Peri-Coronary Sinus Flutter: A Unique Form of an Atypical Atrial Flutter. A Case Report
Article Information
Athanasios Ziakos1*, Harald Greiss1, Angelis Sezenias1, Armin Sause1
1Department of Cardiology, Helios University Heart Center Wuppertal, University Witten/Herdecke, Arrenbergerstrasse 20, 42117, Germany
*Corresponding author: Athanasios Ziakos, Department of Cardiology, HELIOS University Heart Center Wuppertal, University Witten/Herdecke, Arrenbergerstrasse 20, 42117, Germany.
Received: 10 January 2023; Accepted: 18 January 2023; Published: 15 February 2023
Citation: Athanasios Ziakos, Harald Greiss, Angelis Sezenias, Armin Sause. Peri-Coronary Sinus Flutter: A Unique Form of an Atypical Atrial Flutter. A Case Report. Cardiology and Cardiovascular Medicine 7 (2023): 45-49.
Share at FacebookAbstract
The coronary sinus musculature connects to the right and the left atrium, forming electrical interatrial bridges and can be a critical part of various left atrial and biatrial flutter circuits. We herein present a rare case of a macroreentrant tachycardia around only the coronary sinus using interatrial connections at its orifice and the inferolateral left atrium and the adjacent left atrial myocardium. Crucial for the identification of this macroreentrant circuit was the record of low voltage signals of epicardial sleeves through an endocardially placed multipolar HD-mapping catheter with small flat electrodes. The peri-Coronary sinus flutter should always be included in the differential diagnosis of an atypical atrial flutter.
Keywords
Atrial Flutter; Catheter Ablation; Coronary Sinus Flutter; Macroreentrant Tachycardia
Atrial Flutter articles; Catheter Ablation articles; Coronary Sinus Flutter articles; Macroreentrant Tachycardia articles
Article Details
1. Introduction
In recent years, flutter circuits utilising epicardial structures have been a focus of interest, as their occurrence has become more frequent after endocardial catheter ablation and understanding the mechanisms of complex macroreentrant tachycardias has become deeper through high resolution mapping. As already known from several anatomical and invasive studies, the coronary sinus (CS) musculature connects to the right atrium (RA) as well as the left atrium (LA) forming electrical interatrial connections. The connections to the LA can vary from one or two up to a wide continuum [1–3]. The coronary sinus often is a critical part of various forms of left atrial macroreentrant tachycardias, mostly of common perimitral flutter, and biatrial tachycardias, such as large circuits around both mitral and tricuspid valves or biatrial flutter with perimitral circuit using only the right atrial septum [4–6]. The CS musculature with its connections to RA and LA and the adjacent myocardium of LA can serve as substrate for a macroreentrant circuit without utilizing other atrial areas. However, this kind of tachycardia has only been reported once [7]. We herein report a rare case of a peri-CS atrial flutter in a female patient after pulmonary vein isolation (PVI) four years ago and roof line ablation due to roof-dependent atypical flutter during the same procedure.
2. Case Presentation
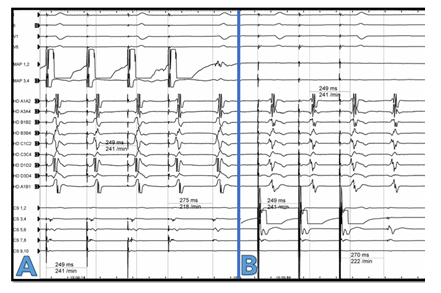
A 77-year-old female patient presented with shortness of breath and tachycardia of 125bpm. The electrocardiogram showed atypical flutter with atrial cycle length (CL) of 240msec. The flutter waves were monophasic positive in II, III, aVF and V1-V2, negative in aVL and isoelectric in I. A PVI-only procedure was performed four years ago. We performed a new electrophysiological study upon admission. Written informed consent was obtained. The procedure was performed under conscious sedation. A steerable decapolar catheter (Inquiry, Abbott, spacing 2-5-2) was introduced into the coronary sinus by the femoral vein and showed a “chevron” pattern. A notch filter was applied for every catheter. An activation map of the left atrium (NavX Ensite Precision, Abbott, St. Paul, MN) was created using a high-density mapping catheter (HD Grid catheter SE, Abbott) with support from a steerable long sheath (Agilis, Abbott). LAT mapping and post pacing interval (PPI) mapping revealed a roof dependant atrial flutter with a “figure of 8” propagation around both pulmonary vein pairs. The pulmonary veins were persistently isolated. Low voltage was documented in the roof of LA. A roof line led to abrupt cycle length prolongation to 270msec with CS propagation distal to proximal. The P-Wave was biphasic negative in II, III, aVF, positive in V1-V2 and isoelectric in I. The decapolar catheter had one large sharp signal per cycle on each bipole Figure 1. An ultrahigh density activation mapping from both LA and RA was performed. In the LA it seemed that the tachycardia was centrifugal propagating from a focal exit in the inferolateral mitral annulus with highly fractionated signals. The entrance of the tachycardia to the RA was at the CS ostium. A focal mechanism of the tachycardia was primarily assumed. Nevertheless, thorough examination of the signals of the bottom of the LA showed double signals directly opposite to the entire CS: one sharper signal simultaneous to the recorded signal in CS (encircled in Figure 1) and in addition highly fractionated signals, which presented a sequence proximal to distal (see stars and arrows in Figure 1). Examination of the propagation pattern of the adjacent LA led to the conclusion that the sharper component presented a near field signal of the LA endocardium and the low voltage fractionated signal presented a far field signal of epicardial myocardial sleeves, i.e. CS musculature. A fast- slow AVNRT using an inferolateral LA-slow pathway is a differential diagnosis. It is although very unlikely due to a) direct transition from a roof-dependant flutter to this tachycardia without a sinus rhythm beat, b) intermittently 3:1 conduction with instable VA-Intervals and c) PPI > TCL+50msec on the septum. Extensive PPI mapping confirmed the exact macroreentrant circuit: The PPI-TCL in the entire CS, including the CS ostium and the inferolateral exit to the LA with fractionated signals, was <10msec Figure 2. Moreover, the PPI at several positions in the LA and RA (see exemplary white crosses in Figure 3) excluded a perimitral, a roof-dependant and a biatrial atrial flutter utilizing only the LA and right atrial septum or the LA and the tricuspid valve. The true circuit was a peri-coronary sinus flutter with an activation sequence proximal to distal in the coronary sinus musculature. LA excitation begins inferolateral and proceeds endocardially through the inferior LA back to the CS ostium Figure 3, Supplementary Video. Radiofrequency energy was delivered endocardial in LA at the area of fractionated signals at the inferolateral exit of the CS using an irrigated 3,5mm-tip ablation catheter (TactiCath SE, St Jude Medical) with a power of 40 Watts and a cut-off temperature of 43°C terminating the tachycardia at the first application. On completion of the procedure, bidirectional conduction block at the roof line without further ablation was proven. Noninducibility of the tachycardias was also confirmed by burst pacing down to a CL of 200msec.
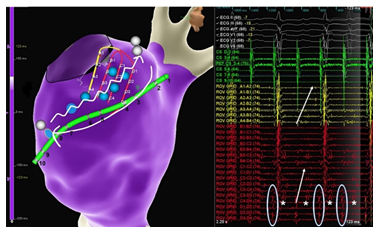
Figure 1: Signals on the grid catheter on the bottom of the LA opposite to CS with two components. Sharp signals simultaneously to the signals on the decapolar catheter (rather posteriorly, encircled). Highly fractionated signals with a sequence proximal to distal (stars and arrows). Blue globes represent exemplary points with double signals adjacent the entire CS. White globes show the exits into the LA and RA. Signals on the CS catheter here not clearly with eccentric sequence because of an inferior deviation of the catheter on the frontal plane, as also seen in Figure 3.
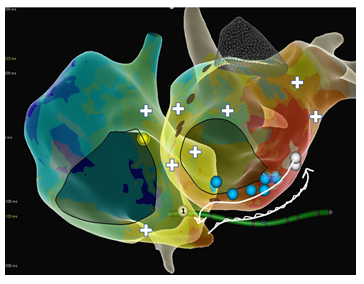
Figure 3: Propagation map of both atria with marked circuit of the peri-CS flutter. Slow conduction in the epicardial sleeves of the coronary sinus from the right to the left and fast backwards through the healthy LA endocardium. Blue spheres show exemplary areas with double signals with one highly fractionated component, white ones exits in the right and left atrium with a PPI equal to the tachycardia cycle length (CL). PPI-CL from all decapolar catheter bipoles was <20msec. White crosses represent sites with PPI-TCL>50msec.
3. Discussion
Atrial arrhythmias involving the musculature of the coronary sinus are common after ablation for atrial fibrillation but also in patients without prior procedures, mostly in the context of a perimitral flutter with a critical isthmus in the LA. Less commonly, but with increasing incidence after atrial ablation, a biatrial flutter that utilizes the CS musculature is diagnosed. The increasing incidence may partly be due to a better understanding of tachycardia mechanisms through high density activation maps. We here describe a rare type of biatrial flutter that utilizes the CS musculature, the adjacent myocardium of the LA and the right atrium in the CS orifice only. To the best of our knowledge, this type of macroreentrant tachycardia has only been described once before. Maruyama et al described a case of peri-coronary sinus flutter after ablation in the proximal CS in the context of AVNRT ablation [7]. The CS musculature was activated from proximal to distal during the tachycardia exiting in the inferolateral LA and reaching the CS orifice endocardially again. The decapolar catheter showed double potentials in the coronary sinus with a dull second component. In our case, the decapolar catheter in coronary sinus with its larger cylindric electrodes, despite the spacing of 2 mm, showed the endocardial activity, epicardial CS sleeves activity was not detected. This could also be due to anteroposterior position discrepancy (see Figure 1). The HD-mapping catheter with the flat small electrodes, though endocardial, was capable of showing signals of the CS musculature. Hence, the signals on the linear catheter in the coronary sinus should not be equated to the activity of the CS musculature, as they can be far field signals of the numerous LA endocardial myocardial cells. The amplitude of a bipolar electrogram depends on the electrode size, the electrode spacing, the angle of incidence between the catheter and tissue, and the orientation of the bipole relative to the wavefront propagation [8]. It has been previously shown in ventricular mapping that the grid catheter with a smaller electrode area, increased electrode density and orthogonal bipole orientations, was capable of detecting activation at the same anatomic sites where a linear catheter failed to record any activity [9]. A recent study showed that depending on the adjacent tissue EGMs acquired from larger electrodes may be composed of far-field components without near-field components [10]. Our case is the first case proving this unique form of peri-CS flutter through extensive biatrial ultra high-density mapping with a multipolar HD-mapping catheter. In our case there was no previous ablation within or in vicinity of the coronary sinus. Unawareness of the possibility of such a flutter circuit could lead to underdiagnosis. Proof of participation of CS musculature in the macroreentrant circuit is ordinarily used as equivalent to confirmation of a perimitral flutter. Extensive activation and entrainment mapping including the coronary sinus and the LA bottom is of substantial importance to verify the exact tachycardia mechanism. The LA bottom, despite the distance to the pulmonary veins and the often present difficult anatomical conditions, should not be neglected. Such a circuit could probably also coexist with a perimitral flutter as a dual loop tachycardia. Especially if during a perimitral flutter double signals with inverse activation sequences are detectable throughout the coronary sinus, such as the cases of perimitral flutter reported from Olgin et al and Jimenez et al [11,12].
4. Conclusion
The CS musculature with its connections to both atria can provide the anatomic substrate for macroreentrant atrial tachycardias. The peri-coronary sinus atrial flutter without utilizing a larger left atrial or biatrial circuit, although rare, should be included in the differential diagnosis of an atypical flutter. Awareness of the existence of this type of atrial flutter is required to prevent underdiagnosis. Despite the precision of the current electroanatomical maps, basic electrophysiological maneuvers such as entrainment mapping should be, if possible, part of every electrophysiological study of a stable macroreentrant tachycardia. A multipolar HD-mapping catheter with small flat electrodes can record crucial low voltage electrocardiograms. This underlines the use of HD-mapping catheters and systems to directly address the tachycardia pathomechanism and prevent needless ablation due to misunderstanding of the tachycardia.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Chauvin M, Shah DC, Haïssaguerre M, et al. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation 101 (2000): 647-652.
- Antz M, Otomo K, Arruda M, et al. Electrical conduction between the right atrium and the left atrium via the musculature of the coronary sinus. Circulation 98 (1998): 1790-1795.
- Saremi F, Thonar B, Sarlaty T, et al. Posterior interatrial muscular connection between the coronary sinus and left atrium: anatomic and functional study of the coronary sinus with multidetector CT. Radiology 260 (2011): 671-679.
- Kitamura T, Martin R, Denis A, et al. Characteristics of Single-Loop Macroreentrant Biatrial Tachycardia Diagnosed by Ultrahigh-Resolution Mapping System. Circ Arrhythm Electrophysiol 11 (2018): e005558.
- Nayak HM, Aziz ZA, Kwasnik A, et al. Indirect and Direct Evidence for 3-D Activation During Left Atrial Flutter: Anatomy of Epicardial Bridging. JACC Clin Electrophysiol 6 (2020): 1812-1823.
- Takigawa M, Derval N, Martin CA, et al. Mechanism of Recurrence of Atrial Tachycardia: Comparison Between First Versus Redo Procedures in a High-Resolution Mapping System. Circ Arrhythm Electrophysiol 13 (2020): e007273.
- Maruyama M, Uetake S, Miyauchi Y, et al. Peri-coronary sinus atrial flutter associated with prior slow pathway ablation. HeartRhythm Case Rep 4 (2018): 10-13.
- Berte B, Zeppenfeld K, Tung R. Impact of Micro-, Mini- and Multi-Electrode Mapping on Ventricular Substrate Characterisation. Arrhythm Electrophysiol Rev 9 (2020): 128-135.
- Jiang R, Beaser AD, Aziz Z, et al. High-Density Grid Catheter for Detailed Mapping of Sinus Rhythm and Scar-Related Ventricular Tachycardia: Comparison With a Linear Duodecapolar Catheter. JACC Clin Electrophysiol 6 (2020): 311-323.
- Takigawa M, Kitamura T, Basu S, et al. Effect of electrode size and spacing on electrograms: Optimized electrode configuration for near-field electrogram characterization. Heart Rhythm 19 (2022): 102-112.
- Jimenez A, Shorofsky SR, Dickfeld TM, et al. Left-sided atrial flutter originating in the coronary sinus after radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol 33 (2010): e96-99.
- Olgin JE, Jayachandran JV, Engesstein E, et al. Atrial macroreentry involving the myocardium of the coronary sinus: a unique mechanism for atypical flutter. J Cardiovasc Electrophysiol 9 (1998): 1094-1099.