D Scopus articles Anti-D impact factor journals Anti-D Scopus journals Anti-D PubMed journals Anti-D medical journals Anti-D free journals Anti-D best journals Anti-D top journals Anti-D free medical journals Anti-D famous journals Anti-D Google Scholar indexed journals Aspirin articles Aspirin Research articles Aspirin review articles Aspirin PubMed articles Aspirin PubMed Central articles Aspirin 2023 articles Aspirin 2024 articles Aspirin Scopus articles Aspirin impact factor journals Aspirin Scopus journals Aspirin PubMed journals Aspirin medical journals Aspirin free journals Aspirin best journals Aspirin top journals Aspirin free medical journals Aspirin famous journals Aspirin Google Scholar indexed journals Low Molecular Weight Heparin articles Low Molecular Weight Heparin Research articles Low Molecular Weight Heparin review articles Low Molecular Weight Heparin PubMed articles Low Molecular Weight Heparin PubMed Central articles Low Molecular Weight Heparin 2023 articles Low Molecular Weight Heparin 2024 articles Low Molecular Weight Heparin Scopus articles Low Molecular Weight Heparin impact factor journals Low Molecular Weight Heparin Scopus journals Low Molecular Weight Heparin PubMed journals Low Molecular Weight Heparin medical journals Low Molecular Weight Heparin free journals Low Molecular Weight Heparin best journals Low Molecular Weight Heparin top journals Low Molecular Weight Heparin free medical journals Low Molecular Weight Heparin famous journals Low Molecular Weight Heparin Google Scholar indexed journals New Treatment articles New Treatment Research articles New Treatment review articles New Treatment PubMed articles New Treatment PubMed Central articles New Treatment 2023 articles New Treatment 2024 articles New Treatment Scopus articles New Treatment impact factor journals New Treatment Scopus journals New Treatment PubMed journals New Treatment medical journals New Treatment free journals New Treatment best journals New Treatment top journals New Treatment free medical journals New Treatment famous journals New Treatment Google Scholar indexed journals Recurrent Pregnancy Loss articles Recurrent Pregnancy Loss Research articles Recurrent Pregnancy Loss review articles Recurrent Pregnancy Loss PubMed articles Recurrent Pregnancy Loss PubMed Central articles Recurrent Pregnancy Loss 2023 articles Recurrent Pregnancy Loss 2024 articles Recurrent Pregnancy Loss Scopus articles Recurrent Pregnancy Loss impact factor journals Recurrent Pregnancy Loss Scopus journals Recurrent Pregnancy Loss PubMed journals Recurrent Pregnancy Loss medical journals Recurrent Pregnancy Loss free journals Recurrent Pregnancy Loss best journals Recurrent Pregnancy Loss top journals Recurrent Pregnancy Loss free medical journals Recurrent Pregnancy Loss famous journals Recurrent Pregnancy Loss Google Scholar indexed journals gestation articles gestation Research articles gestation review articles gestation PubMed articles gestation PubMed Central articles gestation 2023 articles gestation 2024 articles gestation Scopus articles gestation impact factor journals gestation Scopus journals gestation PubMed journals gestation medical journals gestation free journals gestation best journals gestation top journals gestation free medical journals gestation famous journals gestation Google Scholar indexed journals congenital thrombophilias articles congenital thrombophilias Research articles congenital thrombophilias review articles congenital thrombophilias PubMed articles congenital thrombophilias PubMed Central articles congenital thrombophilias 2023 articles congenital thrombophilias 2024 articles congenital thrombophilias Scopus articles congenital thrombophilias impact factor journals congenital thrombophilias Scopus journals congenital thrombophilias PubMed journals congenital thrombophilias medical journals congenital thrombophilias free journals congenital thrombophilias best journals congenital thrombophilias top journals congenital thrombophilias free medical journals congenital thrombophilias famous journals congenital thrombophilias Google Scholar indexed journals prothrombin mutation articles prothrombin mutation Research articles prothrombin mutation review articles prothrombin mutation PubMed articles prothrombin mutation PubMed Central articles prothrombin mutation 2023 articles prothrombin mutation 2024 articles prothrombin mutation Scopus articles prothrombin mutation impact factor journals prothrombin mutation Scopus journals prothrombin mutation PubMed journals prothrombin mutation medical journals prothrombin mutation free journals prothrombin mutation best journals prothrombin mutation top journals prothrombin mutation free medical journals prothrombin mutation famous journals prothrombin mutation Google Scholar indexed journals first trimester articles first trimester Research articles first trimester review articles first trimester PubMed articles first trimester PubMed Central articles first trimester 2023 articles first trimester 2024 articles first trimester Scopus articles first trimester impact factor journals first trimester Scopus journals first trimester PubMed journals first trimester medical journals first trimester free journals first trimester best journals first trimester top journals first trimester free medical journals first trimester famous journals first trimester Google Scholar indexed journals
Article Details
INTRODUCTION
Recurrent pregnancy loss (RPL) is defined as two or more consecutive pregnancy losses prior to 20–24 weeks gestation. It affects about 1–2 % of pregnancies and is often associated with serious psychological complications. During the last few decades, many factors including age, inheritable and acquired genetic and/or anatomical abnormalities, infections, and endocrine dysfunctions have been identified to play a crucial role in this field. However, the true cause remains unclear in most cases. Until now, there is no specific or even generally accepted treatment in such cases.[1],[2] In general, pharmacological treatment is only recommended for anti-phospholipid syndrome, and factor V mutation.[3-5] Dependent on clinicians and patients, this treatment may also be employed in the presence of other congenital thrombophilias, such as prothrombin mutation, deficiencies in anti-thrombin, protein C, and protein S.[1],[6],[7]
Based on the fact that pregnancy reflects an immunological miracle combining immune defense/response on the one hand and immune tolerance on the other hand, it is not surprising that immune imbalance during pregnancy may result in complications. In fact, several studies have shown that RPL could be related to immune abnormalities in roughly 50% of affected women.[8-12] Current studies have focused on the role of decidual macrophages. These cells mature during early stages of pregnancy and persist during the entire gestation period.[13] In the first trimester, about 40 % of all cells in the decidua are leukocytes and 20–30 % of these cells are macrophages, which have the plasticity to alter their function in response to various environmental signals. They are also sensitive to small changes in the microenvironment[13-15], they represent the most crucial immune cells for pregnancies due to their involvement in regulation of implantation, placentation, fetal development, and most importantly vascular remodeling at the maternal-fetal interface. These properties make those cells attractive as a therapeutic target in immune related diseases.
Polyclonal Anti-D is successfully used to prevent alloimmunization in Rh(D) negative pregnant women as well as in treatment of patients with autoimmune thrombocytopenia (ITP). Until now, the mechanisms by which these antibodies are operating in vivo are poorly understood. In addition to Fc mediated phagocytosis of IgG coated RBCs, Anti-D has exciting immunomodulatory effects, which could not completely be explained yet.[16],[17]
In this retrospective study, Anti-D has been shown to increase the rates of successful pregnancies in Rh(D) positive women who remained abortive despite treatment with LMWH and/or aspirin.
Patients and methods
Serological testing including blood group, antibody screening, and direct antiglobulintest were performed by standard techniques using the gel cards (Biorad, Cressier sur Morat, Switzerland and Grifols SA, Barcelona, Spain). Only Rh(D) positive women who had at least two pregnancy losses have been included in this retrospective study. 59 women (age 23 - 45 years) had inheritable genetic abnormalities (thrombophilias), one woman did not give consent to genetic testing but had thrombophilia history in her anamnesis (table 1).
|
woman no. |
age [years] |
blood group |
mutationa [numbers] |
only LMWH/aspirin |
plus Anti-D |
||||
|
abort |
birth |
injectionsb |
gravidity |
abort |
birth |
||||
|
1 |
23 |
B |
2 |
4 |
0 |
after |
1 |
0 |
1 |
|
2 |
27 |
unk |
3 |
3 |
1 |
pre and after |
0 |
0 |
0 |
|
3 |
29 |
A |
3 |
2 |
1 |
after |
1 |
0 |
1 |
|
4 |
29 |
A |
3 |
3 |
0 |
pre and after |
1 |
1 |
0 |
|
5 |
30 |
A |
2 |
4 |
1 |
pre and after |
1 |
0 |
1 |
|
6 |
31 |
B |
4 |
2 |
1 |
after |
1 |
0 |
0 |
|
7 |
31 |
A |
3 |
4 |
0 |
pre |
0 |
0 |
0 |
|
8 |
32 |
A |
4 |
2 |
0 |
pre and after |
2 |
0 |
2 |
|
9 |
33 |
A |
1 |
2 |
0 |
unk |
1 |
0 |
1 |
|
10 |
33 |
B |
2 |
4 |
0 |
pre and after |
1 |
0 |
0 |
|
11 |
34 |
A |
1 |
3 |
0 |
after |
1 |
0 |
1 |
|
12 |
34 |
A |
3 |
3 |
0 |
after |
3 |
1 |
2 |
|
13 |
34 |
0 |
4 |
3 |
0 |
after |
1 |
0 |
1 |
|
14 |
34 |
B |
2 |
2 |
0 |
after |
1 |
0 |
0 |
|
15 |
35 |
0 |
3 |
2 |
0 |
pre and after |
2 |
0 |
2 |
|
16 |
35 |
0 |
3 |
2 |
1 |
pre and after |
2 |
1 |
1 |
|
17 |
35 |
B |
2 |
2 |
1 |
unk |
1 |
0 |
1 |
|
18 |
35 |
A |
6 |
2 |
1 |
unk |
1 |
0 |
1 |
|
19 |
35 |
A |
4 |
4 |
0 |
after |
3 |
2 |
1 |
|
20 |
35 |
A |
2 |
3 |
0 |
after |
1 |
0 |
1 |
|
21 |
36 |
A |
2 |
2 |
1 |
pre and after |
4 |
1 |
2 |
|
22 |
36 |
0 |
3 |
2 |
0 |
pre and after |
2 |
0 |
2 |
|
23 |
36 |
B |
2 |
4 |
1 |
after |
1 |
0 |
1 |
|
24 |
36 |
A |
2 |
2 |
3 |
pre |
0 |
0 |
0 |
|
25 |
36 |
A |
4 |
4 |
0 |
pre and after |
2 |
2 |
0 |
|
26 |
36 |
AB |
1 |
2 |
1 |
after |
1 |
0 |
1 |
|
27 |
37 |
0 |
2 |
3 |
0 |
pre and after |
1 |
0 |
1 |
|
28 |
37 |
unk |
1 |
5 |
1 |
after |
1 |
0 |
1 |
|
29 |
37 |
A |
2 |
2 |
2 |
pre and after |
1 |
0 |
1 |
|
30 |
38 |
A |
1 |
2 |
0 |
after |
1 |
0 |
1 |
|
31 |
38 |
0 |
4 |
3 |
0 |
after |
1 |
0 |
0 |
|
32 |
39 |
A |
1 |
4 |
0 |
after |
1 |
0 |
1 |
|
33 |
39 |
A |
5 |
4 |
2 |
pre and after |
1 |
0 |
1 |
|
34 |
39 |
AB |
1 |
2 |
1 |
pre |
0 |
0 |
0 |
|
35 |
39 |
0 |
3 |
2 |
1 |
after |
1 |
0 |
1 |
|
36 |
40 |
A |
3 |
6 |
2 |
after |
4 |
3 |
0 |
|
37 |
40 |
B |
2 |
7 |
2 |
pre and after |
2 |
1 |
0 |
|
38 |
40 |
0 |
1 |
4 |
0 |
pre and after |
1 |
0 |
1 |
|
39 |
40 |
A |
2 |
2 |
3 |
unk |
1 |
0 |
1 |
|
40 |
41 |
0 |
1 |
7 |
1 |
after |
2 |
0 |
2 |
|
41 |
41 |
A |
1 |
3 |
0 |
pre and after |
1 |
0 |
1 |
|
42 |
41 |
A |
4 |
5 |
2 |
pre |
0 |
0 |
0 |
|
43 |
41 |
0 |
1 |
2 |
0 |
after |
1 |
0 |
1 |
|
44 |
41 |
unk |
3 |
4 |
1 |
pre |
0 |
0 |
0 |
|
45 |
41 |
A |
1 |
5 |
1 |
after |
1 |
1 |
0 |
|
46 |
42 |
0 |
4 |
2 |
0 |
after |
1 |
0 |
1 |
|
47 |
42 |
A |
2 |
7 |
0 |
after |
1 |
0 |
1 |
|
48 |
42 |
A |
3 |
3 |
5 |
pre and after |
1 |
1 |
0 |
|
49 |
42 |
A |
2 |
3 |
1 |
after |
1 |
0 |
1 |
|
50 |
42 |
0 |
1 |
3 |
0 |
unk |
1 |
0 |
1 |
|
51 |
42 |
A |
3 |
4 |
1 |
pre and after |
1 |
1 |
0 |
|
52 |
43 |
A |
1 |
3 |
1 |
pre and after |
1 |
0 |
1 |
|
53 |
43 |
B |
1 |
3 |
0 |
unk |
1 |
0 |
1 |
|
54 |
43 |
A |
nd* |
4 |
3 |
unk |
1 |
0 |
1 |
|
55 |
43 |
A |
2 |
18 |
0 |
pre and after |
1 |
0 |
1 |
|
56 |
44 |
0 |
3 |
4 |
4 |
after |
1 |
0 |
0 |
|
57 |
44 |
0 |
3 |
5 |
2 |
pre and after |
3 |
1 |
1 |
|
58 |
44 |
AB |
4 |
3 |
0 |
after |
1 |
0 |
1 |
|
59 |
44 |
A |
1 |
4 |
0 |
unk |
1 |
1 |
0 |
|
60 |
45 |
B |
4 |
2 |
0 |
pre and after |
1 |
1 |
0 |
a number of thrombophilia associated mutations; b Anti-D injections prior (pre) or after (after) conception; *patient did not give consent to genetic testing, therefore no proof of inherited thrombophilia, but increased risk of thrombosis by anamnesis); unk: unknown; nd: not done
Table 1: Relevant data of women treated with Anti-D
All women had ineffective pretreatment with LMWH and/or aspirin. They were treated on an outpatient basis between 2016 and 2021 at our practice, the Haemostaseologicum Mitte in Berlin. All medical records and investigations were retrospectively reviewed.
Depending on presentation, unpregnant women were introduced to admit themselves Anti-D (300 µg Rhophylac, CSL Behring) five to ten days prior to the expected ovulation, and two times within the first twelve weeks of pregnancy. All other women were presented within short time when they became aware of their pregnancy, and received, similar to the other group, two times Anti-D.
All women were clinically examined and investigated for underlying diseases which might be responsible for the RPL. In addition, they were tested for thrombophilia.
Informed written consent was obtained for participation, and the local ethics review board was briefed about this study.
Statistical analyses were performed using the IBM SPSS statistics 28.0 software.
Results
As expected, administration of Anti-D resulted in a weakly positive direct antiglobulin test in all cases, but none of the treated women developed significant hemolysis.
Most importantly, the majority of these women had not only unremarkable pregnancy, but also normal delivery. Only six women did not get pregnant (10%), and 40 of the remaining 54 women gave a live birth (60% of all treated women and 74% of all pregnant women, respectively) (figure 1).
Impact of previous aborts/births on outcome
Despite treatment with LMWH and/or aspirin, 36 of the 60 women had two or three aborts prior to Anti-D treatment, and the remaining 24 had four or more aborts (figure 1). Following treatment with Anti-D, 27 women of the first group had successful pregnancies (75%), nine had aborts (25%), and three did not get pregnant (8%). Of the second group of 24 women, 13 gave birth (54%), nine had aborts (38%), and three did not get pregnant (13%).
Prior to treatment with Anti-D, 30 women had no birth, and 30 women had at least one birth (table 2). Of the first group, 22 women had successful pregnancies (73%), eight had aborts (27%), and one woman did not get pregnant (3%). Of the other 30 women, 18 women gave birth (60%), ten had aborts (33%), and five did not get pregnant (8%) (table 2).
A chi-square test was used to compare number of aborts prior to treatment with Anti-D and successful pregnancy under treatment with Anti-D. No expected cell frequencies were below 5, and the results showed no significant correlation with χ²(1) = 0•303, p(χ²) = 0•582, p(Fisher’s exact test) = 0•784, V = 0•071.
Impact of age
There have been 23 women at ages between 23 and 35 years, and 37 at ages between 36 to 45 (table 1). Of the first group, 17 women had successful births (74%), six had aborts (26%), and two women did not get pregnant (9%). Of the second group, 27 women gave births (73%), 11 had aborts (30%), and four did not get pregnant (11%) (table 2).
A chi-square test was used to compare age groups and successful pregnancies under treatment with Anti-D. No expected cell frequencies were below 5, and the results showed no significant correlation with χ²(1) = 0•881, p(χ²) = 0•348, p(Fisher’s exact test) = 0•408, V = 0•121.
Impact of Anti-D dose
Of the 25 women who received Anti-D only after conception, 19 gave birth (76%), and 8 had aborts (32%) (table 2). Of the 21 women who received Anti-D prior to pregnancy, 14 gave birth (67%), ten had aborts (48%). Six women did not get pregnant. For eight women full data of Anti-D administration were not available (table 2).
In summary, the prophylactic administration of Anti-D also prior to pregnancy does not appear to be more effective than the administration only post gestation.
A chi-square test was used to compare pre and after conception injections of Anti-D with successful pregnancies. No expected cell frequencies were below 5. Results showed no significant correlation with χ²(1) = 0•801, p(χ²) = 0•371, p(Fisher’s exact test) = 0•522, V = 0•132.
Impact of blood group
There have been 31 women with blood group A, from whom 28 got pregnant, 21 gave birth (68%), ten had aborts (32%). Of the nine women with blood group B, four gave birth (44%), and 5 had aborts (56%). Of the 14 women with blood group O, 12 gave birth (86%), and four had aborts (29%). Only three women had blood group AB, from whom two gave birth (67%), no woman had an abort (0 %), and one did not get pregnant (table 2).
A chi-square test was used to compare blood group and successful pregnancies. Four cell frequencies were below 5, and the results showed no significant correlation with χ²(1) = 2•401, p(χ²) = 0•493, p(Fisher’s exact test) = 0•518, V = 0•205.
Impact of thrombophilia associated mutations
All women showed thrombophilia associated mutations, except one who did not give consent for this kind of genetic testing. 41 women had a PAI1 mutation, 39 a prothrombin 19911 mutation, 27 a fibrinogen alpha mutation, 19 a fibrinogen gamma mutation, seven a factor V Leiden mutation, six a HR2 mutation, two a prothrombin 20210 mutation, two a FSAP mutation, one a MTHFR mutation, and none had a tPA mutation.
From 16 women with only one mutation 13 gave births (81%), two had aborts (13%), and one did not get pregnant (6%). From 16 women with two mutations 12 gave births (75%), three had aborts (19%), and one did not get pregnant (6%). Three mutations were found with 15 women, of whom seven gave births (47%), seven had aborts (47%), and three did not get pregnant (20%). Four to six mutations were found in 12 women. Of these women, seven gave births (64 %), five had aborts (45%), and one woman did not get pregnant (9%) (table 2).
A chi-square test was used to compare number of thrombophilia associated mutations (one or two versus three or more) and successful pregnancies. No expected cell frequencies were below 5. Results showed no significant correlation with χ²(1) = 1•701, p(χ²) = 0•192, p(Fisher’s exact test) = 0•276, V = 0•170.
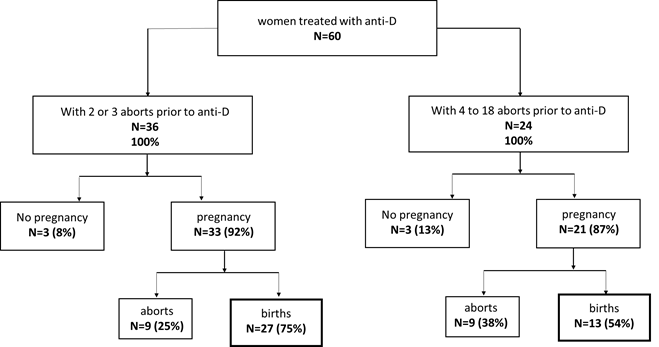
Figure 1: Outcome of women treated with Anti-D in addition to LMWH/ and or aspirin.
As some women had both, birth(s) and abort(s), sum of values for births and aborts can be higher than 100%.
Because some women had birth(s) and abort(s), sum of values for births and aborts can be higher than 100%.
a in relation to time of conception. Six women did not get pregnant, and did only receive Anti-D prior to conception.
Table 2: Efficacy of treatment with Anti-D
Discussion
The vast majority of women treated with Anti-D had unremarkable pregnancy and delivery. More than 70% of all pregnant women delivered healthy children. Interestingly, the efficacy of this treatment does not appear to be dependent on age, thrombophilia, and prior treatment. Nevertheless, by these data there could be a relation between those parameters and outcome of a pregnancy, but case numbers are too low for significant statistical analysis. Treatment with Anti-D was safe with no signs of significant hemolysis.
Worldwide, RPL represents a serious and unresolved problem [1],[2],[6]. Immune pathways play a key role in the pathophysiology of RPL. Already at early stage, gestation induces a major immune response which presumably involves all immune cells, including lymphocytes, macrophages and decidual dendritic cells. Thus, the question whether immunotherapy may help in management of RPL is increasingly focused in new studies[18].
Macrophages are presumably the main player in regulation of pregnancy, including implantation, placentation, fetal development, parturition, and most importantly vascular remodeling at the maternal-fetal interface. Thus, some modulation of macrophages may prevent miscarriages related to immune imbalance. The question then arose to whether subtle attraction of these cells by coating of autologous red blood cells in such affected women may result in successful pregnancies. In fact, the results obtained during observation were encouraging. The vast majority of treated women had unremarkable pregnancy and delivery. Interestingly, the efficacy of this treatment does not appear to be dependent on age, thrombophilia, and prior treatment. Nevertheless, by these data there could be a relation between those parameters and outcome of a pregnancy, but case numbers are too low for significant statistical analysis. Since all treated women received simultaneously LMWH and/or aspirin, the question remains open whether the beneficial effect was solely related to Anti-D or to a combined effect of both drugs. This is supported by the finding that LWMH beyond its anti-coagulating effect may induce immunomodulation.[19] Until now, a monotherapy with Anti-D has not been undertaken. However, it has been shown that treatment with intravenous immunoglobulins (IvIg) that somewhat resembles Anti-D, may improve the outcome in pregnant women with miscarriages.
Similarly, there is evidence that polyclonal Anti-D has an immunomodulatory effect, which could not fully be explained yet.[20],[21] In our experience, this treatment is safe when it is given subcutaneously or at least slowly by intravenous route.[22] In previous studies we have demonstrated that the administration of LMWH and/or aspirin results in live births in most women with RPL.[7] In this retrospective study, we used low Anti-D doses (2 to 3 times 300 µg) for treatment of RPL in Rh(D) positive women who remained abortive despite treatment with LMWH and/or aspirin.
At first glance, the question may arise whether this treatment could be justified in Rhesus positive pregnant women. Based on the following facts, this treatment is, we think, rational as long as no alternative therapy is available for such affected patients. Anti-D is used in treatment of adults and children for four decades with little or no side effects, if the drug was carefully injected.[22] The injection of 300 µg is harmless compared to that dose given in ITP (50-75 µg/kg body weight), and to that amount reaching Rh(D) positive infants in Rh(D) negative pregnant women who receive at least two times 100 - 300 µg prophylaxis.
The positive influence of Anti-D on RPL is evident as reflected by the significantly increased rates of successful pregnancies in the majority of treated women in our practice. This effect does not appear to be dependent on age, inherited hemophilias or blood group. The question whether Anti-D per se or only in combination with LMWH is effective is a matter of speculation. Since the mechanisms by which LMWH influences the outcome in RPL is largely unknown, a synergic effect cannot be excluded. Ultimately, LMWH has been shown to induce in vitro and in vivo a proinflammatory profile on RPL. It has been suggested that this effect could be valuable at the implantation stage.[7] Thus, it remains unclear whether Anti-D alone could also be effective as well as the combination with LMWH.
The question why this treatment was ineffective in 26% of the treated pregnant women remains to be answered. A possible explanation could be related to unknown predisposition factors that cannot be influenced by immunological modulations. Similarly, the phenomenon that the treatment may again result in miscarriage in some women who had been shown to be responsive remains also obscure. Whether this ineffectiveness could be related to the giving dose or timing of Anti D administration remains to be determined.
Though, the most possible explanation for the successful pregnancy following treatment with Anti-D may be attributed to an immunomodulatory effect, some other questions remain open. For example, when and by which mechanisms Anti-D may induce the tolerance of the semiallogeneic fetus. There is no doubt that pregnancy is highly controlled by immune reactions which simultaneously maintain immune response (defense) and tolerance (semi-allogeneicity), respectively. [10],[11],[23].
Changes during pregnancy include an increase in phagocytic cells and their functional capacity to ingest IgG coated RBCs,[24] an increase of T and B regulatory cells, reduction of natural killer cells, and polarization of decidual macrophages.[23],[25-28] It has been demonstrated that RPL is associated with an imbalance of pregnancy related immune haemostasis,[10],[11],[29] which could, at least in part, be normalized by immunomodulation.[18],[30]
Anti-D administration into Rh(D) positive pregnant women results in coating of autologous RBCs with IgG antibodies. This happening represents a dangerous signal, and attracts macrophages to recognize IgG opsonized RBCs. Although the attracted macrophages do not appear to ingest a significant amount of the IgG coated RBCs, they could be influenced in the presence of IgG-coated RBCs.
In addition, polyclonal Anti-D may contain antibodies other than Anti-D which may have influence on macrophages, independent of phagocytosis, i.e. HLA and anti-idiotype antibodies. Thus, it would not be surprising that the reactions to Anti-D may lead to polarization of decidual macrophages. These cells exhibit wide plasticity and are sensitive to small microenvironmental changes, including nutrition, smoking, toxins, inflammation, and physiological stress.[13-15]
It seems that the M1 subtype predominates over the M2 subtype in RPL. Coating of autologous RBCs with antibodies represents unphysiological condition in vivo, which may lead to polarization of M1 to M2 phenotype. The latter cells seem to be responsible for immunotolerance, which is a prerequisite for successful implantation a fetal development.
This hypothesis can only be proved by phenotyping of decidual macrophages prior to and following treatment. Alternatively, an extended investigation of cytokines and changes of peripheral immune cells may give an explanation of this phenomenon.[8-11],[26],[28]
Conclusions
We are aware of the limitation of the presented study. It is mainly based on retrospective data. In addition, the number of treated women is relatively small and too low for significant statistical analysis, and an optimal dose and timing of Anti-D remained speculative.
Anti-D could be a new therapeutic option in women with recurrent pregnancy loss who are not responding well to low molecular heparin and/or aspirin alone.
Thus, new well-designed studies may gain deeper insights in this field, and into the maternal-fetal immune interface.
Statement of Ethics
This is a retrospective analysis of patient data. The Ethics Committee of the Berlin Medical Association has confirmed that no ethical approval is required.
Author Contribution statement
FR: literature search, data collection, data analysis, data interpretation, writing, critical revision, accessed and verified all data; FL: data collection; HK: treatment of patient, critical revision; JS: writing, critical revision; BH: treatment of patients, critical revision; AK: data collection; RH: treatment of patient, critical revision; CFS: treatment of patient, critical revision, SM: treatment of patient, critical revision; GH: treatment of patient, critical revision; AS: treatment of patient, literature search, data interpretation, writing, critical revision, accessed and verified all data
All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication of the manuscript.
REFERENCES
- Hong Li Y, Marren A. Recurrent pregnancy loss: A summary of international evidence-based guidelines and practice. Aust J Gen Pract 2018; 47(7): 432-436.
- Green DM, O'Donoghue K. A review of reproductive outcomes of women with two consecutive miscarriages and no living child. J Obstet Gynaecol 2019; 39(6): 816-821.
- Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012; 98(5): 1103-1111.
- Homer HA. Modern management of recurrent miscarriage. Aust N Z J Obstet Gynaecol 2019; 59(1): 36-44.
- Henkel A, Shaw KA. Advances in the management of early pregnancy loss. Curr Opin Obstet Gynecol 2018; 30(6): 419-424.
- Rai R, Regan L. Recurrent miscarriage. Lancet 2006; 368(9535): 601-11.
- Monien S, Kadecki O, Baumgarten S, Salama A, Dorner T, Kiesewetter H. Use of heparin in women with early and late miscarriages with and without thrombophilia. Clin Appl Thromb Hemost 2009; 15(6): 636-644.
- Luan X, Kang X, Li W, Dong Q. An investigation of the relationship between recurrent spontaneous abortion and memory T follicular helper cells. Am J Reprod Immunol 2017; 78(5).
- Qian J, Zhang N, Lin J, et al. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci Trends 2018; 12(2): 157-167.
- Yang F, Zheng Q, Jin L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front Immunol 2019; 10: 2317.
- Deshmukh H, Way SS. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu Rev Pathol 2019; 14: 185-210.
- Saini V, Arora S, Yadav A, Bhattacharjee J. Cytokines in recurrent pregnancy loss. Clin Chim Acta 2011; 412(9-10): 702-708.
- Jena MK, Nayak N, Chen K, Nayak NR. Role of Macrophages in Pregnancy and Related Complications. Arch Immunol Ther Exp (Warsz) 2019; 67(5): 295-309.
- Zhang YH, He M, Wang Y, Liao AH. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front Immunol 2017; 8: 120.
- Yao Y, Xu XH, Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol 2019; 10: 792.
- Lazarus AH, Crow AR. Mechanism of action of IVIG and anti-D in ITP. Transfus Apher Sci 2003; 28(3): 249-55.
- Brinc D, Denomme GA, Lazarus AH. Mechanisms of anti-D action in the prevention of hemolytic disease of the fetus and newborn: what can we learn from rodent models? Curr Opin Hematol 2009; 16(6): 488-496.
- Carp H. Immunotherapy for recurrent pregnancy loss. Best Pract Res Clin Obstet Gynaecol 2019; 60: 77-86.
- Rasmark Roepke E, Bruno V, Nedstrand E, et al. Low-molecular-weight-heparin increases Th1- and Th17-associated chemokine levels during pregnancy in women with unexplained recurrent pregnancy loss: a randomised controlled trial. Sci Rep 2019; 9(1): 12314.
- Coopamah MD, Freedman J, Semple JW. Anti-D initially stimulates an Fc-dependent leukocyte oxidative burst and subsequently suppresses erythrophagocytosis via interleukin-1 receptor antagonist. Blood 2003; 102(8): 2862-2867.
- Crow AR, Lazarus AH. The mechanisms of action of intravenous immunoglobulin and polyclonal anti-d immunoglobulin in the amelioration of immune thrombocytopenic purpura: what do we really know? Transfus Med Rev 2008; 22(2): 103-116.
- Salama A. Emerging drugs for immune thrombocytopenia (ITP). Expert Opin Emerg Drugs 2017; 22(1): 27-38.
- Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC. Immune Cells at the Fetomaternal Interface: How the Microenvironment Modulates Immune Cells To Foster Fetal Development. J Immunol 2018; 201(2): 325-334.
- Davis D, Kaufmann R, Moticka EJ. Nonspecific immunity in pregnancy: monocyte surface Fcgamma receptor expression and function. J Reprod Immunol 1998; 40(2): 119-128.
- Huang N, Chi H, Qiao J. Role of Regulatory T Cells in Regulating Fetal-Maternal Immune Tolerance in Healthy Pregnancies and Reproductive Diseases. Front Immunol 2020; 11: 1023.
- Pei CZ, Kim YJ, Baek KH. Pathogenetic factors involved in recurrent pregnancy loss from multiple aspects. Obstet Gynecol Sci 2019; 62(4): 212-223.
- Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest 2018; 128(10): 4224-4235.
- Esteve-Sole A, Luo Y, Vlagea A, et al. B Regulatory Cells: Players in Pregnancy and Early Life. Int J Mol Sci 2018; 19(7).
- Matthiesen L, Kalkunte S, Sharma S. Multiple pregnancy failures: an immunological paradigm. Am J Reprod Immunol 2012; 67(4): 334-340.
- Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol 2018; 80(4): e13018.
