Perioperative Outcomes of Acute Type-A Aortic Dissection Repair was Unaffected by COVID-19 Testing Delay
Article Information
Felix Orelaru1, Elizabeth L Norton2, Rana-Armaghan Ahmad3, Aroma Naeem3, Karen M Kim3, Shinichi Fukuhara3, Himanshu J Patel3, G Michael Deeb3, Bo Yang3*
1Department of General Surgery, St. Joseph Mercy, Ann Arbor, Michigan, USA
2Division of Cardiothoracic Surgery, Department of Surgery, Emory University, Atlanta, Georgia
3Department of Cardiac Surgery, Michigan Medicine, Ann Arbor, Michigan, USA
*Corresponding author: Bo Yang, Department of Cardiac Surgery, Michigan Medicine, Ann Arbor, Michigan, USA.
Received: 11 March 2022; Accepted: 21 March 2022; Published: 05 April 2022
Citation:
Felix Orelaru, Elizabeth L Norton, Rana-Armaghan Ahmad, Aroma Naeem, Karen M Kim, Shinichi Fukuhara, Himanshu J Patel, G Michael Deeb, Bo Yang. Perioperative Outcomes of Acute Type-A Aortic Dissection Repair was Unaffected by COVID-19 Testing Delay. Cardiology and Cardiovascular Medicine 6 (2022): 100-110.
Share at FacebookAbstract
Background: This study assesses impact of COVID-19 testing delay on perioperative outcomes of Acute Type A Aortic Dissection (ATAAD) repair at a single institution.
Methods: From January 2010 - May 2021, 539 ATAAD patients underwent open aortic repair at our institution. Sixty-five of these patients had open aortic repair during COVID (March 2020 - May 2021) and 474 patients were pre-COVID (January 2010 - February 2020).
Results: Compared to the pre-COVID group, patients During-COVID had a higher proportion of previous myocardial ischemia [9/65 (14%) vs 28/474 (5.9%), p=0.03], chronic obstructive pulmonary disease [14/65 (22%) vs 55/474 (12%), p=0.02], and renal malperfusion syndrome [11/65 (17%) vs 30/474 (6.4%), p=0.01]. There was no significant difference in surgical outcomes between groups, including operative mortality (7.6% vs 9.2%, p=0.64). The median admission-to-Operating Room (OR) time was 107 minutes in the During-COVID group compared to 87 minutes in pre-COVID group, p=0.88. During COVID, the median admission-to-OR time was significantly longer in the Waiting group compared to the No-waiting group (209 min vs 75min, p=0.0009). Only one patient had positive COVID test. There were no aortic ruptures while awaiting COVID testing results. There was a total of 6 reported deaths in the During-COVID group: 1 patient died post-surgery due to ARDS caused by COVID, and others due to ischemic stroke (3 patients) and organ failure (2 patients).
Conclusions: Perioperative outcomes of ATAAD patients were similar during-COVID compared to pre-COVID. Waiting for COVID testing results did not significantly affect the perioperative outcomes among ATAAD patients after repair.
Keywords
Aortic dissection; COVID-19; Outcomes; Thoracic aortic repair
Aortic dissection articles; COVID-19 articles; Outcomes articles; Thoracic aortic repair articles
Article Details
Abbreviations:
ATAAD: Acute type A aortic dissection; CPB: Cardiopulmonary bypass; SARS CoV-2 (COVID-19): Severe acute respiratory syndrome coronavirus 2
1. Introduction
SARS CoV-2 (COVID-19) virus is a highly infective pathogen associated with significantly increased operative mortality in cardiac surgery patients and high transmission risks to the medical team [1,2]. Guidelines to mitigate virus propagation has included COVID-19 virus testing for all patients prior to surgical procedures such as Acute Type A Aortic Dissection (ATAAD) repair [3-5]. However there is a dilemma if COVID-19 testing should take precedence over emergent ATAAD repair, and there is currently no evidence in literature to guide in this scenarios. Acute type A aortic dissection is a critical condition that requires swift surgical intervention due to high mortality rate of 1-2% per hour if left untreated after the onset of symptoms [6]. This study assesses impact of COVID-19 testing delay on perioperative outcomes in ATAAD patients after repair at a single institution.
2. Materials and Methods
This study was approved by the Institutional Review Board at Michigan Medicine (HUM001118517) and a waiver of informed consent was obtained.
2.1. Study Population
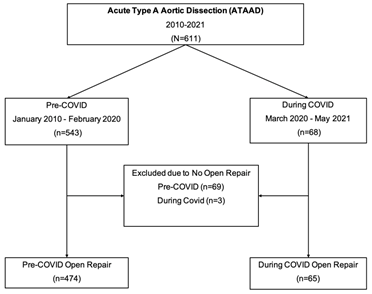
Between January 2010 - May 2021, 611 patients presented with acute type A aortic dissection at our institution. Sixty-eight patients during COVID (March 2020 - May 2021) and 543 patients pre-COVID (January 2010 - February 2020). In total only 539 patients had open ATAAD repair: During-COVID (n=65) and pre-COVID (n=474). All patients who did not undergo open aortic repair or poor surgical candidate were excluded from the study cohort: During-COVID group (n=3) and pre-COVID group (n=69), (Figure 1). All patients above 18years old who presented with ATAAD and underwent open surgical repair were included in the study. All patients during the COVID pandemic had COVID-19 testing using Reverse Transcriptase Polymerase Chain Reaction (RT-PCR). Waiting group included patients who waited for COVID-19 results prior to any open aortic repair or endovascular fenestration/stenting by interventional radiologist. Patients in the no-waiting group had COVID-19 testing done before transfer and the results of COVID test was known on admission. Data was retrieved through the University of Michigan Aortic Database to identify patients who underwent open surgical repair of ATAAD. Data from the Aortic Database was augmented with data from the Society of Thoracic Surgeons (STS) Michigan Medicine Cardiac Surgery Data Warehouse to determine preoperative, intraoperative, and postoperative characteristics. Univariate comparisons between groups were performed using Wilcoxon rank-sum tests for continuous data. The primary outcome was defined as perioperative outcomes in acute type A aortic dissection patients who waited for COVID-19 test results during the COVID-19 pandemic.
3. Statistical Analysis
Data were presented as median (25th, and 75th percentile) for continuous data and n (%) for categorical data. Comparisons of univariate characteristics were performed using Wilcoxon rank-sum tests for continuous data and chi-square tests for categorical data. Statistical calculations were performed using SAS 9.4 (SAS Institute, Cary, NC).
4. Results
4.1. Pre-COVID versus During-COVID group
4.1.1. Preoperative demographics data
Compared to the pre-COVID group, patients During-COVID had a significantly higher proportion of chronic obstructive pulmonary disease (22% vs 12%), previous myocardial ischemia (14% vs 5.9%), peripheral vascular diseases (55% vs 28%), celiac malperfusion syndrome (9.2% vs 1.5%), renal malperfusion syndrome (17% vs 6.4%), lower extremity malperfusion syndrome (15% vs 7.5%), and DeBakey Class II (20% vs 10%) but lower proportion of patients with DeBakey Class I (80% vs 90%). Otherwise, there was no difference between groups (Table 1).
4.1.2. Intraoperative and Perioperative Data
The median admission-to-operating room (OR/IR) time was similar between groups: 107 minutes in the During-COVID group compared to 87 minutes in pre-COVID group (p=0.88). The During-COVID group had higher proportion of aortic valve replacement (9.2% vs 2.4%), otherwise all other procedures such as aortic root replacement, root repair, arch replacement and concomitant mitral valve or tricuspid replacement were similar between groups. Compared to the pre-COVID group, patients During-COVID had shorter surgical incision-to-cardiopulmonary bypass (CPB) time (49minutes vs 76minutes), CPB time (163minutes vs 219 minutes), aortic cross clamp time (121minutes vs 144minutes) and hypothermic circulatory arrest (HCA) time (22minutes vs 31minutes), (Table 2). Lastly, compared to the pre-COVID group, the During-COVID group had higher proportion of deep sternal wound infection (4.7% vs 0.2%). Otherwise, there was no significant difference in surgical outcomes between groups, including operative mortality, acute renal failure requiring dialysis and stroke, among others (Table 3). Only one patient had positive COVID test. There was a total of 6 reported deaths in the During-COVID group: 1 patient died post-surgery due to ARDS caused by COVID, and others due to ischemic stroke (3 patients) and organ failure (2 patients).
|
Pre-COVID |
During-COVID |
p-value |
|
|
(n=474) |
(n=65) |
||
|
Patient age (years) |
60 (51, 69) |
57 (48, 70) |
0.65 |
|
Sex, male |
313 (66) |
44 (68) |
0.79 |
|
BMI (kg/m2) |
28 (25, 33) |
27 (24, 31) |
0.07 |
|
Pre-existing comorbidities |
|||
|
Hypertension |
367 (77) |
57 (88) |
0.06 |
|
Diabetes |
39 (8.2) |
10 (15) |
0.06 |
|
Smoking status |
0.41 |
||
|
Never |
191 (41) |
20 (31) |
0.12 |
|
Former |
157 (34) |
27 (42) |
0.2 |
|
Current |
120 (26) |
18 (28) |
0.72 |
|
CAD |
81 (18) |
6 (9.2) |
0.09 |
|
COPD |
55 (12) |
14 (22) |
0.02 |
|
History of MI |
28 (5.9) |
9 (14) |
0.03 |
|
History of renal failure |
18 (3.8) |
6 (9.2) |
0.06 |
|
History of CVA |
22 (79) |
6 (9.2) |
0.13 |
|
PVD |
132 (28) |
36 (55) |
<0.0001 |
|
Connective tissue disorder |
16 (3.4) |
2 (3.1) |
1.0 |
|
Previous cardiac surgery |
37 (7.8) |
6 (9.2) |
0.69 |
|
Aortic Insufficiency |
|||
|
Trace |
49 (11) |
8 (12) |
0.71 |
|
Mild |
125 (28) |
14 (22) |
0.31 |
|
Moderate |
77 (17) |
12 (18) |
0.76 |
|
Severe |
86 (19) |
15 (23) |
0.43 |
|
Ejection fraction |
58 (55, 65) |
60 (55, 65) |
0.23 |
|
Acute MI |
19 (4.0) |
3 (4.6) |
0.74 |
|
Acute stroke |
42 (8.9) |
7 (11) |
0.63 |
|
Acute renal insufficiency |
37 (7.8) |
7 (11) |
0.43 |
|
Acute paralysis |
12 (2.6) |
4 (6.2) |
0.12 |
|
Cardiogenic shock |
49 (10) |
8 (12) |
0.63 |
|
Tamponade |
62 (14) |
9 (14) |
0.96 |
|
CPR |
8 (1.7) |
3 (5.3) |
0.11 |
|
Preoperative creatinine |
1.0 (0.8, 1.3) |
1.1 (0.9, 1.4) |
0.09 |
|
Malperfusion syndrome |
116 (25) |
20 (31) |
0.29 |
|
Coronary |
20 (4.3) |
2 (3.1) |
1 |
|
Cerebral |
40 (8.5) |
6 (9.2) |
0.85 |
|
Spinal cord |
7 (1.5) |
2 (3.1) |
0.3 |
|
Celiac |
7 (1.5) |
6 (9.2) |
0.002 |
|
Mesenteric |
34 (7.2) |
7 (11) |
0.32 |
|
Renal |
30 (6.4) |
11 (17) |
0.01 |
|
Lower extremity |
35 (7.5) |
10 (15) |
0.03 |
|
Delayed operation |
48 (10) |
9 (14) |
0.37 |
|
DeBakey Class I |
416 (90) |
52 (80) |
0.02 |
|
DeBakey Class II |
47 (10) |
13 (20) |
0.02 |
|
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. |
|||
Table 1: Demographics and pre-existing conditions of Pre-COVID versus During-COVID group.
|
Pre-COVID |
During-COVID |
p-value |
|
|
(n=474) |
(n=65) |
||
|
Admission to OR/IR time (minutes) |
87 (51, 251) |
107 (42, 308) |
0.88 |
|
Number of COVID tests per patient |
0 |
1 (1,1) |
|
|
Waited for COVID test results |
0 |
20 (31) |
|
|
Aortic Root Procedure |
0.02 |
||
|
None |
38 (8.1) |
2 (3.1) |
0.21 |
|
AVR only |
11 (2.4) |
6 (9.2) |
0.01 |
|
Root replacement |
143 (30) |
13 (20) |
0.11 |
|
Root repair |
275 (59) |
44 (68) |
0.18 |
|
Arch Replacement |
0.35 |
||
|
None |
26 (5.5) |
1 (1.5) |
0.23 |
|
Hemiarch |
288 (61) |
46 (71) |
0.14 |
|
Zone 1 Arch |
41 (8.7) |
5 (7.7) |
0.78 |
|
Zone 2 Arch |
88 (19) |
8 (12) |
0.2 |
|
Zone 3 Arch |
26 (5.5) |
5 (7.7) |
0.57 |
|
Frozen Elephant Trunk |
94 (20) |
10 (15) |
0.37 |
|
Concomitant Procedures |
|||
|
CABG |
24 (5.1) |
1 (1.5) |
0.34 |
|
Mitral valve |
7 (1.5) |
1 (1.5) |
1 |
|
Tricuspid valve |
6 (1.3) |
1 (1.5) |
0.6 |
|
Surgical incision to CPB (min) |
76 (61, 96) |
49 (35, 72) |
0.02 |
|
CPB time (min) |
219 (181, 271) |
163 (126, 217) |
<0.0001 |
|
Cross-clamp time (min) |
144 (110, 198) |
121 (82, 153) |
0.0001 |
|
HCA |
445 (95) |
63 (98) |
0.25 |
|
HCA time (min) |
31 (23, 40) |
22 (17, 33) |
<0.0001 |
|
Cerebral perfusion |
0.21 |
||
|
None |
22 (4.7) |
1 (1.6) |
0.34 |
|
Antegrade |
286 (61) |
38 (59) |
0.77 |
|
Retrograde |
109 (23) |
21 (33) |
0.1 |
|
Both antegrade and retrograde |
50 (11) |
4 (6.3) |
0.27 |
|
Lowest temperature (°C) |
19 (18, 24) |
25 (18, 27) |
0.007 |
|
Blood transfusion (PRBCs), units |
1.0 (0.0, 4.0) |
0.0 (0.0, 2.5) |
0.002 |
|
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. |
|||
Table 2: Intraoperative Outcomes of Pre-COVID versus During-COVID group.
4.2. Waiting versus no-waiting group during covid time
4.2.1. Preoperative demographics data
Compared to the Waiting group, the No-waiting group had more patients with malperfusion syndrome (40% vs 10%) but patients with interventional radiology (IR) amenable malperfusion syndrome such as celiac, mesenteric, renal, and lower extremity malperfusion syndrome were similar between groups. Also, there was no difference in demographics between groups including chronic obstructive pulmonary disease, acute myocardial infarction, connective tissue disorder, cardiac tamponade, among others, (Table 4).
4.2.2. Intraoperative and perioperative data
During COVID, the median admission-to-OR time was significantly longer in the Waiting group compared to the No-waiting group (209 minutes vs 75minutes). The average wait time for COVID-19 test result was 168 minutes. There were no aortic ruptures while awaiting COVID testing results. Furthermore, compared to the No-Waiting group, the Waiting group had less aortic root repair (50% vs 76%), but all other open aortic procedures including aortic root replacement, arch replacement, concomitant mitral and tricuspid valve replacement, and other intraoperative outcomes were similar between groups, (Table 5). Finally, postoperative outcomes such as operative mortality, pneumonia, prolonged ventilation, or acute renal failure requiring dialysis, were similar between the Waiting and No-Waiting group, (Table 6).
|
Pre-COVID |
During-COVID |
p-value |
|
|
(n=474) |
(n=65) |
||
|
Reoperation for bleeding |
22 (4.7) |
5 (7.8) |
0.36 |
|
Tamponade |
6 (1.3) |
2 (3.1) |
0.25 |
|
Deep sternal wound infection |
1 (0.2) |
3 (4.7) |
0.006 |
|
Sepsis |
13 (2.8) |
4 (6.3) |
0.14 |
|
Postoperative MI |
3 (0.6) |
0 (0) |
1 |
|
Atrial fibrillation |
156 (33) |
24 (38) |
0.52 |
|
Cerebrovascular accident |
30 (6.4) |
4 (6.3) |
1 |
|
TIA |
1 (0.2) |
0 (0) |
1 |
|
Acute renal insufficiency |
59 (13) |
12 (19) |
0.18 |
|
Requiring dialysis |
25 (5.4) |
7 (11) |
0.09 |
|
Permanent |
12 (2.6) |
2 (3.1) |
0.68 |
|
Gastrointestinal complications |
43 (9.2) |
9 (14) |
0.22 |
|
Pneumonia |
69 (15) |
14 (22) |
0.14 |
|
Prolonged ventilation (>24 hours) |
249 (53) |
30 (47) |
0.32 |
|
Hours intubated |
28 (12, 74) |
25 (10, 61) |
0.3 |
|
Reintubation |
31 (6.6) |
6 (9.4) |
0.43 |
|
Tracheostomy |
10 (2.1) |
2 (3.1) |
0.65 |
|
Postoperative LOS (days) |
10 (7, 16) |
10 (7, 20) |
0.68 |
|
Intraoperative mortality |
3 (0.6) |
0 (0) |
1 |
|
In-hospital mortality |
33 (7.0) |
6 (9.2) |
0.45 |
|
30-day mortality |
36 (7.6) |
6 (9.2) |
0.62 |
|
Operative mortality* |
36 (7.6) |
6 (9.2) |
0.64 |
|
Data presented as median (25 %, 75 %) for continuous data and n (%) for categorical data. |
|||
Table 3: Postoperative Outcomes of Pre-COVID versus During-COVID group.
|
No-Waiting |
Waiting |
p-value |
|
|
(n=45) |
(n=20) |
||
|
Patient age (years) |
56 (50, 69) |
57 (48, 71) |
0.84 |
|
Sex, male |
28 (62) |
16 (80) |
0.15 |
|
BMI (kg/m2) |
27 (23, 31) |
28 (25, 31) |
0.53 |
|
Pre-existing comorbidities |
|||
|
Hypertension |
37 (82) |
0 (0) |
0.05 |
|
Diabetes |
5 (11) |
5 (25) |
0.26 |
|
Smoking status |
0.49 |
||
|
Never |
12 (27) |
8 (40) |
0.28 |
|
Former |
14 (31) |
4 (20) |
0.36 |
|
Current |
14 (29) |
4 (25) |
1 |
|
CAD |
6 (13) |
0 (0) |
0.17 |
|
COPD |
12 (27) |
2 (10) |
0.19 |
|
History of MI |
8 (18) |
1 (5.0) |
0.25 |
|
History of renal failure |
5 (11) |
1 (5.0) |
0.66 |
|
History of CVA |
4 (8.9) |
2 (10) |
1 |
|
PVD |
27 (60) |
9 (45) |
0.26 |
|
Connective tissue disorder |
1 (2.2) |
1 (5.0) |
0.52 |
|
Previous cardiac surgery |
5 (11) |
1 (5.0) |
0.66 |
|
Pre-operative AI |
|||
|
Trace |
3 (6.7) |
5 (25) |
0.09 |
|
Mild |
11 (24) |
3 (15) |
0.52 |
|
Moderate |
10 (22) |
2 (10) |
0.32 |
|
Severe |
11 (24) |
4 (20) |
0.76 |
|
Ejection fraction |
58 (55, 65) |
65 (55, 67) |
0.35 |
|
Acute MI |
2 (4.4) |
1 (5.0) |
1 |
|
Acute stroke |
4 (8.9) |
3 (15) |
0.67 |
|
Acute renal insufficiency |
4 (8.9) |
3 (15) |
0.46 |
|
Acute paralysis |
2 (4.4) |
2 (10) |
0.58 |
|
Cardiogenic shock |
7 (16) |
1 (5.0) |
0.42 |
|
Tamponade |
7 (16) |
2 (10) |
0.71 |
|
Preoperative creatinine |
1.1 (0.9, 1.4) |
1.2 (1.0, 1.3) |
0.75 |
|
Malperfusion syndrome |
18 (40) |
2 (10) |
0.02 |
|
Coronary |
2 (4.4) |
0 (0) |
1 |
|
Cerebral |
5 (11) |
1 (5.0) |
0.66 |
|
Spinal cord |
2 (4.4) |
0 (0) |
1 |
|
Celiac |
5 (11) |
1 (5.0) |
0.66 |
|
Mesenteric |
5 (10) |
2 (13) |
1 |
|
Renal |
8 (20) |
2 (10) |
0.48 |
|
Lower extremity |
8 (18) |
2 (10) |
0.71 |
|
Delayed operation |
7 (16) |
2 (10) |
0.71 |
|
DeBakey Class |
|||
|
I |
37 (82) |
15 (75) |
0.52 |
|
II |
8 (18) |
5 (25) |
0.52 |
|
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. |
|||
Table 4: Demographics and Preoperative Outcomes of During-COVID Group.
|
No-Waiting |
Waiting |
p-value |
|
|
(n=45) |
(n=20) |
||
|
Admission to OR/IR time (minutes) |
75 (50, 176) |
209 (187, 318) |
0.0009 |
|
Number of COVID tests |
1 (1,1) |
1 (1,1) |
1 |
|
Wait time for COVID test results (minutes) |
0 (0,0) |
168 (80, 186) |
<0.0001 |
|
Aortic Root Procedure |
0.02 |
||
|
None |
0 (0) |
2 (10) |
0.09 |
|
AVR only |
5 (11) |
1 (5.0) |
0.66 |
|
Root replacement |
6 (13) |
7 (35) |
0.09 |
|
Root repair |
34 (76) |
10 (50) |
0.04 |
|
Arch Replacement |
0.9 |
||
|
None |
1 (2.2) |
0 (0) |
1 |
|
Hemiarch |
32 (71) |
14 (70) |
0.93 |
|
Zone 1 Arch |
4 (8.9) |
1 (5.0) |
1 |
|
Zone 2 Arch |
5 (11) |
3 (15) |
0.69 |
|
Zone 3 Arch |
3 (6.7) |
2 (10) |
0.64 |
|
Frozen Elephant Trunk |
8 (18) |
2 (10) |
0.71 |
|
Concomitant Procedures |
|||
|
CABG |
0 (0) |
1 (5.0) |
0.31 |
|
Mitral valve |
1 (2.2) |
0 (0) |
1 |
|
Tricuspid valve |
1 (2.2) |
0 (0) |
1 |
|
Surgical incision to CPB (min) |
58 (47, 81) |
31 (26, 35) |
0.18 |
|
CPB time (min) |
167 (126, 219) |
149 (125, 204) |
0.33 |
|
Cross-clamp time (min) |
121 (82, 152) |
115 (82, 175) |
0.97 |
|
HCA |
|||
|
HCA time (min) |
24 (17, 33) |
19 (17, 33) |
0.44 |
|
Cerebral perfusion |
0.14 |
||
|
None |
1 (2.2) |
0 (0) |
1 |
|
Antegrade |
29 (64) |
9 (47) |
0.2 |
|
Retrograde |
14 (31) |
7 (37) |
0.66 |
|
Both antegrade and retrograde |
1 (2.2) |
3 (16) |
0.07 |
|
Lowest temperature (°C) |
24 (18, 27) |
26 (22, 28) |
0.09 |
|
Blood transfusion (PRBCs), units |
0.0 (0.0, 3.0) |
0.0 (0.0, 2.0) |
0.21 |
|
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data. |
|||
Table 5: Intraoperative Outcome for During-COVID Group.
|
No-Waiting |
Waiting |
p-value |
|
|
(n=45) |
(n=20) |
||
|
Reoperation for bleeding |
5 (11) |
0 (0) |
0.31 |
|
Tamponade |
2 (4.4) |
0 (0) |
1 |
|
Deep sternal wound infection |
2 (4.4) |
1 (5.3) |
1 |
|
Sepsis |
2 (4.4) |
2 (11) |
0.58 |
|
Postoperative MI |
0 (0) |
0 (0) |
1 |
|
Atrial fibrillation |
16 (36) |
8 (42) |
0.62 |
|
Cerebrovascular accident |
4 (8.9) |
0 (0) |
0.31 |
|
TIA |
0 (0) |
0 (0) |
1 |
|
Acute renal insufficiency |
10 (22) |
2 (11) |
0.48 |
|
Requiring dialysis |
6 (13) |
1 (5.3) |
0.66 |
|
Permanent |
2 (4.4) |
0 (0) |
1 |
|
Gastrointestinal complications |
7 (16) |
2 (11) |
0.71 |
|
Pneumonia |
11 (24) |
3 (16) |
0.53 |
|
Prolonged ventilation (>24 hrs) |
24 (53) |
6 (32) |
0.11 |
|
Hours intubated |
26 (10, 63) |
16 (9, 61) |
0.64 |
|
Reintubation |
6 (13) |
0 (0) |
0.17 |
|
Tracheostomy |
2 (4.4) |
0 (0) |
1 |
|
Postoperative LOS (days) |
10 (7, 22) |
9 (6, 17) |
0.63 |
|
Intraoperative mortality |
0 (0) |
0 (0) |
1 |
|
In-hospital mortality |
6 (13) |
0 (0) |
0.17 |
|
30-day mortality |
6 (13) |
0 (0) |
0.17 |
|
Operative mortality* |
6 (13) |
0 (0) |
0.17 |
|
Data presented as median (25 %, 75 %) for continuous data and n (%) for categorical data. |
|||
Table 6: Postoperative Data for During-COVID Group.
5. Discussion
It is well documented in literature that ATAAD is a catastrophic event and a surgical emergency with associated mortality rate of 1-2% per hour when untreated, but average operative mortality rate of 20-25% after repair [6,7]. Therefore, ATAAD patients historically undergo emergent surgical intervention to improve their survival outcome. However, the COVID-19 pandemic steered many hospitals and surgeons to require COVID-19 virus testing results prior to operative ATAAD repair [4,5]. One of the reasons for this decision is because COVID-19 virus has been associated with significantly increased operative mortality and morbidity in cardiac surgery patients, and knowledge of pre-operative infection status could help optimize patients to improve surgical outcomes [8,9]. Additionally, a multinational collaborative study (COVIDSurg Collaborative) reported a 34.0% 30-day postoperative mortality and 94.1% risk of pulmonary complication (pneumonia and acute respiratory distress syndrome) in COVID-19 positive cardiac surgery patients [2]. Furthermore, other rationales to wait for COVID-19 testing result is based on the evidence that preoperative patient testing limits healthcare workers exposure, minimize hospital acquired COVID-19 transmission to vulnerable patients and facilitate contact tracing in cases of exposure [10,11]. However, International Registry of Acute Aortic Dissection (IRAD) data showed that delayed ATAAD repair greater than 24 hours from symptom onset is associated with a 17.1% operative mortality in ATAAD patients [12]. Also, other studies have shown up to a 66.7% operative mortality in ATAAD patients delayed beyond 8-12 hours prior to repair [13]. Considering the risk associated with delayed surgical intervention in ATAAD patients as well as the risk and benefit of COVID-19 virus testing prior to ATAAD repair, our study sought to clarify the dilemma in the surgical management of acute Type A aortic dissection patients during COVID. In our study, all ATAAD patients had COVID-19 testing done at outside hospital before transfer or at our hospital before they went to the operating room except one patient in the early COVID pandemic. That patient eventually died of COVID respiratory failure after a straightforward open type A aortic dissection repair. Patients who waited for COVID-19 test had almost three times prolonged admission-to-operating room time compared to No-waiting group due to high COVID testing turnaround time (168 minutes). The prolong testing time could be because early technology and laboratory processes had not been streamlined. A major concern of delayed diagnosis and management of acute type A aortic dissection is the risk of aortic rupture [14]. However, in our study, there were no aortic ruptures while awaiting COVID testing results. This observation could be because of adequate medical stabilization such as blood pressure control prior to operative intervention. Also, we found that only one patient (0.02%) had a positive COVID-19 result prior to ATAAD repair. This patient did not wait for COVID result prior to intervention and died on postoperative day twelve due to Acute Respiratory Distress Syndrome (ARDS) and subsequent cardiac arrest. This case happened at early stage of pandemic. Risk of ARDS has been shown to be significantly increased in COVID-19 positive ATAAD patients after surgery due to the use of Cardiopulmonary Bypass (CPB). It is reported that CPB pump have non-endothelial surfaces that trigger proinflammatory responses mediated by tumor necrosis factor α and interleukin-10 [1,15]. This literature finding suggest that efforts could be directed towards perioperative management of inflammatory response in COVID-19 positive ATAAD patients to improve mortality outcomes associated with ARDS.
Finally, our study shows that operative mortality (i.e., both 30-day mortality and/or in-hospital mortality) was similar between ATAAD patients that waited for COVID-19 result prior to repair and No-waiting group. Our findings support that the operative delay in ATAAD patients due to prolonged COVID-19 testing did not worsen the surgical outcomes of ATAAD patients during COVID-19 pandemic at our institution. It is noteworthy that our results should be interpreted with caution due to small sample size in the waiting group and low study power, which could have resulted in our inability to delineate differences between groups. Also, our study is limited because it is a single center retrospective study and the timeline for the during-COVID group is short (March 2020-May 2021). We acknowledge that the COVID-19 pandemic is still ongoing and long-term studies would be warranted. In conclusion, our study showed that obtaining and waiting for COVID-19 test results during the COVID-19 pandemic delayed admission-to-operating room time in acute type A dissection patients but there was no change in perioperative outcomes. Institutional policy requiring mandatory COVID testing prior to acute type A dissection repair is appropriate and could protect vulnerable patients, limit healthcare workers exposure to symptomatic or asymptomatic COVID-19 carrier and facilitate contact tracing in cases of exposure.
Acknowledgements
Our sincere gratitude to the entire staff at the University of Michigan Cardiac Surgery Department for their support through this project. Dr. Yang is supported by the NHLBI of NIH K08HL130614, R01HL141891, and R01HL151776, Phil Jenkins and Darlene & Stephen J. Szatmari Funds
References
- Harky A, Poole G, Axiaq A, et al. COVID-19 and cardiac surgery: do outcomes differ? J Card Surg 35 (2020): 3391-3394.
- Archer JE, Odeh A, Ereidge S, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 396 (2020): 27-38.
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Information for Healthcare Professionals About Coronavirus (COVID-19) (2019).
- Engelman D, Lother S, George I, et al. Ramping Up Delivery of Cardiac Surgery During the COVID-19 Pandemic: A Guidance Statement from The Society of Thoracic Surgeons COVID-19 Task Force. The Annals of Thoracic Surgery 110 (2020): 712-717.
- Mehta C, Malaisrie S, Budd A, et al.. Triage and management of aortic emergencies during the coronavirus disease 2019 (COVID-19) pandemic: A consensus document supported by the American Association for Thoracic Surgery (AATS) and Asian Society for Cardiovascular and Thoracic Surgery (ASCVTS). Asian Cardiovascular and Thoracic Annals (2020).
- Lindsay J, Hurst J. Clinical Features and Prognosis in Dissecting Aneurysm of the Aorta. Circulation. 35 (1967): 880-888.
- Berretta P, Patel H, Gleason T, et al. IRAD experience on surgical type A acute dissection patients results and predictors of mortality. Ann Cardiothorac Surg 5 (2016): 346-351.
- Nguyen T, Thourani V, Nissen A, et al. The Effect of COVID-19 on Adult Cardiac Surgery in the United States in 717 103 Patients. The Annals of Thoracic Surgery 113 (2022): 738-746.
- Sanders J, Akowuah E, Cooper J, et al. Cardiac surgery outcome during the COVID-19 pandemic: a retrospective review of the early experience in nine UK centres. Journal of Cardiothoracic Surgery 16 (2021): 43.
- Ortoleva J, Dalia A. Preoperative COVID-19 Testing for Cardiovascular Procedures in Endemic Areas Should be Mandatory. Journal Of Cardiothoracic and Vascular Anesthesia 34 (2020): 3180-3181.
- Kovoor J, Tivey D, Williamson P, et al. Screening and testing for COVID-19 before surgery. ANZ Journal of Surgery 90 (2020): 1845-1856.
- Trimarchi S, Nienaber C, Rampoldi V. Contemporary Results of Surgery in Acute Type A Aortic Dissection: The International Registry of Acute Aortic Dissection Experience. ACC Current Journal Review 14 (2005): 45-46.
- Matthews C, Madison M, Timsina L, et al. Impact of time between diagnosis to treatment in Acute Type A Aortic Dissection. Scientific Reports 11 (2021).
- Patel HJ, Williams DM, Dasika NL, et al. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: A long-term analysis. J Thorac Cardiovasc Surg 135 (2008): 1288-1296.
- Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology 11 (2020): 1446.