Cytomegalovirus Infection (CMV) and Human Immunodeficiency Virus: Diagnostic and Therapeutic Challenges in Virology
Article Information
Jorge Aponte1, Juan David Vera2, Aldair Chaar3, Daniel Martin3, Forero Yency4
1Internal Medicine, Department of critical care,?Internal medicine La?Samaritana?and Santa Clara Hospital, Bogota, Colombia
2Internal medicine PGY2, La Sabana University, Bogota, Colombia
3 General Medicine Practitioner. LA Sabana University. Bogota, Colombia
4Internal medicine PGY3, La Sabana University, Bogota, Colombia
*Corresponding Author: Aponte Jorge E, Internal Medicine, Department of critical care,?Internal medicine La?Samaritana?and Santa Clara Hospital, Bogota, Colombia, Tel: +573205036036;
Received: 19 April 2018; Accepted: 04 May 2018; Published: 07 May 2018
Share at FacebookAbstract
Human immunodeficiency virus and co-infection by cytomegalovirus (CMV) is a life threatening association with increased risk of complications and high impact in morbidity and mortality due to immunosenescence, disproportionate inflammatory response and increased viral replication of HIV if not treatment is started [1]. Multisystemic compromise can even produce retinal, gastrointestinal, hematological, pulmonary and central nervous compromise by CMV. The purpose of this review is to provide a diagnostic and therapeutic approach based on current literature and pharmacotherapeutics.
Keywords
Cytomegalovirus; Diagnostic; Treatment
Article Details
List of abbreviations: CMV: ??Citomegalovirus; HIV: Human immunodeficiency virus; ART: Antiretroviral therapy; AIDs: Acquired Immune Deficiency Syndrome; Ig: Immunoglobulin; PCR: Polymerase chain reaction
HSV: Herpes simplex virus; VZV: Varicella zoster virus; CSF: Cerebrospinal fluid
1. Introduction
CMV is a human parasite with a high prevalence worldwide, 90% in underdeveloped countries and 60% in developed countries [2]. Unlike immunocompetent patients where the infection could be self-limited, patients with immunosuppression are at increased risk of complications, disability and mortality. Co-infection with CMV in HIV patients is associated with worse outcomes and increased HIV viral replication with reduction of T-lymphocytes CD4 <50 cel/ mL leading to reactivation of latent CMV infections with subsequent viremia, colonization and compromise of different organs (retina, digestive tract, central nervous system, lungs, among others) [3]. Diagnosis of CMV in patients with AIDS defining state is not always easy and requires clinical suspicious. The purpose of this paper is to review the natural history of co-infection with HIV and CMV, its pathophysiology, prognosis as well as the systemic compromise in various organs. We also comment about diagnostic methods according to the clinical scenario, diagnostic performance and therapeutics.
2. Pathophysiology and Impact of HIV Replication in CMV Coinfection
Cytomegalovirus was initially known as cytomegalic inclusions disease virus and is one of the three B-herpes viruses that are responsible for infections in humans. It contains a double-stranded DNA molecule and is characterized as being the largest compared to other species [4]. It can be differentiated by the number of genes it contains and its additional functions for trascription to occur, most of the strains studied are AD169 and the Toledo strain.
Host response to CMV, involves the activation of human leukocyte antigen (HLA) which involves immune responses to infection by this type of herpes virus. Presentation of some peptides by the virus through the major histocompatibility complex allows activation of CD8 cytotoxic lymphocytes, recognition and destruction of infected cells [5]. On the other hand, HIV and CMV act synergistically inducing the expression and release of cytokines by CMV and activation of HIV DNA leading to increased viral load and faster progression to an immunodeficiency state [6].
Replication cycle begins with the insertion of the virus to target cell thanks to the fusion of the viral envelope with the plasma membrane of the host cell in a neutral environment/neutral pH [4]. This process can explain the entry of CMV to most cells, especially in pigmented epithelium in the retina and endothelium due to its receptors and a low pH allowing for endocytosis of viral particles. Surface glycoproteins allow fusion and/or endocytosis, more than 50 have been described and the more prominent are: glycoprotein B (gB), gM/gN and gH/gL. Glycoprotein gM has the greater proportion and it acts with the gN to be able to be united to the receiver. Likewise, gH and gL act together to achieve the entry of the virus as a fusion receptor and as a chaperone [7] It is essential to know some of the receptors that allow the entry of this type of herpes. viruses, among the most important are heparan sulfate proteoglycans, major histocompatibility complex, annexin II, ?2?1, among others. The entry of the virion through these mechanisms not only allows replication to take place but also activates and inflammatory cascades that allows the release of cytokines and interleukins that mediate the activation of the innate immune response, through gene activation. This activation not only allows the perpetuation of the damage but also facilitates the survival of the virus inside the cell, inhibiting, for example, the pathways of the caspases. which under "normal" conditions are activated in order to cause apoptosis and thus prevent the replication of the virus. However, this virus has a large number of pathways through which it inhibits apoptosis pathways such as caspase and allows the virus to remain inside the cell long enough to replicate. Additionally, another example of genetic regulation and signal cascades is the modulation of the metabolic processes of the host, where by different mechanisms, some not yet known at all, CMV is able to increase the supply of nutrients to the cell, in order to have enough substrate to carry out the replication, where the control of the translation (process that requires the use of energy) plays an important role in this process of metabolic regulation, given that although it is clear that for different mechanisms the CMV manages to increase the contribution of substances such as glucose, it is also important to emphasize that this virus must achieve a balance between the increase of nutrients, anabolic processes and the inhibition of stress produced in the host cell, since the increase in Stress can eventually promote apoptosis of it and therefore be left without a place where to perform the replication process [8-10].
Simultaneously, the virus is replicating, synthesizing nucleocapsid proteins so that later from the nucleus the DNA inside the capsid, together with some proteins of the tegument leave the nucleus towards the cytoplasm and the Golgi apparatus to through vesicles, where other tegument proteins and membrane glycoproteins adhere, necessary to complete the complete formation of the virus (Figure 1).
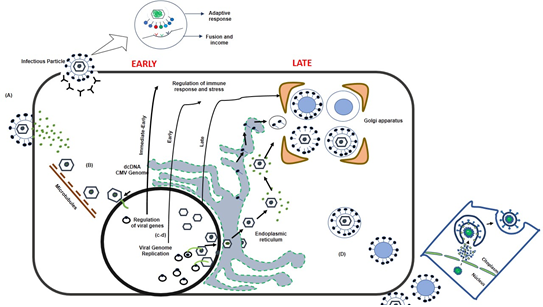
Figure 1: Pathophysiology and impact of HIV replication in CMV coinfection.
Regarding the response of the host to the presence of CMV, it is known that human leukocyte antigen (HLA) is involved in the body's immune response to infection by this herpes virus. The presentation of some peptides of the virus by means of the major histocompatibility class complex, allows CD8 cytotoxic lymphocytes to recognize and destroy the infected cells. The latter, evidences the relationship that exists with VIH and the pathological events that take place after the severe immunosuppression caused by VIH. These two viruses act synergistically, for example, CMV stimulates the expression and release of a series of cytokines that allow the activation of VIH DNA and additionally activate the proviral DNA of VIH, which translates into a higher VIH viral load and a rapid progression to acquired immunodeficiency syndrome. (eleven)
3. Prognosis and Complications
The coinfection of VIH, CMV is related to greater replication, transmission and progression of VIH infection [12], various immunological mechanisms have been described that explain the severity of this coinfection, on the other hand, CMV coinfection in a prognostic factor as demonstrated by Brandsaeter AB, Johannessen A, Holberg-Petersen M, et al, where the viral load for CMV> 200 copies / ml was an independent factor of mortality [13]; It is important to note that the impact on survival is not only seen in patients who are not receiving antiretroviral treatment or with advanced AIDS disease (14), but there is a clear impact on mortality in patients receiving antiretroviral therapy [15]. has linked VIH -CMV coinfection with increased cardiovascular and cerebrovascular morbidity, resulting in increased morbidity and mortality [16], white organ involvement in patients with VIH -AIDS makes the prognosis of patients less encouraging, therefore the patient must be evaluated. - infection of CMV and VIH in order to establish an early and timely treatment in this group of patients.
3.1 Retinal involvement
Cytomegalovirus retinitis is the most frequent ocular infection due to opportunistic germs and is the main cause of visual loss in patients with AIDS [9]. It is estimated that before the beginning of the ART era, this retinopathy was present in 30% of patients with VIH, although this figure decreased by approximately 80% after the start of ART therapy in 1966 [17].
CMV is a neurotropic pathogen that has an important affinity towards neural tissues and the retina. It is characterized by causing a necrotizing retinitis which typically presents cytomegaly with two types of inclusions, basophilic cytoplasm and eosinophilic intranuclear [18]. Within the spectrum of lesions caused by CMV to the retina, hemorrhages and areas of necrosis may be evidenced in the periphery, posterior pole both unilaterally and bilaterally. Those lesions that affect the posterior pole (macula and optic nerve) and are accompanied by vitreitis can present photophobia, defects in the visual field, progressive loss of visual acuity and floaters [19].
The clinical signs that can be evidenced are the following: cotton wool spots randomly which come together as the CMV progresses through the circulation of the retina; Along with this, granular lesions can also be superimposed, areas of hemorrhage without a certain pattern, which can compromise the optic nerve and the macula. All these findings can be summarized in three injury patterns that are typical of CMV involvement, the first is retinopathy in "pizza pie" or "pepperoni pizza", the second are granular lesions in the periphery of the retina. and the last one the frozen branch angitis.
Similarly, to describe the retinal lesions, this can be divided into 3 zones: Zone one refers to the posterior pole, the second includes the edge of zone 1 to the vertex of the veins and in the last zone the space delimited between the periphery and zone 2 [20]. In the same way, this division has been used to evaluate the progression of the lesions in the clinical studies and also to correlate the clinical manifestations with the degree and type of retinal involvement. This is how, for example, when CMV affects zone 1, it is known that there is macular, papillomacular or optic nerve involvement, which is related to an immediate impairment of vision and therefore the commitment of this area is the most important determinant. important to define the urgent requirement of an intervention (Figure 2).
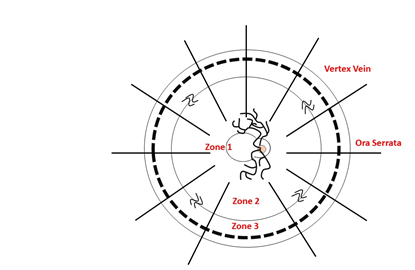
Figure 2: Image made by Jorge Aponte, Juan David Vera, Aldair Chaar, Daniel Martin. Modified of Arch Ophthalmol. 1989; 107: 1759-1766.
On the other hand is systemic therapy, in which valganciclovir is the antiretroviral of choice, given its great oral bioavailability, low risk of toxicity, complications and easy administration. Therapy with this drug consists of an induction phase of 14 to 21 days at a dose of 900 mg every 12 hours and a maintenance phase in which 900 mg are administered daily until the CD4 count is greater than or equal to 100 cells / microL for at least 3 consecutive months, the viral load is suppressed, it has received at least 3 months of therapy against CMV and the retinitis is in a quiescent phase [21]. However not only oral valganciclovir is available, but on the other hand ganciclovir or foscarnet can be used, mainly in those patients unable to tolerate the oral route. It is known that ganciclovir compared to valganciclovir is equally effective as induction therapy [22]. On the other hand, when comparing ganciclovir vs foscarnet, some type of superiority was not shown in the outcomes studied, so the choice of antiretroviral will depend on the patient's comorbidities, bioavailability and, in general, the pharmacokinetic and / or pharmacodynamic profile that is most suitable for the patient. patient [23]. All this referring to the period of induction, since there is no study comparing antiretrovirals in the maintenance phase, so the use of valganciclovir, cidofovir, foscarnet or ganciclovir will depend on the pharmacological aspects, risk of adverse effects more that the superiority of one over the other since the evidence is null as to the comparison of these.
For the follow-up of the treatment it is essential to perform an indirect ophthalmoscopy at the diagnosis, two weeks after the induction phase and one month after the induction phase, monthly monitoring of the fundus to determine the response to treatment.
It is clear that, the retinal compromise caused by CMV is facilitated by the inefficiency of T cells to eliminate the virus, which generally occurs when the TCD4 lymphocyte count is less than 50 cells / ul [24]. However, despite the diversity of damage to the retina caused by this virus, patients may be asymptomatic or may manifest nonspecific symptoms such as difficulty handling (44%), blurred vision (42%), difficulty reading ( 40%) among others [25].
The diagnosis in most cases is made with the set of clinical manifestations and ophthalmological findings made during the complete approach of the patient. In contrast to what was previously believed, the detection and measurement of CMV viremia through the polymerase chain reaction (PCR) or by blood cultures does not have a good sensitivity or specificity to correlate infection with this virus. the organic commitment [26]. In the same way it happens with the detection of antibodies against the CMV, where the negativity of the IgG-CMV diminishes considerably the probability of infection by this microorganism, nevertheless it does not rule out in the case of being negative, the latter happens more commonly in those patients with advanced VIH infection, which is one of the most common false negatives in this type of patient [27].
On the other hand, the treatment of this type of retinitis is determined by the findings found in the retina, this referring to the location and types of injuries found. Treatment is divided into menacing and non-threatening lesions, the former refers to those found in zone 1, that is, those located less than 1500 microns from the fovea or adjacent to the optic nerve and the second ones to the zone 2 and 3, previously explained. When zone 1 is affected, intravitreous therapy is used given that systemic antiretroviral administration takes a long time to reach stable levels intraocularly. Two drugs are available for intraocular administration, ganciclovir and foscarnet, the most used is the first and a dose of 2.5 mg in 0.05 ml is administered once a week for 3 weeks to prevent the increase in intraocular pressure and for both the complications derived from this.
3.2 Gastrointestinal involvement
Gastrointestinal involvement due to CMV in patients with VIH remains frequent despite the rise in current antiretroviral therapy. There are several manifestations that CMV coinfection can produce at this level, including esophageal ulcers that are usually manifested by dysphagia and odynophagia, generating a picture of esophagitis whose diagnosis depends on the demonstration of intranuclear inclusions or immunohistochemical staining or culture or the detection by PCR of CMV from the tissue samples taken in biopsies [28]. In patients with AIDS, watery explosive diarrhea suggestive of colitis is frequent as a presentation in which fever is frequent. The diagnosis of this condition is made through sigmoidoscopy in which there is presence of pseudomembranes, erosions and serpiginous ulcers; In many cases cytomegalovirus colitis can be observed as a tumor lesion that produces partial obstruction or as lesions similar to Kaposi's sarcoma. In this type of immunosuppressed patients, CMV is frequently present in the colon along with other pathogens, such as the Mycobacterium avium-intracellulare complex and Cryptosporidium. In cases of severe colitis, perforation and gangrene may occur. The diagnosis of CMV colitis is carried out in the same way by means of biopsy, demonstrating the typical bodies of inclusion, or by means of culture from the material taken in biopsies [28]. It is important to clarify that the inclusion bodies are generally observed in the epithelium or in the crypts of the intestinal mucosa; and that maintenance treatment with antivirals such as ganciclovir has not been shown to be useful in preventing recurrence of colitis by this agent [29]. Patients with AIDS can contract acute CMV pancreatitis since cases of cholecystitis have been associated with the presence of CMV in the common bile duct, gallbladder and biliary tree, associated with the development of pathologies such as acalculous cholecystitis, papillary stenosis and Sclerosing cholangitis [30]. Finally, it is important to define that the digestive compromise by CMV in patients with VIH generally occurs in cases of greater chronicity of retroviral infection, manifesting itself later than another type of commitment such as fungal, parasitic, tuberculous or neoplastic
3.3 Hematological involvement
VIH can alter the three hematological cell lines: leukocytes, erythrocytes and platelets. Neutropenia and anemia are observed especially in advanced stages of the disease; On the other hand, thrombocytopenia can appear from the onset of the disease. Neutropenia may be due to both VIH infection itself and an adverse effect of ART treatment. Thrombocytopenia is a relatively common hematologic complication in VIH disease occurring in approximately 10% of infected patients and in approximately one third of patients with AIDS. Clinically it behaves as an immune-mediated thrombocytopenia, similar to that which occurs in seronegative patients. It often occurs in mild form with counts above 20 × 10 at 3 ul and moderate to severe in 5-10% of cases. Two main mechanisms have been described that would explain the reduction in the platelet count: 1) Increase of the immune-mediated peripheral destruction, 2) Platelet production altered by the infection of the megakaryocytes in the bone marrow [31].
Cytomegalovirus, on the other hand, infects a wide range of cell types, such as epithelial, endothelial, neuronal, smooth muscle cells, fibroblasts, monocytes and macrophages [32]. Similar to VIH, it is directly related to a decrease in the cell line count, especially and early in the platelet line through an immune-mediated mechanism. Few studies have directly evaluated the hematological relationship of coinfection between CMV / VIH, however Masanori Furuhata et.al report a case of CMV / VIH confection with platelet counts in range of severity, which improve substantially until normalize after treatment for CMV, which could demonstrate potentiation in platelet destruction [33].
On the other hand, case reports have been written about increased hypercoagulability status in patients with CMV / VIH, which generates a considerable increase in the risk of arterial and venous thrombosis. Mulder et.al reported in a randomized clinical trial the increased risk of venous thrombosis in patients with VIH who suffer coinfection with CMV by measuring coagulation factors (Factor VIII and Fibrinogen), which were elevated in 100% of patients with CMV / VIH, unlike 78% of patients with isolated VIH [34] Cases of alteration of cell lines have been reported with the antiviral treatment itself, mainly with Ganciclovir and Valganciclovir, as well as reports of Hemophagocytic Syndrome [35], however These are situations that can only occur due to VIH infection, which proves the lack of studies in this field.
3.4 Lung involvement
Pulmonary involvement of CMV in patients with VIH at the pulmonary level is very infrequent, unlike patients with hematological neoplasms, or immunosuppression in transplant patients where the scenario and prognosis is ominous. In patients with VIH, co-infection with P. jirovecci or other fungi is common [36], clinical manifestations are variable: fever, cough, physical examination, evidence of rales, and oxygenation compromise from mild dyspnea to respiratory failure [37], viremia can cause arthralgias, elevated aminotransferases, leukopenia and thrombocytopenia. The radiological findings in this group of patients are alveolar opacities, frosted glass, or bilateral nodular or reticular opacities, the size of the nodules is generally less than 5 mm and in 20% of patients, pleural effusions can be documented [38]. The diagnosis in this case is given by the evidence of the virus in bronchoalveolar lavage (BAL) or transbronchial biopsy, either by direct detection: immunohistochemistry, histopathology, electron microscopy, pp65 antigenemia; molecular detection by PCR or in situ hybridization or isolation by culture, the findings in immunohistochemistry are three: cytomegaly, eosinophilic cytoplasmic inclusions and amphiphilic cytoplasmic inclusions, this is important when evaluating differential diagnosis of pulmonary compromise by other viruses that produce the same cytopathic changes as herpes simplex virus (HSV) and varicella zoster virus (VZV).
It is important to note that as previously explained that CMV infection at the pulmonary level is usually related to coinfection by other opportunistic germs, so demonstrating the evidence of the virus in the studies does not determine a causal relationship with pulmonary involvement, and only in the case in which other opportunists were discarded could be related as a precise etiology, that is, it is a diagnosis of expulsion in this group of patients. (twenty-one)
3.5 Neurological involvement
Neurological diseases caused by CMV are rare, however, those could be fatal. Encephalitis /Ventriculoencephalitis, Polyradiculitis, Myelitis, Dementia and Multifocal peripheral neuropathy are found most frequently in the spectrum of neurological diseases caused by VIH / CMV co-infection. The definitive diagnosis of CNS infections by CMV is based on the clinical presentation and demonstration of CMV in CSF or brain tissue. The detection of CMV DNA by PCR in the CSF has become an important diagnostic tool for CMV encephalitis with sensitivity and specificity of 80 and 90% respectively. avoiding the need for brain biopsy. CMV is rarely isolated in CSF, possibly due to the low number of infectious particles [39].
3.5.1 Encephalitis / Ventriculoencephalitis: Autopsy reports have revealed cytomegalic cells in 10-20% of the brains studied [40]. There are two types of presentation of encephalic disease due to CMV / VIH. The first scenario is diffuse micronodular encephalitis. These are patients who are often asymptomatic whose diagnosis is made by the microscopic finding in autopsies of small nodules of microglial infiltrates in the gray matter of the cortex, brainstem, cerebellum and basal ganglia. Only 10% of said nodules contain CMV inclusion bodies. Occasionally, it may present as a mild neurocognitive alteration [41]. The second scenario is the CMV encephalitis that occurs in advanced VIH infection such as lethargy, confusion, paralysis of cranial nerves and nystagmus. Seizures, ataxia, hyperreflexia and headache often occur. The presence of weakness in the lower limbs associated with hyporeflexia may be a sign of concomitant radiculopathy, the CSF study reveals mononuclear pleocytosis, hypoglucorrhachia and hyperproteinorrachia. However, in some cases the CSF is indeterminate. CT or MRI typically show periventricular uptake to contrast [42].
3.5.2 Polirradiculomielopatias: The Polirradiculo CMV myelopathies present a subacute onset of bilateral ascending weakness in the lower limbs that can progress to arreflexic paraplegia in weeks. Urinary retention and anal sphincter dysfunction are commonly presented in the initial evaluation. Nerve conduction studies and electromyography show signs of acute denervation in the affected muscle groups. Histologically, it is an inflammatory polyradiculitis in which there is necrosis of the ganglionar motor neuron of the dorsal and ventral horns of the marrow, a condition that explains the motor dysfunction [43]. This syndrome has occasionally been called polyradiculous myelopathy or myeloradiculitis due to the involvement of the medullary corticospinal tract represented by flexor plantar response (positive Babinski's sign) [39] CSF analysis reveals polymorphonuclear pleocytosis, hyperproteinorrachia and hypoglucorrhakia, imaging studies may be normal; however, it can be evidenced by nerve root enhancement, which is associated with leptomeningeal involvement [44].
3.6 Dementia: Taken in some reports as a form of encephalitis, it is characterized by the presence of microglial nodules and usually presents with delirium, confusion, apathy and focal neurological deficit. CSF evaluation reveals lymphocytepredominant pleocytosis, hypoglucorraquia or euglucorraquia and euproteinorraquia or hiperproteinorraquia [45].
3.6.1 Multifocal neuropathy: Usually the peripheral neuropathic component begins with painful paresthesias in a characteristic way, there is an asymmetric compromise of the upper and lower extremities that follow the peripheral nerve distribution. It commonly affects the radial, ulnar, peroneal and lateral cutaneous nerves. The progression of sensory dysfunction to motor symptoms may occur in a few weeks. The involvement of cranial nerves is not usual, although cases have been reported [41].
4. Diagnosis
Diagnosis almost always depends on laboratory confirmation, since it could not be done based solely on clinical findings. To make the diagnosis in this group of patients it is necessary to highlight that cytomegalovirus commits heavily immunosuppressed patients with VIH (T-CD4 lymphocytes <100 cells / mL) [46]; and that in this same population, asymptomatic viral replication is frequent.
The usual clinical samples for the study are: serum for antibody detection; whole blood for the study of cellular immunity and for direct detection techniques; and urine, saliva, whole blood, plasma, serum, bronchoalveolar lavage, cerebrospinal fluid and tissue for the study of CMV by direct detection techniques.
Generally, and simply, CMV retinitis is usually diagnosed from the recognition of characteristic retinal changes during an ophthalmologic examination. The diagnosis in this context has a positive predictive value of 95%, however in some cases the diagnosis can be difficult so PCR of samples of aqueous or vitreous humor can be useful to establish it.
CMV colitis is diagnosed from the visualization of typical mucosal ulcerations during an endoscopic study in addition to the histopathological demonstration of characteristic intranuclear and intracytoplasmic inclusions. The diagnosis of CMV esophagitis is based on the presence of ulcers in the distal esophagus and the evidence by means of biopsy of inclusion bodies in the endothelial cells with inflammatory reaction at the edge of the ulcer. Inclusion bodies can be found in large numbers or in isolation. The culture of CMV from biopsy of cells in brush of the colon or esophagus is insufficient to establish the diagnosis of colitis or esophagitis in the absence of histopathological changes since a significant number of patients with low CD4 TL counts can have positive cultures without clinical disease.
Performing the diagnosis of pneumonitis is difficult and requires both clinical and radiological findings, such as diffuse interstitial infiltrates, fever, cough or dyspnea. Likewise, the identification of multiple inclusion bodies in lung tissue should be achieved during cytological examination and the presence of other pathogens that more frequently can cause pneumonitis in this group of patients should be ruled out. On the other hand, the neurological commitment is established in the context of a compatible clinical syndrome and the presence of CMV in CSF or brain tissue, generally determined by PCR.
5. Immunoglobulin Measurement
It is based on the evaluation of the humoral immune response from the measurement of IgG and IgM specific antibodies against CMV. The demonstration of specific IgM antibodies or seroconversion of IgG antibodies is the traditional method to detect primary infection, however, the determination of IgM often leads to false positives, does not allow demonstrating recurrent infection and its values ??remain detectable for months. or years [21].
When there is a primary infection there is an IgM and IgG antibody response, the latter detectable for life in most cases, however the serological response in reactivation or reinfection is less predictable since in the normal subject no antibodies are produced specific IgM, while in immunocompromised patients there may be an increase in the IgG titer that, in some cases, is accompanied by specific IgM antibodies [46]. The presence of IgG antibodies is a sensitive marker of past infection, so negative levels of IgG make CMV infection unlikely [21]. Based on the above, the variability of the humoral response in immunosuppressed patients limits the real utility of the serological diagnosis in the face of a suspicious clinical picture.
An avidity test can help determine if the infection is recent [47]. During the first weeks of the primary infection, the IgG antibodies show very low avidity for the antigen, but as they mature, the avidity increases progressively. The lower or higher degree of avidity is determined with the serum sample treated and not treated with urea, which is able to dissociate the antigen-antibody complexes. The percentage of the quotient between the measurement of the treated sample and that of the untreated sample determines the avidity of the antibodies. A low percentage of avidity (<35%) indicates recent infection and percentages> 65% are indicative of past infection [21].
5.1 Culture
Cytomegalovirus can be easily isolated in urine, buccal samples, cervical tissue and tissue obtained by biopsy or autopsy. The growth of CMV from samples taken from the pharynx, urine or blood is a pathological finding, but only the culture from blood is very suggestive of pathogenic infection by CMV, since the presence of the virus in the pharynx or urine is often associated with asymptomatic infection.
The clinical importance of positive CMV cultures is difficult to determine in immunosuppressed patients. For example, CMV may be present in saliva or urine of up to 60-90% of VIH patients with AIDS, and the presence of the virus in these locations is not proof that CMV has been the cause of the disease. the patient's illness; in the same way, these patients can persistently eliminate virus in pharyngeal or bronchoalveolar washes. In these cases, the presence of histological changes such as intranuclear inclusions must be observed to establish the diagnosis of CMV involvement [20]. Cytological and histological abnormalities are not sensitive parameters of infection but are specific and indicative of CMV disease [47].
Although CMV can be grown easily, its growth is usually slow, so typical cytopathic changes can occur in cell cultures between 1 and 4 weeks. CMV produces characteristic, large, rounded infected cells that contain cytoplasmic inclusions with a "frosted glass" appearance. Given this consideration, although in the past it was the reference test for diagnosis, it has been replaced by faster, more effective and more modern tests.
Antigenemia test: It is based on the use of monoclonal antibodies to detect the viral protein of the pp65 tegument, product of the UL83 gene, which is the majority viral antigen present in peripheral blood leukocytes during CMV infection. Immunoenzymatic or immunofluorescence techniques have been used to perform this detection. It is a useful diagnostic technique (fast, simple, sensitive, specific) in immunocompromised patients; and the number of cells expressing the antigen correlates with the viral load of CMV [46]. The presence of at least one positive nucleus is indicative of infection.
This test provides a direct measure of the presence of CMV and can detect virus antigens in the cerebrospinal fluid of patients with CMV polyradiculopathy, and also in the peripheral blood of patients with immunosuppression (15). In patients with VIH infection, a clear cut-off point has not been established and values ??greater than 20 nuclei that give a sensitivity of 77%, specificity of 97%, PPV 91% and NPV of 92% are applied as significant [46].
In spite of being a relatively simple and highly standardized technique for the follow-up and management of CMV infection in other groups of patients as well as in those transplanted, the poor interpretation in lymphopenic and neutropenic patients as well as the greater simplicity and degree of automation of the Recent quantitative molecular techniques have replaced the classical tests for antigenemia by DNAemia for the control of CMV infection.
Molecular diagnosis: For the diagnosis and monitoring of immunosuppressed patients, quantitative blood or plasma techniques (DNAemia) should be used. Nowadays, PCR is the molecular technique most used for the quantification of CMV DNA. It is a quantitative technique for measuring viral load that plays a central role in the diagnosis in the modern laboratory. Specifically, real-time PCR is the method of choice for its sensitivity, specificity and ease of automation, it is more sensitive than culture and is the best tool for early detection of the disease. The test quantifies the viraemia and allows monitoring of the treatment [47].
The use of plasma instead of whole blood has been recommended because of its greater correlation with active replication of CMV, since the detection of CMV DNA in whole blood may only reflect the presence of the virus in lymphocytes where CMV remains in a dormant state. after the primary infection. In immunosuppressed patients, the viral load of CMV correlates with the presence of symptoms attributable to the virus and is a prognostic factor in the subsequent appearance of complications [18]. Quantitative PCR has allowed us to prove that a high number of copies / ml of CMV DNA in plasma correlates with the activity of CMV disease in patients with AIDS [30]. It is not yet clear if there is a correlation between the levels of viral load in bronchoalveolar lavage and the development of respiratory disease since the possibility of contamination with saliva, the main route of excretion of CMV, is important. It is for this reason that the viral load in BAL should be higher than that determined in pharyngeal washings to demonstrate local replication of CMV in the lower respiratory tract that allows to attribute an etiological role in pneumonic processes.
In VIH patients who start treatment with highly active antiretroviral therapy and CD4 lymphocytes <50 / mL, both qualitative and quantitative detection of CMV DNA has been shown to be one of the best predictors of retinitis risk several months later [20]. Patients with CMV retinitis have viral DNA detected in the blood in 70% of cases, while detection can be performed from the vitreous in 80% of cases.
Diagnostic Method |
Advantages |
Disadvantages |
Serology (IgG-IgM) |
Screening for latent infection |
Low S and E for acute infection |
PCR assays |
• Gold standard |
• Preferred in total blood sample |
Antigenemia |
• Quick and easy to perform |
• Less than PCR in leukopenia |
Viral culture |
• Highly specific |
• Dependent time |
Biopsy |
• Highly specific |
• Invasive |
Table 1: Advantages and disadvantages of the different diagnostic methods available for the detection of CMV.
Diagnostic Method |
Primary Infection |
Cmv Disease |
IgM |
+ |
Non-applicable |
IgG (seroconversion) |
++ |
Non-applicable |
IgG (serological status) |
Non-applicable |
Non-applicable |
IgG (avidity test) |
+ |
Non-applicable |
Antigenemia |
Non-applicable |
+ |
Cell culture |
Non-applicable |
+++ |
PCR quantified |
Non-applicable |
+++ |
Table 2: Utility of the different diagnostic methods in the detection of CMV according to the clinical situation.
5.2 Treatment
VIH-CMV coinfection, as previously mentioned, requires iniation of treatment as soon as possible to improve survival and decrease complications related to the progression of the disease. The therapeutic options include ganciclovir, valganciclovir, foscarnet and cidofovir.
Retinitis by CMV requieres induction with valganciclovir 900 mg every 12 hours or ganciclovir 5 mg/kg/every 12 hours during 14 to 21 days and continued maintenance with valganciclovir or ganciclovir. If CMV infection threatens visual capacity, intravitreal treatment with ganciclovir or foscarnet should be given concomitantly with systemic treatment. Some minor injuries can be resolved with ARV therapy, however the control of CMV replication can take from 3 to 6 months so maintenance treatment should always be continuied and the choice of treatment is valganciclovir 900 mg day which is equivalent to 5 mg/kg/day of ganciclovir. Although there is no specific time regarding maintenance therapy duration, four parameters are considered factors if antivirals are suspended: quiescent retinitis, treatment of lesions for at least 3 months, suppression of VIH replication, and CD4 lymphocyte count> 100. Among relapsing factors that can result in vision loss the clinician shoulg take into account: absence of immune reconstitution, thickening of the retinal blood barrier that prevents the entry of drugs and poor adherence to anti CMV treatment. The risk of immune reconstitution syndrome has been seen in these patients, the initiation of ARV therapy is suggested 2 weeks after anti CMV therapy [21].
In gastrointestinal compromise (colitis - esophagitis) the choice is induction with ganciclovir 5 mg / kg / iv every 12 hours for 21 - 42 days until the symptoms resolve, treatment with valganciclovir can be started in patients with mild disease, and after induction and tolerance to switch to oral valganciclovir is possible, however in patients with moderate to severe disease it is suggested to complete 14 days of induction therapy with ganciclovir IV, since there are no induction studies in this group of patients with valganciclovir, in cases of resistance or toxicity to ganciclovir the choice is foscarnet. Maintenance therapy will be maintained until a CD4 count> 100 is reached. The initiation of ARV therapy should be done as soon as retinitis is ruled out and the patient is tolerated orally [21].
In pulmonary compromise the choice is therapy with ganciclovir or foscarnet, there are no studies with valganciclovir and its effectiveness in these cases is unknown. In neurological compromise, given their high mortality, some experts suggest initiation of IV therapy combining ganciclovir and Foscarnet, despite the risk of toxicity, in these cases there are no guidelines on the duration of therapy or the role of valganciclovir (Table 3).
Target Organ |
Induction |
Maintenance |
Monitoring |
Retinitis |
- Intravitreal ganciclovir 2.5 mg in 0.05 ml + Valganciclovir 900 mg bid 14-21 days |
-Valganciclovir 900 mg qd |
- Start ART 1 - 2 weeks of anti CMV treatment |
- Valganciclovir 900 mg bid |
|
- Surveillance of leukopenia, thrombocytopenia |
|
- Ganciclovir 5 mg/kg/iv every 12 hours |
-Complete three months of treatment |
- Suspend if: quiescent retinitis, treatment of lesions for at least 3 months, suppression of VIH replication, and CD4 lymphocyte count> 100 |
|
- Foscarnet 60 mg / kg / iv every 8 hours, 90 mg / kg / iv every 12 hours |
- |
- |
|
Colitis |
- Ganciclovir 5 mg iv every 12 hours, Valganciclovir 900 mg every 12 hours orally for 21 - 42 days |
-Valganciclovir 900 mg day |
- |
- Alternative: |
FALSE |
||
- Foscarnet Foscarnet 60 mg / kg / iv every 8 hours, 90 mg / kg / iv every 12 hours |
-Vigilance of neutropenia, thrombocytopenia |
||
- Oral valganciclovir in mild compromise |
- |
||
Pneumonitis |
- Ganciclovir iv or Foscarnet iv |
The role of valganciclovir is unknown |
- |
Neurological |
Combination therapy ganciclovir iv and Foscarnet iv |
The role of valganciclovir as well as the duration of therapy is unknown |
Hematological and renal toxicity |
Table 3: CMV infection treatment in patients with HIV.
6. Conclusion
CMV and VIH co ninfection, independent of the state of the disease, CD4 count, is related to worse descents, given the inflammatory state and the higher immunological senescence, making it necessary to perform an active search of the disease, using the available diagnostic tools , making an early and timely diagnosis, guaranteeing the specific treatment according to the target organ, and achieving an impact on the survival and risks of complications in this group of patients.
Acknowledgements
Dr. Jorge Aponte for his dedication to his medical practice
Availability of Data and Materials
All the data were collected and analyzed by the authors and are not ready to share their data because the data have not been published.
Author Contributions and Acknowledgements
AJ, VJ, CA, MD participated in the recolection of data, search of literature and writing of the first draft. AJ and FY drafted and translated the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests
Consent for Publication
Not applicable
This review is done with the authors' own resources, without any source of external financing
Ethical Standards and Consent
This study was approved by the Institutional Ethical Committee. All techniques were performed according to the Helsinki declaration of 1975 and all modifications about it. Informed consent was obtained.
References
- Gianella S, Letendre S. Cytomegalovirus and VIH: A Dangerous Pas de Deux. J Infect Dis 214(2016): S67?S74.
- Gkrania-Klotsas E, Langenberg C, Sharp SJ, et al. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based european prospective investigation of cancer-norfolk cohort. Clin Infect Dis 56 (2013): 1421-1427.
- Chaisson RE, Moore RD, Richman DD, et al. Incidence and natural history of Mycobacterium avium-complex infections in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. Am Rev Respir Dis 146 (1992): 285-289.
- Halary F, Amara A, Lortat-Jacob H, et al. Human Cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17 (2002): 653-664.
- Varnum SM, Streblow DN, Monroe ME, et al. Identification of Proteins in Human Cytomegalovirus ( HCMV ) Particles: the HCMV Proteome Identification of Proteins in Human Cytomegalovirus ( HCMV ) Particles?: the HCMV Proteome. J Virol 78 (2004): 10960-10966.
- Yurochko AD, Hwang E-S, Rasmussen L, et al. The Human Cytomegalovirus UL55 (gB) and UL75 (gH) Glycoprotein Ligands Initiate the Rapid Activation of Sp1 and NF-?B during Infection. J Virol 71 (1997): 5051-5059.
- Navarro L, Mowen K, Rodems S, et al. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol Cell Biol 18 (1998): 3796-3802.
- Dhuruvasan K, Sivasubramanian G, Pellett PE. Roles of host and viral microRNAs in human cytomegalovirus biology. Virus Res 157 (2011): 180-192.
- Mohr I. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res 119 (2006): 89-99.
- Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus Infection: A Driving Force in Human T Cell Immunosenescence. Ann N Y Acad Sci 1114 (2007): 23-35.
- M EF. Cytomegalovirus Disease in Patient with VIH Infection. J Antimicrob Agents 2 (2016): 1-5.
- Grønborg HL, Jespersen S, Hønge BL, et al. Review of cytomegalovirus coinfection in HIV-infected individuals in Africa. Rev Med Virol 27 (2017): 1-14.
- Brantsæter AB, Johannessen A, Holberg-Petersen M, et al. Cytomegalovirus viremia in dried blood spots is associated with an increased risk of death in HIV-infected patients: A cohort study from rural Tanzania. Int J Infect Dis 16 (2012): e879-885.
- Spector SA, Hsia K, Crager M, et al. Cytomegalovirus (CMV) DNA Load Is an Independent Predictor of CMV Disease and Survival in Advanced AIDS. J Virol 73 (1999): 7027-7030.
- Deayton JR, Prof Sabin C, Johnson M, et al. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 363 (2004): 2116-2121.
- Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 211 (2015): 178-186.
- Goldberg DE, Smithen LM, Angelilli A, et al. HIV-associated retinopathy in the HAART era. Retina 25 (2005): 633-49-3.
- Schinstock B, Schaffer B. Occupational exposure to HIV. Sixth Edit. Vol. 92, Am J Med. Elsevier Inc. (1992): 115-116.
- Cmv C. 7.5 - Ocular Infections with Cytomegalovirus (CMV) [Internet]. Fourth Edi. Ophthalmology. Elsevier Ltd (2017): 704-708.
- Holland GN, Buhles WC, Mastre B, et al. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. UCLA CMV Retinopathy. Study Group. Arch Ophthalmol 107 (1989): 1759-1766.
- AIDSinfo. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults (2013): 408.
- Martin DF, Sierra-Madero J, Walmsley S, et al. A Controlled Trial of Valganciclovir as Induction Therapy for Cytomegalovirus Retinitis. N Engl J Med 346 (2002): 1119-1126.
- The New England Journal of Medicine Downloaded from nejm.org on October 14, 2015. For personal use only. No other uses without permission. Copyright © 1992 Massachusetts Medical Society. All rights reserved (1992).
- Carmichael A. Cytomegalovirus and the eye. Eye 26 (2012): 237-240.
- Mills RP. Correlation of QoL with clinical symptoms and signs at the time of glaucoma diagnosis. Tr Am Ophth Soc (1998): 754-812.
- Spector SA, Wong R, Hsia K, et al. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest 101 (1998): 497-502.
- Ross SA, Novak Z, Pati S, et al. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets 11 (2011): 466-474.
- Cmv C, Ii CSC, Capítulo EDEL. Citomegalovirus (CMV) (2017).
- Saenz JLP, Álvarez CC, Marimont MB. Capítulo 288 - Infecciones causadas?por el citomegalovirus y los virus del herpes?humano de tipos 6, 7 y 8. Farreras Rozman. Medicina Interna + StudentConsult Elsevier (2017).
- Meyer-Olson D, Schmidt RE, Bollmann BA. Treatment and prevention of cytomegalovirus-associated diseases in HIV-1 infection in the era of HAART. HIV Ther 4 (2010): 413-436.
- Scaradavou A. HIV-related thrombocytopenia. Blood Rev 16 (2002): 73-76.
- Plachter B, Sinzger C, Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv Virus Res 46 (1996): 195-261.
- Furuhata M, Yanagisawa N, Nishiki S, et al. Severe Thrombocytopenia and Acute Cytomegalovirus Colitis during Primary Human Immunodeficiency Virus Infection. Intern Med 55 (2016): 3671-3674.
- Mulder R, Tichelaar YIG V, Sprenger HG, et al. Relationship between cytomegalovirus infection and procoagulant changes in human immunodeficiency virus?infected patients. Clin Microbiol Infect 17 (2011): 747-749.
- Ohkuma K, Saraya T, Sada M, et al. Evidence for cytomegalovirus-induced haemophagocytic syndrome in a young patient with AIDS. BMJ Case Rep (2013): 1-3.
- Fraire AE, Woda BA, Welsh RL, et al. Viruses and the Lung (2013): 117-122.
- Lee FE-H, Treanor JJ. Viral Infections [Internet]. Sixth Edit. Murray and Nadel’s Textbook of Respiratory Medicine. Elsevier Inc (2013): 527-556.
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 4 (2004): 725-738.
- Cohen BA, McArthur JC, Grohman S, et al. Neurologic prognosis of cytomegalovirus polyradiculomyelopathy in AIDS. Neurology 43 (1993): 493-499.
- Lang W, Miklossy J, Deruaz JP, et al. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol 77 (1989): 379-390.
- Anders HJ, Goebel FD. Neurological manifestations of cytomegalovirus infection in the acquired immunodeficiency syndrome. Int J STD AIDS 10 (1999): 151-191.
- Albarillo F, O’Keefe P. Opportunistic Neurologic Infections in Patients with Acquired Immunodeficiency Syndrome (AIDS). Vol. 16, Current neurology and neuroscience reports (2016): 10.
- Albarillo F, O’Keefe P. Opportunistic Neurologic Infections in Patients with Acquired Immunodeficiency Syndrome (AIDS). Curr Neurol Neurosci Rep 16 (2016): 10.
- Bowen LN, Smith B, Reich D, et al. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 12 (2016): 662-674.
- Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. Vol. 8, Journal of NeuroVirology (2002): 158-167.
- Gimeno Cardona C, del Remedio GS, Sanbonmatsu Gamez S, et al. Enfermedades Infecciosas y Microbiología Clínica Enfermedades Infecciosas y Microbiología Clínica Infección por citomegalovirus humano. Enferm Infecc Microbiol Clin 32 (2014): 1522.
- Lasso BM. Diagnóstico y tratamiento de infecciones oportunistas en el paciente adulto con infección por VIH/SIDA. Rev Chil infectología 28 (2011): 440-460.
Citation: Jorge Aponte, Juan David Vera, Aldair Chaar, Daniel Martin, Forero Yency. Cytomegalovirus Infection (CMV) and Human Immunodeficiency Virus: Diagnostic and Therapeutic Challenges in Virology. Archives of Microbiology & Immunology 2 (2018): 018-035.
