Pembrolizumab Induced « Polyosis-Like » Hair Discoloration in a Patient with Metastatic Lung Adenocarcinoma
Article Information
El Ali Ziad*
Department of Oncology and Hematology, IRIS-Sud Hospitals, Brussels, Belgium
*Corresponding author: El Ali Ziad, MD, PhD, Department of Oncology and Hematology, Rue Jean Paquot 63
1050 Bruxelles, Belgium
Received: 07 June 2021; Accepted: 16 June 2021; Published: 24 June 2021
Citation: El Ali Ziad. Pembrolizumab Induced « Polyosis-Like » Hair Discoloration in a Patient with Metastatic Lung Adenocarcinoma. Archives of Clinical and Biomedical Research 5 (2021): 452-456.
Share at FacebookKeywords
Pembrolizumab; Lung Adenocarcinoma
Pembrolizumab articles
Article Details
1. Introduction
Immune-checkpoint inhibitors (ICIs) are currently one of the most important developments in cancer therapy. Due to the growing use of these drugs, clinicians will increasingly be confronted with different immune-related adverse events (irAEs).
In studies assessing the safety profile of ICIs, it was reported that >60% of patients develop side effects that could involve any organ leading to thyroiditis, hepatitis, pneumonitis, hypophysitis, uveitis, polyneuritis, colitis, pancreatitits, myocarditits, and cutaneous eruptions.
Cutaneous irAEs occur in > 30% of patients, which makes skin side effects the most common [1, 2].
2. Case Presentation
This is the case of a 60 years old, heavy smoker, male patient with a stage IV lung cancer treated currently in our institution. Staging PET-CT scan performed in July 2020 showed mediastinal lymph-nodes, third right rib infiltration, and multiple bilateral lung metastasis.
Biopsy confirmed the diagnosis of lung adenocarcinoma and immunohistochemistry showed 1% PDL-1 expression in tumor cells. Tumor sequencing (Ampliseq Cancer Hotspot Panel V2 (Illumina)) showed no EGFR, KRAS, or BRAF-V600E gene mutation. FISH analysis was negative for ROS-1 rearrangement and ALK rearrangement.
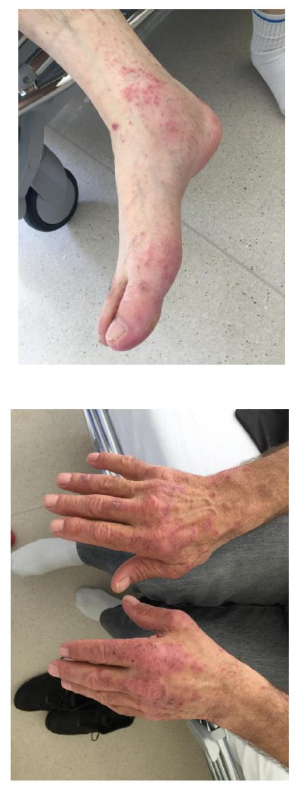
The patient started treatment with combined chemotherapy (Cisplatin-Pemetrexed) with immunotherapy (Pembrolizumab). After three cycles, he developed chemotherapy induced grade II alopecia and Pembrolizumab related acute multiforme erythema (Figures 1 and 2). No biopsy was performed and the patient started antibiotic, with an improvement noted few days later. Two weeks after the onset of the skin irAE, the patient presented to our clinic with joints pain. Clinical exam revealed hand and foot joints swelling and laboratory tests were strongly positive for rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Pembrolizumab induced rheumatoid polyarthritis was diagnosed and the patient started corticotherapy.
In addition to all this, our patient presented an interesting poliosis-like hair discoloration (Figures 3 and 4). Before chemo-immunotherapy, He had gray, close to black hair, but after the onset of autoimmune side effects, strangely, the scalp looked like a « dalmatian ». Blood test was positive for skin basement membrane antibodies. The identification showed no antibodies against BP180, BP 230, desmoglein 3, nor desmoglein1. PET-CT scan performed after three cycles of combined therapy showed no response to treatment. No second ligne treatment started because patient had requested a therapeutic break.
3. Discussion
Cutaneous irAEs affect between one-third and more than half of all patients receiving ICIs. Rash, pruritus, and vitiligo are the most widely reported skin toxicities. These cutaneous irAEs are typically mild, and can usually be treated without interruption of immunotherapy [2-4]. There are several uncommon and potentially life-threatening dermatologic irAEs that must be considered in patients with severe presentations. Immunobullous eruptions, usually mimicking bullous pemphigoid, occurs in 1% of patients receiving PD-1 or PDL-1 inhibitors. Diagnosis is made by skin biopsy, which often demonstrates IgG and C3 deposits along the dermoepidermal junction, and positive BP180 autoantibody. In addition to the bullous pemphygoid, severe cutaneous adverse reactions can occur in ICI therapy, but are fortunately rare: acute generalized exanthematous pustulosis, DRESS syndrome, Stevens-Johnson syndrome [5, 6].
Vitiligo-like depigmentation is a common cutaneous irAE that may be seen in up to a quarter of patients treated for melanoma, and rarely in patients with other malignancies. Depigmentation typically first presents several months into therapy, and may be preceded by an inflammatory phase [7, 8]. The distribution is often symmetric and photo distributed, distinguishing it from the periorificial and acral presentation of classical vitiligo. Coincident poliosis of scalp, eyebrow, eyelash, and body hair may occur. Treatment is not necessary, but topical steroids or calcineurin inhibitors and phototherapy may be attempted [8].
The occurrence of vitiligo has been linked to better tumor responses and outcomes in patients with advanced melanoma. In a retrospective study the emergence of lichenoid and spongiotic histopathological pattern of dermatitis was associated with favourable outcomes in a small cohort of patients receiving antiPDL-1 and anti PD-1 antibodies for the treatment of various malignancies. Several guidelines have been published, providing comprehensive algorithms for the treatment of most frequently occurring skin irAEs, with clear recommendations regarding the type of immunosuppressive drugs to use and the duration of the treatment based on the severity [5-8].
Our case presents an interesting dermatologic, probably Pembrolizumab induced, hair « dalmatian like » discoloration. Laboratory serum analysis showed only the presence of circulating skin basement membrane antibodies, with no antibodies specific for other autoimmune skin disease. We suppose that, a non-identified a specific, immunotherapy induced antibodies against the epidermal basement membrane led to the dysfunction of the melanogenesis process. This hypothesis needs to be confirmed.
References
- Mrtins F, Latifyan S, Sykiotis GP, et al. Adverse effects of immune-checkpoints inhibitors: epidemiology, management and surveillance. Nature Review Clinical Oncology 16 (2020): 563-580.
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 4 (2015): 560-575.
- Sanlorenzo M, Vujic Igor, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatology 151 (2015): 1206-1212.
- Chen WS, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: A spectrum of immune reactions from paraneoplastic pemphigus-like to grover-like lesions. J Cutan Pathol 45 (2018): 764-773.
- Jour G, Glitza IC, Ellis RM, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathology 43 (2016): 688- 696.
- Schmidgen MI, Butsch F, Schadmand-Fischer S, et al. Pembrolizumab-induced lichen planus pemphigoides in a a ptient with metastatic melanoma. J Dtsch Dermatol Ges 15 (2017): 742-747.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol 19 (2018): 345-361.
- Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol 76 (2017): 863-870.