Perfusion Index as a Diagnostic Tool for Patent Ductus Arteriosus in Preterm Infants
Article Information
Md. Nazmus Sihan1*, Sharmin Reza Suchi2, Mahboba Akther3, Tareq Rahman4, Humayra Akter5, Mosammad Alpana Jahan6, Md. Arif Hossain7, Md. Shahidullah8, Md. Abdul Mannan9
1Resident Physician (Paediatrics), Cumilla Medical College Hospital, Cumilla, Bangladesh
2MD Resident, Department of Radiology and Imaging, Sir Salimullah Medical College & Mitford Hospital, Dhaka, Bangladesh
3Junior Consultant (Paediatrics), Mugda Medical College Hospital, Dhaka, Bangladesh
4Neonatologist, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
5Registrar, Bangladesh Institute of Tropical and Infectious Diseases, Chattogram, Bangladesh
6Neonatologist, Subject Matter Specialist, RRT & NKC, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
7Medical Officer of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
8Professor of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
9Professor of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding Author: Md. Nazmus Sihan, Resident Physician (Paediatrics), Cumilla Medical College Hospital, Cumilla, Bangladesh
Received: 23 May 2022; Accepted: 20 June 2022; Published: 19 July 2022
Citation: Md. Nazmus Sihan, Sharmin Reza Suchi, Mahboba Akther, Tareq Rahman, Humayra Akter, Mosammad Alpana Jahan, Md. Arif Hossain, Md. Shahidullah, Md. Abdul Mannan. Perfusion Index as a Diagnostic Tool for Patent Ductus Arteriosus in Preterm Infants. Journal of Pediatrics, Perinatology and Child Health 6 (2022): 338-346.
Share at FacebookAbstract
Background: Patent ductus arteriosus (PDA) is common among preterm infants. Preterm infants with patent ductus arteriosus have left-to-right shunt across PDA causing less blood flow to the lower legs. Echocardiogram is the gold standard for diagnosing PDA but is not available in all NICU. Perfusion index (PI) reflects the peripheral circulation which can be measured using a pulse oximeter and it could aid in diagnosing PDA. Objective: To evaluate the accuracy of Delta perfusion index (Delta PI; pre ductal – post ductal PI) in diagnosing PDA in preterm babies.
Methods: Preterm infants with gestational age <37 weeks were assessed for pre and post ductal perfusion index on days 1 and 3 of life and difference between pre and post ductal perfusion index (Delta PI) were calculated. All the patients were undergone echocardiographic examination on day 3. Based on echocardiography, each infant was categorized into PDA and no-PDA group. Mean delta perfusion index were compared between two group. Receiver operating characteristic (ROC) curve analysis with associated area under the curve (AUC) was conducted to explore the discriminative ability of delta perfusion index level in predicting PDA with selection of the most suitable cut-off point.
Results: Seventy infants with median age 32.84 ± 2.230 weeks and weight 1613 ± 420 grams were analyzed. The baseline characteristics did not differ significantly between the groups. The mean delta perfusion index in the PDA group was significantly higher than the mean delta perfusion index of non-PDA group in Day 1 (0.680 ± 0.164 vs 0.414 ± 0.129). Also, on day 3 the significantly higher mean delta perfusion index in the PDA group was observed than the mean delta perfusion index of the non-PDA group (0.820 ± 0.216 vs 0.516 ± 0.255). The mean delta perfusion index on both days 1 and 3 showed that HsPDA group had signi
Keywords
Perfusion Index, Diagnostic Tool, Patent Ductus Arteriosus, Preterm Infants
Perfusion Index articles; Diagnostic Tool articles; Patent Ductus Arteriosus articles; Preterm Infants articles
Article Details
1. Introduction
Patent ductus arteriosus (PDA) is common among premature infants (Gestation <37 weeks) with reports ranging from 25%–85% depending on population and diagnostic criteria at first week of life [1]. It is still a major cause of morbidity and mortality in neonates especially in preterm infants causing approximately 30% of the estimated 4 million neonatal deaths annually [2]. Early diagnosis and a proper supportive therapy are crucial to improve survival and to reduce morbidity as well as long-term sequelae [3, 4]. Currently, echocardiography is the best tool to diagnose PDA. In preterm infants the ductus arteriosus (DA) often fails to constrict and in those, whom constriction occurs, complete occlusion does not occur as profound hypoxia required to achieve this, fails to develop. It is the profound hypoxia that drives the inflammatory cascade that results in cell death and remodeling. In the preterm infant the ductus is so thin walled that the vasa vasorum are not required for transport of oxygen and nutrients to the vessel wall. These are derived from the lumen of the ductus instead and therefore the wall of the ductus is unable to develop the hypoxia required to achieve remodeling [5]. This enables the DA to reopen with its associated hemodynamic consequences. Risk factors for a preterm neonate developing PDA have been well-described. Patent ductus arteriosus (PDA) is diagnosed when the DA fails to close after 72 hours. Symptoms will develop in 50-70% of infants at <28 weeks gestation or with a birthweight of <1000gm [6]. Clinical complications will depend on the degree of left to right shunting through DA, which causes increased left ventricular output and a redistribution of blood flow that can result in metabolic acidosis, intracranial hemorrhage, necrotizing enterocolitis and pulmonary edema or hemorrhage [7, 8]. In those babies with a PDA, prophylactic closure of DA would seem attractive but has been and remains one of the most hotly debated topics in neonatal medicine [1]. Concern remains about administration of a drug known to have deleterious side effects on cerebral, renal and splanchnic blood flow. Yet there is strong clinical and experimental evidence to support the fact that the efficacy of indomethacin decreasing with advancing postnatal age [8]. This dilemma is worsened by the fact that it is very difficult to predict which babies will go on to develop clinical complication as a result of PDA [9]. Colour doppler echocardiography is by far the most sensitive means of diagnosing PDA [10], and provides further information about the hemodynamic significance of PDA. Echocardiographic evaluation includes direct visualization of the ductus itself and the Doppler evaluation of the pulmonary artery, the ductus and the descending aorta. Conventional echocardiographic markers applied at 72 hours of life, such as ductal diameter, ductal velocity, left atrium/aorta (LA/Ao) ratio, diastolic retrograde flow and pulmonary anterograde flow determined the severity of PDA. Echocardiogram may not be immediately available for diagnosing a hemodynamically significant PDA (HsPDA) in many neonatal intensive care units (NICU). In addition, it needs an expert cardiologist to perform echocardiography. However, treatment based on these parameters has not led to improved neurodevelopmental outcome at 2 years in preterm infants [10-13]. Several biomarkers have been identified in patients with myocardial injury and cardiac dysfunction. B-type natriuretic peptide (BNP) and its inactive by-product N terminal Pro-BNP (NTpBNP) are released by the stressed neonatal myocardium in response to volume and pressure loading [9], BNP and NTpBNP levels are good markers of PDA and fall following successful treatment [14], Cardiac specific troponins T and I have become established as the best biochemical markers for myocardial necrosis. They start to increase two hours after myocardial infarction, and concentrations can remain raised for up to two weeks after a full thickness infarct. Indeed, the assays for cardiac troponin I are now so sensitive and specific, mainly because of the use of the latest third generation assays, that a concept of minimal myocardial damage has arisen in adult medicine. But it may not be available in all laboratory round the clock and it does not identify PDA but predicts morbidity related to PDA [2]. Perfusion index (PI) is a noninvasive measure for monitoring the general hemodynamic status of the preterm infant [15-17]. PI provides assessment for the pulse strength and is derived from pulse oximetry. It is the ratio of the pulsatile blood flow to the non-pulsatile static blood flow in a patient’s peripheral tissue, such as fingertip, toe or ear lobe. A compromised arterial flow can be detected if the PI is low. PI, by a simple pulse oximeter using signal extraction technique, has been studied and found to be independent of heart rate variability, saturations, oxygen consumption and temperature. In neonates, PI has clinical application. PI has a lower value in infants with critical left heart obstructive disease [18]. In addition, PI is decreased in infants born to mothers with chorioamnionitis [19]. It has been studied in various clinical settings such as effectiveness as predictor of severity of illness in newborns [20], and local organ perfusion in newborn [17]. Reports are inconsistent as to the value of PI in the assessment of PDA. This may be attributed in part to location and the duration of PI measurements. In the presence of a hemodynamically significant patent ductus arteriosus (hsPDA) the perfusion to post-ductal regions is compromised, as there is steal of blood from the dorsal aorta and that the difference of pre- and post-ductal regions of this index might give a clue toward a significant PDA. The peripheral perfusion of the lower extremities (postductal) is decreased compared to the right arm (preductal) in preterm infants with hsPDA [21]. It is reported that a difference in PI between the upper and the lower extremity, or delta PI (ΔPI), of more than 1.05% strongly correlated with the echocardiographic diagnosis of hsPDA. Currently in newborn care units of our country, there is scarcity of echocardiography machine as well as cardiologist to perform echo. In this circumstance, PI may play a vital role to identify hemodynamically significant patent ductus arteriosus (hsPDA). So far there is no study in Bangladesh showing the validity of PI in diagnosing PDA. This study is, therefore, to evaluate the accuracy of ∆PI (DPI; pre ductal – post ductal PI) in diagnosing PDA in preterm babies. This study will be the 1st prospective analysis of PI in Bangladesh.
2. Materials and Methods
2.1 Study design
Prospective observational study.
2.2 Place of study
Department of Neonatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Shahbag, Dhaka, Bangladesh.
2.3 Study period
From August 2018 to July 2019 (Total 12 months).
2.4 Study population
Preterm neonates (gestation <37 weeks) admitted at NICU, BSMMU during study period.
2.5 Inclusion criteria
- Inborn preterm neonates (gestation <37 weeks) admitted at NICU, BSMMU during study period.
2.6 Exclusion criteria
- Babies with antenatally or postnatally diagnosed major congenital heart disease.
- Any morbidity or mortality (ex- PNA, respiratory distress, acidosis, sepsis, shock, AKI) occurring before 72 hours of life were excluded.
- Suspected critical congenital heart disease.
- Parents refused to participate in the study.
2.7 Operational definition
2.7.1 Gestational age: The duration of gestation is measured from the first day of the last normal menstrual period. Gestational age is expressed in completed days or completed weeks [22].
2.7.2 Term neonate: From 37 completed weeks to less than 42 completed weeks (259 to 293 days) of gestation [22].
2.7.3 Preterm neonate: Less than 37 completed weeks (less than 259 days) of gestation [22].
2.7.4 Birth weight: The first weight of the fetus or newborn obtained after birth. For live births, birth weight should preferably be measured within the first hour of life before significant postnatal weight loss has occurred [22].
2.7.5 Fetal growth at birth can be assessed by using chart: Appropriate for gestational age (AGA) is defined as a birth weight between 10th and 90th percentile for infant’s gestational age. Small for gestational age (SGA) is defined as a birth weight below 10th percentile for infant’s gestational age. Large for gestational age is defined as a birth weight above 90th percentile for infant’s gestational age [23].
2.7.6 APGAR score: APGAR score a measure of the physical condition of a newborn infant. It is obtained by adding points (2, 1, or 0) for heart rate, respiratory effort, muscle tone, response to stimulation, and skin coloration; a score of ten represents the best possible condition [23]. Patent ductus arteriosus Refers to the failure of the closure of ductus arteriosus and continued patency of this fetal channel [23].
2.8 Study procedure
This prospective observational study was conducted in the Department of Neonatology, BSMMU, Dhaka city after approval by Research Ethics Committee over a one-year period from August 2018 to July 2019. Preterm neonates with gestation < 37 weeks admitted at NICU, BSMMU satisfying the inclusion and exclusion criteria was enrolled for the study. A written informed consent was taken from guardian and assured about confidentiality. Face-to-face interview with the mother or caregivers was taken to all enrolled neonates. History included demographic and socioeconomic information, date of admission, mode and place of delivery, resuscitation at birth, gestational age, birth weight, Maternal and perinatal risk factors that might influence the presence of PDA, such as intrauterine growth restriction (IUGR) status of the fetus, gestational diabetes mellitus, pregnancy induced hypertension, maternal hypothyroid, antenatal corticosteroid used etc was noted. Clinical features were assessed twice a day, such as presence of tachycardia, hyperdynamic precordium, excessive weight gain, blood pressures, and wide pulse pressures, bounding pulses, heart murmur, respiratory deterioration, apnea, respiratory distress and hepatomegaly. Investigations other than echocardiography such as chest radiography for cardio-thoracic ratio, arterial blood gas for metabolic acidosis and cranial ultrasonogram for any intraventricular hemorrhage (IVH) was done if clinically indicated. Perfusion Index (PI) was measured on Days 1 (12–24 h) and 3 (48–72 h) in pre-ductal (right hand) and post-ductal (right/left foot) sites. PI at each site was calculated as an average of 30 readings taken every 20 s for 10 min.
2.9 Sample size
So, 72 cases were enrolled for the study.
2.10 Data analysis
Quantitative/Continuous data were expressed as mean ± standard deviation. Qualitative/Categorical data were presented as frequency and proportion. All quantitative variables were compared by unpaired t-test; categorical variables were compared by Chi-square test. Receiver operating characteristic (ROC) curve analysis with associated area under the curve (AUC) was conducted to explore the discriminative ability of delta perfusion index (∆PI) level in predicting PDA and No PDA with selection of the most suitable cut-off point with the best sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy. An area under curve (AUC) value of 0.5 indicates no ability to discriminate and larger values indicate increasing ability. A value of 0.8 is considered good. P-values < 0.05 was considered statistically significant. Data entry and analysis was carried out using the Statistical Package of Social Science Software program, version 20 (SPSS) (IBM Corporation, New York, USA).
3. Results
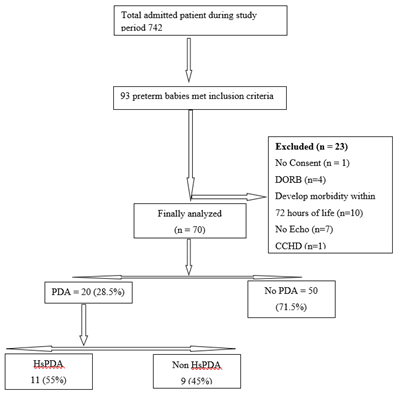
During the study period, total 93 preterm babies were assessed for eligibility. Among these 93 infants 23 were excluded due to 1 having no consent, 10 babies develop morbidity within 72 hours after initial enrollment, 7 having no echocardiography, 1 due to critical congenital heart disease and 4 babies took DORB. Finally, 70 patients were analyzed. Out of them 20 (28.5%) have PDA and 50(71.5%) have no PDA (Figure 1). Among PDA group 11(55%) were HsPDA and the rest were 9(45%) non HsPDA. Baseline characteristics of the studied infants are presented in Table 1. Mean gestational age was 32.84 ± 2.23 weeks and mean birth weight was 1613.53 ± 420gm. Gender distribution reflected slight male predominance (61.4%). Three-fourth of the infants were non-IDM 55(78.6%), most of them had no H/O PIH 46(65.7%). Only 14(20%) had H/O PROM. Most of the infants were AGA 54(77.1%) followed by SGA 14(20%) and LGA 2(2.9%). All the included neonates were inborn. Comparison between baseline characteristics of the two groups PDA and No PDA is presented in Table 2. Prevalence of PDA in this study was 28.5%. 14 (70%) infants having PDA were late preterm and regarding birth weight there was no significant difference. Gender distribution reflected slight male predominance (60%) in PDA group. About three-fourth of the infants having PDA were non IDM 14 (70%), most of them had no h/o PIH 15(75%) as well as PROM 15(75%). 50% infants having PDA had not received Antenatal Corticosteroid. Most of the infants having PDA were AGA 17(85%). Comparison of preductal and post ductal perfusion index on day 1 between PDA and no PDA group is presented in Table 3. The pre-ductal PI was higher in the PDA group on Day 1 than non-PDA group (1.615 ± 0.370 vs. 1.518 ± 0.428). The Post ductal PI of PDA group was lower than non-PDA group (0.935 ± 0.343 vs. 1.104 ± 0.427) and it was significantly lower than pre-ductal PI. The mean Delta PI in the PDA group was higher than the mean DPI in the non-PDA group in Day 1 (0.680 ± 0.164 vs 0.414 ± 0.129) indicating the steal occurring in the PDA group.
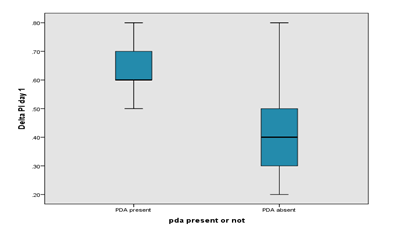
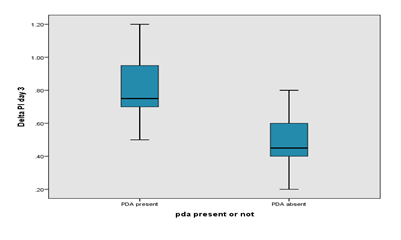
Box plot showing the significant difference in delta Perfusion Index on day 1 between PDA and no PDA group. The mean delta Perfusion Index in the PDA group was higher than the mean delta PI in the non-PDA group in Day 1 (0.680 ± 0.164 vs 0.414 ± 0.129). Comparison of preductal and post ductal perfusion index on day 3 between PDA and no PDA group is presented in Table 4. The pre-ductal PI was slightly higher in the PDA group on Day 3 than non-PDA group (1.585 ± 0.363 vs. 1.566 ± 0.461). The Post ductal PI of PDA group was significantly lower than preductal PI (0.765 ± 0.380 vs. 1.585 ± 0.363). The mean Delta PI in the PDA group was higher than the mean Delta PI in the non-PDA group in Day 3 (0.820 ± 0.216 vs 0.516 ± 0.255) indicating the steal occurring in the PDA group. Box plot showing the significant difference in delta perfusion index (delta PI) on day 3 between PDA and no PDA group. The mean delta PI in the PDA group was higher than the mean delta PI in the non-PDA group in Day 3 (0.820 ± 0.216 vs 0.516 ± 0.255). Comparison of ductal diameter between HsPDA and Non HsPDA group is presented in Table 5. The ductal diameter of HsPDA group was significantly higher than non-HsPDA group (2.409 ± 0.610 vs. 0.805 ± 0.487).
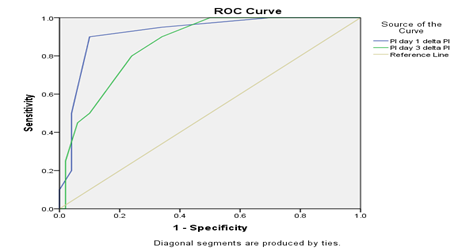
Comparison of preductal and post ductal perfusion index on day 1 between HsPDA and non HsPDA group is presented in Table 6. The pre-ductal PI was slightly higher in the HsPDA group on Day 1 than non-HsPDA group (1.845 ± 0.304 vs. 1.489 ± 0.407). The Post ductal PI of HsPDA group was significantly lower than preductal PI (1.090 ± 0.361 vs. 1.845 ± 0.304). The mean Delta PI in the HsPDA group was higher than the mean DPI in the non-HsPDA group in Day 1 (0.754 ± 0.175 vs 0.440 ± 0.139) indicating the more steal occurring in the Hs PDA group and it is statistically significant. Comparison of preductal and post ductal perfusion index on day 3 between HsPDA and non HsPDA group is presented in Table 4.7. The pre-ductal PI was slightly higher in the HsPDA group on Day 3 than non-HsPDA group (1.736 ± 0.317 vs. 1.540 ± 0.446). The Post ductal PI of HsPDA group was significantly lower than preductal PI (0.845 ± 0.425 vs. 1.736 ± 0.317). The mean Delta PI in the HsPDA group was higher than the mean DPI in the non-HsPDA group in Day 3 (0.890 ± 0.246 vs 0.549 ± 0.253) indicating the more steal occurring in the Hs PDA group and it is statistically significant. Delta perfusion index (ΔPI) cutoff 0.5 on Day 1 offered a good sensitivity (85%), specificity (89%), positive predictive value (78%), and negative predictive value (93%) with an overall accuracy of 88.5%. The delta perfusion index (ΔPI) cutoff on Day 3 also followed similar trends with 0.7 had good specificity (90%) but less sensitivity (50%), positive predictive value (66.7%), negative predictive value (81.8%) with an overall accuracy of 78.5%.
|
Characteristics |
Finding |
|
Gestational age (weeks), mean ± SD |
32.84 ± 2.230 |
|
Birth weight (grams), mean ± SD |
1613 ± 420 |
|
Gender |
|
|
Male |
43 (61.4%) |
|
Female |
27 (38.6%) |
|
IDM |
|
|
Yes |
15 (21.4%) |
|
No |
55 (78.6%) |
|
PIH |
|
|
Yes |
24 (34.3%) |
|
No |
46 (65.7%) |
|
PROM |
|
|
Yes |
14 (20%) |
|
No |
56 (80%) |
|
Antenatal steroid therapy |
|
|
None |
32 (45.7%) |
|
Incomplete |
14 (20%) |
|
Complete |
24 (34.3%) |
|
Fetal growth |
|
|
Small for gestational age |
14 (20%) |
|
Appropriate for gestational age |
54 (77.1%) |
|
Large for gestational age |
2 (2.9%) |
Data are presented as number (percentage) unless otherwise indicated. IDM: Infant of diabetic mother, PIH: Pregnancy induced hypertension, PROM: Premature rupture of membrane, ACS: Antenatal corticosteroid.
Table 1: Baseline characteristics of studied neonates (n=70).
|
Variable |
PDA, no (%) (n=20) |
No PDA, no (%) (n=50) |
p value |
|
Gestational age |
|||
|
<32 weeks |
6 (30) |
14 (28) |
0.867ns |
|
32-<37 weeks |
14 (70) |
36 (72) |
|
|
Birth weight |
|||
|
<1500 gm |
8 (40) |
26 (52) |
0.364 ns |
|
1500-<2500 gm |
12 (60) |
24 (48) |
|
|
Gender |
|||
|
Male |
12 (60) |
31 (62) |
0.877 ns |
|
Female |
8 (40) |
19 (38) |
|
|
IDM |
|||
|
Yes |
6 (30) |
9 (18) |
0.269 ns |
|
No |
14 (70) |
41 (82) |
|
|
PIH |
|||
|
Yes |
5 (25) |
19 (38) |
0.301 ns |
|
No |
15 (75) |
31 (62) |
|
|
PROM |
|||
|
Yes |
5 (25) |
9 (18) |
0.508 ns |
|
No |
15 (75) |
41 (82) |
|
|
ACS |
|||
|
None |
10 (50) |
22 (44) |
0.411 ns |
|
Incomplete |
2 (10) |
12 (24) |
|
|
Complete |
8 (40) |
16 (32) |
|
|
Fetal growth |
|||
|
SGA |
3 (15) |
11 (22) |
0.503 ns |
|
AGA |
17 (85) |
37 (74) |
|
|
LGA |
0 (0) |
2 (4) |
|
Statistical test: Chi square test, IDM: Infant of diabetic mother, PIH: Pregnancy induced hypertension, PROM: Premature rupture of membrane, ACS: Antenatal corticosteroid, s: Significant, ns: not significant.
Table 2: Comparison of baseline characteristics of studied neonates (n=70).
|
PI Level |
PDA(n=20) |
No PDA (n=50) |
p value |
|
Pre ductal PI |
1.615 ± 0.370 |
1.518 ± 0.428 |
0.378 ns |
|
Post ductal PI |
0.935 ± 0.343 |
1.104 ± 0.427 |
0.120 ns |
|
Delta PI |
0.680 ± 0.164 |
0.414 ± 0.129 |
<0.001s |
Data are presented as (mean ± standard deviation). Statistical test: Independent t-test. PI level: Perfusion index level. Delta PI level: Difference between pre and post ductal Perfusion index. s: significant. ns: not significant
Table 3: Perfusion Index level among studied group on Day 1.
|
PI Level |
PDA (n=20) |
No PDA (n=50) |
p value |
|
Pre ductal PI |
1.585 ± 0.363 |
1.566 ± 0.461 |
0.870 ns |
|
Post ductal PI |
0.765 ± 0.380 |
1.050 ± 0.387 |
<0.01 s |
|
Delta PI |
0.820 ± 0.216 |
0.516 ± 0.255 |
<0.01s |
Data are presented as (mean ± standard deviation). Statistical test: Independent t-test. PI level: Perfusion index level. Delta PI level: Difference between pre and post ductal Perfusion index. s: significant, ns: not significant.
Table 4: Perfusion Index level among studied group on Day 3.
|
PDA size |
HsPDA |
Non HsPDA |
P value |
|
Ductal diameter |
2.409 ± 0.610 |
0.805 ± 0.487 |
<0.01s |
Data are presented as (mean ± standard deviation). Statistical test: Independent t-test. s: significant, ns: not significant.
Table 5: Echocardiographic finding among PDA group.
|
PI Level |
HsPDA (n=11) |
Non HsPDA (n=9) |
p value |
|
Pre ductal PI |
1.845 ± 0.304 |
1.489 ± 0.407 |
0.035ns |
|
Post ductal PI |
1.090 ± 0.361 |
1.049 ± 0.421 |
0.759 ns |
|
Delta PI |
0.754 ± 0.175 |
0.440 ± 0.139 |
<0.001s |
Data are presented as (mean ± standard deviation).Statistical test: Independent t-test, PI level: Perfusion index level, Delta PI level: Difference between pre and post ductal Perfusion index, HsPDA: Hemodynamically significant Patent ductus arteriosus. s: significant, ns: not significant.
Table 6: Perfusion Index level among HsPDA and Non HsPDA group on Day1.
|
PI Level |
HsPDA (n=11) |
Non HsPDA (n=9) |
p value |
|
Pre ductal PI |
1.736 ± 0.317 |
1.540 ± 0.446 |
0.171ns |
|
Post ductal PI |
0.845 ± 0.425 |
0.991 ± 0.399 |
0.274ns |
|
Delta PI |
0.890 ± 0.246 |
0.549 ± 0.253 |
<0.01s |
Data are presented as (mean ± standard deviation), Statistical test: Independent t-test , PI level: Perfusion index level, Delta PI level: Difference between pre and post ductal Perfusion index , HsPDA: Hemodynamically significant Patent ductus arteriosus. s: significant, ns: not significant.
Table 7: Perfusion Index level among HsPDA and Non HsPDA group on Day3.
|
Cut-off |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
|
|
point |
(%) |
(%) |
(%) |
(%) |
(%) |
|
|
ΔPI D1 |
0.5 |
85% |
89% |
78% |
93% |
88.50% |
|
ΔPI D3 |
0.7 |
50% |
90% |
66.70% |
81.80% |
78.50% |
Table 8: Screening analysis of delta PI (ΔPI) in diagnosing PDA using the most suitable cut-off point.
4. Discussion
Patent ductus arteriosus (PDA) is common among preterm babies and a major cause of morbidity and death. When PDA is hemodynamically significant, survival is further compromised. A reduction in PDA related mortality may be possible by identifying PDA earlier and targeting them for optimum management. Currently, echocardiography is the gold standard tool to diagnose PDA. Echocardiography is costly and requires expert cardiologist to perform this procedure. Echocardiography may not be immediately available in many neonatal intensive care units (NICU) of Bangladesh. All neonatal units do have pulse oximeters, which could provide a clue toward the presence of PDA by measuring Perfusion index (PI). This prospective observational study was conducted with an objective to identify PDA by calculating Perfusion index (PI) using pulse oximeter. In this study, total 93 preterm babies were assessed for eligibility. Among these 93 infants 23 were excluded and finally 70 preterm babies were analyzed. Out of them 28.5% had PDA which was similar to a study finding conducted by Benitz W.E. et al., [1] who reported the incidence of PDA ranging from 25%–85% depending on population and diagnostic criteria at first week of life. In our study the incidence of HsPDA was 55% which was also consistent with a study conducted by Hammerman and Kaplan et al., [24]. The increased incidence of hemodynamically significant PDA in this population possibly owing to high incidence of premature delivery (22.3%) and growth restriction (21%) according to Rashed Shah et al. [25] and Bishnupada Dhar et al. [26]. As the availability of echocardiography is not yet available across the country, Perfusion Index, a simple clinical adjunct has been used to suspect its presence.
Specificity and sensitivity of Perfusion Index is proven by several studies and this makes Perfusion Index, a sensitive bedside simple marker important in the early diagnosis of PDA to improve outcomes. In this study, babies <37 weeks old were monitored for the presence of PDA by monitoring Perfusion Index serially. In this present study, the right hand was used to represent preductal site and the right/left foot to represent post ductal site. The baseline characteristics did not differ significantly between the groups. In this study, the pre-ductal PI was higher in the PDA group on Day 1 than non-PDA group (1.615 ± 0.370 vs. 1.518 ± 0.428). The Post ductal PI of PDA group was lower than non-PDA group (0.935 ± 0.343 vs. 1.104 ± 0.427) and it was significantly lower than pre-ductal PI. The mean Delta perfusion index (delta PI) in the PDA group was higher than the mean Delta perfusion index (delta PI) in the non-PDA group in Day 1 (0.680 ± 0.164 vs 0.414 ± 0.129) indicating the steal occurring in the PDA group. Our study finding was consistent with Bianchi et al. [27] who found lower Post ductal PI in babies who required medical closure of PDA (PI 1.29 ± 0.35) or multiple medical treatment/surgical ligation of PDA (PI 1.19 ± 0.20) as compared with babies with spontaneous closure of PDA (PI 1.56 ± 0.55).
This study had higher pre-ductal perfusion index in PDA as well as HsPDA group. Table 4.4 showed the Comparison of preductal and post ductal perfusion index on day 3 between PDA and no PDA group. The pre-ductal PI was slightly higher in the PDA group on Day 3 than non-PDA group (1.585 ± 0.363 vs. 1.566 ± 0.461). The Post ductal PI of PDA group was significantly lower than preductal PI (0.765 ± 0.380 vs. 1.585 ± 0.363). The mean delta perfusion index (delta PI) in the PDA group was higher than the mean delta PI in the non-PDA group in Day 3 (0.820 ± 0.216 vs 0.516 ± 0.255) indicating the steal occurring in the PDA group. Terek et al. [28] also found lower post ductal PI on Day 1 in babies with HsPDA which was similar to our study findings. They also concluded that it becomes normalized after treatment. The mean delta perfusion index (delta PI) in the HsPDA group (Day 1: 0.754 ± 0.175, Day 3: 0.890 ± 0.246) was higher than the mean delta perfusion index in the non HsPDA group (Day 1: 0.440 ± 0.139, Day 3: 0.549 ± 0.253), indicating the more steal occurring in the HsPDA group. This finding was consistent with Khositseth et al. [21] who studied Delta Perfusion Index in 30 preterm infants on Days 1, 3 and 7. On Days 1 and 3 of life, the Delta perfusion index of infants with HsPDA [1.57%, interquartile range (IQR) 0.28–2.32, n ¼ 14, and 1.32%, IQR 0.28–1.83, n ¼ 10] was significantly higher than those without HsPDA (0.14%, IQR 0.03 to 0.30, n ¼ 16, and 0.08%, IQR 0.07 to 0.26, n ¼ 20), p ¼ 0.009 and 0.005, respectively. Granelli and Ostman-Smith et al. [18] found a median perfusion index of 1.70% (IQR, 1.18–2.50) in 10,000 normal newborns at 1st week of age. Cresi et al. [29] reported perfusion index measured at either foot in clinically and hemodynamically stable preterm infants in the first week of life (day 1, 3, and 7 of life) and concluded that perfusion index could be used as an indirect and noninvasive measurement of peripheral perfusion at specific monitoring sites. To use Delta Perfusion Index (delta PI) as a bedside tool for diagnosing PDA, there is need of having a cutoff value. The Delta Perfusion Index (delta PI) cutoff of 0.5 on Day 1 offered a good sensitivity (85%), specificity (89%) and predictive values, while decreasing the cutoff to 0.4 the sensitivity increases to 95% at the cost of specificity 66%.
In this study considering use of 0.4 cutoff in NICU where it is possible to confirm PDA by echo before treatment. However, if echo is not possible or if interpretation of echo is difficult, it would be safer to use the 0.5 cutoff with specificity of 89% before treatment. The Delta perfusion index cutoff on Day 3 also followed similar trends with 0.7, who had good specificity (90%) but less sensitivity (50%). While consider a cutoff of 0.65 had improved sensitivity (80%) at the cost of specificity (76%). However, Day 1 Delta Perfusion Index was more sensitive and specific than Day 3 Delta Perfusion Index. This suggests that it is possible to predict the occurrence of PDA on the first day of life. Khositseth et al. [21] showed that at all time points (Days 1, 3 and 7 of life), Delta Perfusion Index >1.05% had sensitivity, specificity, positive predictive value and negative predictive value of 66.7%, 100%, 100% and 86.4%, respectively. In our study to detect HsPDA, cutoff for delta perfusion index to diagnose HsPDA was lower than other study. This study adds to the existing evidence that Delta Perfusion Index could be useful adjunct for bedside diagnosis of PDA. This study shows that delta perfusion index is a good surrogate marker for diagnosing PDA as well as HsPDA. If ductus arteriosus steal a large volume of left ventricular output, it would be at the cost of systemic distal perfusion and thereby reflected in Perfusion Index. When pre-ductal and post-ductal sites are monitored for perfusion, a difference could give insights into the amount of steal and thereby hemodynamic significance.
This study, to the best of my knowledge, is the first study of Perfusion Index in PDA in Bangladesh. In a country where rate of prematurity is high, and preterm infants are being managed in NICU and other centers without universal access to echocardiography but with pulse oximeter, this study could identify the group of babies who would need referral for evaluation and management of PDA. This would result in better and cost-effective management. The other strength of the study is that all perfusion indexes were recorded by the principal investigator. There was no inter-observer bias. Despite some limitations, this study had identified a simple bedside investigation available to all secondary and tertiary care centers, not requiring intensive training for diagnosing PDA.
5. Conclusion
Assessment of the difference between pre ductal perfusion index and post ductal perfusion index (Delta Perfusion Index -∆PI) can be used as a diagnostic tool to identify Patent Ductus Arteriosus in preterm infants.
Limitation of the Study
- Single centered study.
- Echocardiographic examination was not done by same cardiologist.
Recommendation
- Delta perfusion index (∆PI) can be a diagnostic tool to identify PDA in preterm neonates where echocardiography is not available.
- Further multicenter study with large sample size is recommended to validate the present study.
References
- Benitz WE. Committee on Fetus and Newborn: Patent Ductus Arteriosus in Preterm Infants. Pediatrics 137 (2016): 1-6.
- James ED, Pharm D, Jatinder B. Patent Ductus Arteriosus: An Overview. J Pediatr Pharmacol Ther 12 (2007): 138-146.
- Kluckow M, Jeffery M, Gill A, et al. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 99 (2014): 99-104.
- Owlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 7 (2010): CD000174.
- Clyman RI, Chan CY, Mauray F. Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation’, Pediatr Res 45 (1999): 19-29.
- Hammerman C. Patent ductus arteriosus: Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment’, Clin Perinatol 22 (1995): 457-479.
- Brooks JM, Travadi JN, Patole SK, et al. Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed 90 (2005): 235-239.
- Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate 89 (2006): 330-335.
- El-Khuffash A, Higgins M, Walsh K, et al. Quantitative assessment of the degree of ductal steal using celiac artery blood flow to left ventricular output ratio in preterm infants. Neonatology 93 (2007): 206-212.
- Evans N. Current controversies in the diagnosis and treatment of patent ductus arteriosus in preterm infants. Adv Neonatal Care 3 (2003): 168-177.
- Knight DB. The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomized trials. Semin Neonatol 6 (2001): 63-73.
- Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev (2006): CD004213.
- Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 344 (2001): 1966-1972.
- El-Kuffash A, Molloy EJ. Are B-type natriuretic peptide (BNP) and N-terminal-pro-BNP useful in neonates?. Arch Dis Child Fetal Neonatal Ed 92 (2007): 320-324.
- Alderliesten T, Lemmers PM, Baerts W, et al. Perfusion index in preterm infants during the first 3 days of life: reference values and relation with clinical variables. Neonatology 107 (2015): 258-265.
- Piasek CZ, Van BF, Sola A. Perfusion index in newborn infants: a noninvasive tool for neonatal monitoring. Acta Paediatr 103 (2014): 468-473.
- Zaramella P, Freato F, Quaresima V, et al. Perfusion index co-relates with calf muscle perfusion as measured by NIRS. J Perinatol 25 (2005): 417-422.
- Granelli AW, Ostman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatr 96 (2007): 1455-1459.
- De Felice C, Goldstein MR, Parrini S, et al. Early dynamic changes in pulse oximetry signals in preterm newborns with histologic chorioamnionitis. Pediatr Crit Care Med 7 (2006): 138-142.
- Felice D, Latini G, Vacca P, et al. Perfusion index as a severity marker for high illness severity among newborns. Eur J Pediatr 161 (2002): 561-562.
- Khositseth A, Muangyod N, Nuntnarumit P. Perfusion index as a diagnostic tool for tool for patent ductus arteriosus in preterm infants. Neonatology 104 (2013): 250.
- Steiner TJ, Birbeck GL, Jensen R, et al. The global campaign, world health organization and lifting the burden: collaboration in action. The Journal of Headache and Pain 12 (2011): 273-274.
- Gomella TL, Cunningham MD, Eyal FG, et al. Neonatology: management, procedures, on-call problems, diseases, and drugs. New York: McGraw-Hill Education Medical (2013): 565-570.
- Kaplan CA, Simon HA. In search of insight. Cognitive psychology 22 (1990): 374-419.
- Rashed S, Luke C M, Gary L, et al. Incidence and risk factors of preterm birth in a rural Bangladeshi cohort. BMC Pediatrics 14 (2014): 112.
- Dhar B, Hossain K, Bhadra S, et al. Maternal Anthropometry and Intrauterine Growth Retardation (IUGR)A Hospital Based Study. Journal of the Bangladesh College of Physicians and Surgeons 28 (2010).
- Bianchi A, Ossola S, Stifani A, et al. Newborns 32 weeks gestational age with patent ductus arteriosus: is perfusion index predictor of ductal closure?. In Pediatric Academic Societies Annual Meeting (2012).
- Terek D, Altun KO, Uygur O. et al. Can low perfusion index predict the treatment need in premature infants with PDA?. Arch Dis Child 97 (2012): 7-8.
- Cresi F, Pelle E, Calabrese R, et al. Perfusion index variations in clinically and hemodynamically stable preterm newborns in the first week of life. Ital J Pediatr 36 (2010): 6