Antifleas Activity and Safety of Tithonia diversifolia and Senna didymobotrya Extracts
Article Information
Githinji James Maina*, Maitho Timothy, Mbaria James Muchunu
Department of Public Health Pharmacology and Toxicology, Nairobi, Kenya
*Corresponding Author: Githinji JM, Department of Public Health Pharmacology and Toxicology, University Of Nairobi, P.O. BOX 29053-00625, Nairobi, Kenya
Received: 27 August 2018; Accepted: 07 September 2018; Published: 15 September 2018
Citation: Githinji James Maina, Maitho Timothy, Mbaria James Muchunu. Antifleas Activity and Safety of Tithonia diversifolia and Senna didymobotrya Extracts. J Pharm Pharmacol Res 2 (2018): 078-092.
Share at FacebookAbstract
Background: Tithonia diversifolia and Senna didymobotrya have been used traditionally as bio-pesticides. Their use in the control of fleas, and also their aqueous extract safety is not adequately documented.
Methods: Antifleas activity of Tithonia diversifolia and Senna didymobotrya were compared with Chrysanthemum cinerariifolium, and also their acute toxicity in Wister rats and dermal and eye irritation in Newzealand albino rabbit were studied using a method reported previously. Crude aqueous extracts of flowers and leaves for T. diversifolia, leaves of S. didymobotrya and flowers of C. cinerariifolium were prepared and serial dilutions of the crude extracts and control drugs were prepared. Whatman’s filter paper no.1 stripes were coated with plant extracts which were used to investigate antifleas activity, using fleas obtained from mongrel dogs. The activities were observed after 24 and 48 hours by counting the number of life fleas in the polypylene tubes.
Results: T. diversifolia showed most (93%) antifleas activity, C. cinerariifolium (90%) and S. didymobotrya 66.3%. The three plants extracts studied did not show any signs of eye or dermal toxicity and had LD50 of above 2000 mg/kg.
Conclusions: Further studies should be conducted in order to find out if T. diversifolia flowers can be used to control jigger flea.
Keywords
T diversifolia flowers; Fleas control; Jiggers
Article Details
1. Introduction
Fleas are ecto-parasite which are passed from domestic animals to humans and cause similar effects as in the animal hosts. Fleas have exclusive ability to reproduce in households, are present all year round, regardless of the seasons, which means that dogs living in cold areas are also infested. Flea bites can cause pruritus and their key pathogenic effect is flea allergic dermatitis (FAD) as well as other health effects. FAD prevention relies on the control of the flea infestation, involving regular and continued use of anti-flea chemical agents, which are normally topical formulations but occasionally can be given orally [1].
Fleas are pests which are a nuisance and they are vectors of diseases. Three types of plagues in human are associated with fleas. These are bubonic plague, pneumonic plague and septicemic plague. Rat and cat fleas are associated with murine typhus fever which is caused by rickettsia typhi which is mainly transmitted by the rat and cat fleas, where humans acquire the infection as result of contamination from the dried feaces and crushed bodies of the fleas. The sand fleas, chigoes or jigger fleas are only a millimeter long and are nuisance as the females burrow into the skin. Mostly, a person may be affected by one or two jiggers at a time, but Infestations with hundreds of jiggers also occurs. Under favourable conditions a complete cycle from eggs through larvae, pupa and finally adult, can take 18 days [2].
Arthropods like fleas and ticks determine the community composition of bacterial [3]. Fleas host many vector-borne pathogens and their diseases [4]. The common disease causing bacterium which is Yersinia pestis causes plague in man. None of the flea types is specific to humans and only a fraction of the fleas come into contact with human on regular basis. Abundance of human associated fleas (Pulex irritans, Ctenocephalides felis, and X.cheopis) however, has been described in human dwellings in plague-endemic regions of Africa [5]. Human (P irritans) fleas are associated with plague transmission from human to human, and Cat fleas (C fellis) are suspected in the plague outbreaks for example in Northwest Uganda [4]. Clinical manifestations of plague are; bubonic plague which is most common, septic plague without bubo, and pneumonic plague, meningitis and pharyngitis [6]. Pneumonic plague is rapidly fatal if untreated. Fleas and ticks should be controlled in order to reduce risk of disease transmission and also reduce economic losses associated with parasitization of domesticated animals [7]. Rickettsia typhi is associated with fleas, ticks mites and body lice. The obligate intracellular gram negative bacteria are transferred from rodents’ reservoir by an arthropod to humans [8].
Bitam et al., [4] associated Tunga penetrans fleas with secondary bacterial infections in the lesions among them; Clostridium tetani, Streptococcus pyogens, Sataphylococcus aureus, Klebsiella aerogenes, Enterobacter agglomerans, Escherichia coli as well as other enterobacteriaceae. A study in Kandara Sub-Counties in Murang’a County Kenya reported that 6,200 school going children are infested by Tungiasis in 2014 [9]. Most of the affected patients use mechanical means to remove jiggers with sharp objects such as needles [10]. Tithonia species contains sesquiterpene lactones and diterpenoids which have biological activities against insects [11]. Tithonia diversifolia is
used in Ikolomani Division of Kakamega County, Kenya, in management and control of jigger fleas [12]. There is
limited information on the effects of crude plant extracts like Tithonia diversifolia and Senna didymobotrya on fleas which are hosted in animals. Additionally, the toxicity and safety profiles of these plants have not been reported adequately in various animals. It is therefore important to investigate the LD50 of these plants’ extracts as insecticides have been associated with accidental poisoning. If the two plants’ extracts are found to be effective on fleas, more investigations should be conducted in order to check if the same extracts can be effective on the sand flea, which is a jigger causing flea and is reported as a menace to the poor residents of Murang’a County and other regions of Kenya.
The current study was conducted in order to investigate in vitro antiflea activity of Tithonia diversifolia flowers and leave crude extracts and also Senna didymobotrya leaves crude extracts. The other objective of the study was to determine skin sensitization and irritation as well as determine the acute toxicity of these extracts.
2. Materials and Methods
2.1 Approvals of the study
The study was approved by National Commission for Science Technology and Innovations (NACOSTI) and also Biosafety, Animal Use and Ethics Committee (BAUEC) of University of Nairobi.
2.2 Experimental animals
Young adult female nulliparous and non-pregnant Newzealand albino rabbits were obtained from the University of Nairobi animal house and were used for the determination of acute dermal irritation/corrosion (OECD, 404) and eye irritation test (OECD, 405). Adult (8 to 12 weeks old), female, nulliparous and non-pregnant Wister rats weighing 90-130 g were obtained from the University of Nairobi animal house and were used for acute toxicity tests of the extracts. All animals in these studies were individually housed and acclimatized for 5 days to laboratory conditions before beginning the study [13]. Fleas used in the study were obtained from the local mongrel dogs. The animals were housed under the standard laboratory conditions (temperatures of 25°C ± 3°C, natural light and relative humidity of 50 - 60%). They were fed on standard pellet diet and on unlimited supply of water [14].
2.3 lant materials
Tithonia diversifolia and Senna didymobotrya plants specimens which included aerial parts and roots were collected from Maragua area of Murang’a County. The collected specimens were photographed, identified and authenticated with the aid of a taxonomist at the National Museums of East Africa where the voucher specimens were prepared and deposited. The plant materials were transported to the Department of Public Health, Pharmacology and Toxicology laboratories and were washed thoroughly with running tap water, chopped into small pieces and then air- dried under the shade for a period of 14 days and then grounded. Extractions of plant materials were done at the Kabete campus, of the University of Nairobi using a previous reported method [15-16]. The crude methanolic and aqueous extracts were dissolved separately in dimethyl sulfoxide (DMSO) and distilled water respectively at a concentration of 1000 mg/10 ml. Serial dilutions were prepared logarithmically under sterile conditions by adding
calculated amounts of distilled water in order to obtain working concentration ranging from 100 mg/ml ? 1 mg/ ml. All the prepared crude extract solutions were stored at 40°C and retrieved only during use.
2.4 Determination of in vitro anti-flea activity of Tithonia diversifolia and senna didymobotrya
Polypropylene (15 ml) centrifuge tubes contact assay against Ctenocephalides felis and Ctenocephalides canis were used in the study as previously reported [17], but with minor modifications. Methanolic crude extracts were not tested although in piloting study they were found to be more active, reason being the fact that herbalists use water as the solvent for their concoctions. Three concentrations of each crude aqueous extract (Senna didymobotrya leaves- SLAE, Tithonia diversifolia leaves-TLAE, Tithonia diversifolia flowers-TFAE) were prepared for testing in-vitro anti-flea activity. For each extract, 1 mg/ml, 10 mg/ml and 100 mg/ml concentrations were made. A Whatman filter paper no.1 strip measuring 10 cm by 1.5cm was saturated with the extract concentration to be tested and was later allowed drying up, thus leaving the paper evenly coated with the extract at the concentration being tested. The coated stripe was fitted into the 15 ml polypropylene centrifuge tube. A total of ten fleas held by the small loose cotton wool were randomly picked and transferred into these polypropylene tubes and screw cap with holes replaced to hold the fleas inside, avoiding suffocation.
Similarly, a positive control using pyrethrum flower aqueous extract (PFAE) at similar concentrations were included for comparison. Viability of fleas in each tube was tested as previously described Dryden et al., [18]. Adult fleas Ctenocephalides canis and felis from the natural habitat were obtained from the mongrel dogs obtained from Ndumboini households. The fleas were held in a large container with small pieces of cotton wool which served as holding grounds for them. Each piece of cotton wool held an average of ten fleas. A total of 10 fleas were picked randomly and were transferred into each tube. The tubes were closed with an untreated screw cap with needle- punctured holes in the center in order to hold the fleas inside, avoiding suffocation. These tubes were kept at ambient temperatures and humidity, horizontally in order to ensure maximum contact between the fleas and the filter paper surface in the tube.
2.5 Acute dermal toxicity
The acute dermal toxicity of the crude aqueous extracts was tested using OECD [19] guidelines 404. Nine (three rabbits per test substance) healthy, adult female, nulliparous and non-pregnant Newzealand albino rabbits weighing 2.0-3.0 Kg were used. The test extract was applied onto the skin of the test rabbits as a single application of 2000 mg/kg. Eye irritation tests were also conducted using the Newzealand albino rabbits as described in the OECD the guidelines 405.
2.6 Determination of acute toxicity levels of the active crude extract in rats
Evaluation of acute toxicity and LD50 of the crude aqueous extracts was done using Wister rats and OECD [13] method as described in guidelines 425. A total of 15 rats, four for Senna didymobotrya leave extracts, four for Tithonia diversifolia leave extracts, four for Tithonia diversifolia flower extracts and three which served as controls,
each per test group of adult female nulliparous and non-pregnant Wister rats weighing 90-130 g were used to investigate acute toxicity of active crude extracts as described in the limit test.
2.7 Data Analysis
Data analysis was done using Student’s t-test, R version 3.4.3 and graphs were drawn using Microsoft Excel for 2010 year. The data obtained from In vitro anti-flea studies was expressed as a mean ± standard error of the mean (SEM) of the two independent experiments. The data from acute toxicity studies was analyzed qualitatively and quantitatively using suitable statistical tools. The LD50 values were calculated using the Acute Oral Toxicity Guidelines (425) Statistical Program Version: 1.0) [14].
3. Results
The findings of the study are presented below
3.1 In Vitro Antifleas Activity of Tithonia diversifolia and Senna didymobotrya Crude Extracts in Comparison with Pyrethrum Flowers Aqueous Crude Extract.
Means of the aqueous extracts are shown in Table 1.
|
Extract |
Concentration |
Means of dead fleas |
Means of life fleas N2* |
Initial Number of fleas N2 |
Efficacy percentage |
|
TLAE |
100 mg/ml |
8.67 |
1.33 |
10 |
86.7 |
|
10 mg/ml |
5.33 |
4.77 |
10 |
53.3 |
|
|
1 mg/ml |
3.67 |
6.33 |
10 |
36.7 |
|
|
TFAE |
100 mg/ml |
9.33 |
0.77 |
10 |
93.3 |
|
10 mg/ml |
8.0 |
2.0 |
10 |
80 |
|
|
1 mg/ml |
3.67 |
6.33 |
10 |
36.7 |
|
|
SDLAE |
100 mg/ml |
6.63 |
3.37 |
10 |
66.3 |
|
10 mg/ml |
4.33 |
5.77 |
10 |
43.3 |
|
|
1 mg/ml |
4.0 |
6.0 |
10 |
40.0 |
|
|
PFAE |
100 mg/ml |
9.0 |
1.0 |
10 |
90 |
|
10 mg/ml 1 mg/ml |
7.33 6.0 |
2.67 4.0 |
10 10 |
73.3 60.0 |
KEY: N2- Initial number of fleas; N1-Mean Number of Dead Fleas; N2*-Means of life fleas; TDFA-Tithonia
diversifolia flowers aqueous extract; TDLA- Tithonia diversifolia leaves aqueous extract; SDLA-Senna didymobotrya leaves aqueous extract; PFAE-Pyrethrum Flowers aqueous extract
Table 1: Means of fleas for all aqueous extract (TLAE, TFAE, SDLAE, and PFAE). Results of in vitro antifleas activity of all extracts in the study are compared and are shown in Figure 1.
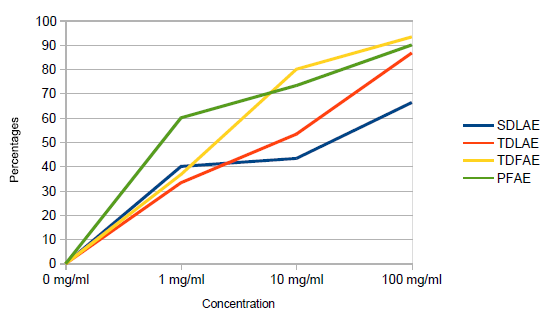
Figure 1: In vitro activity of SLAE, TDLAE, and TDFAE compared with PFAE extracts.
3.2 Acute Dermal Toxicity Profile of Tithonia diversifolia and Senna didymobotrya Crude Extracts in Newzealand Albino Rabbits
3.2.1 Acute dermal irritation: Effects of the extracts on the weight of the rabbits are shown in Table 2.
|
Rabbits |
means weight in Kg at the beginning |
means weight in kg at the end |
p Value |
Significant or not significant |
|
2.13 |
2.14 |
0.424 |
Not significant |
|
|
TDLA |
1.82 |
1.82 |
0.943 |
Not significant |
|
SDLA |
1.75 |
1.77 |
0.94 |
Not significant |
Table 2: Rabbits mean weights before and 14 days after application of the extract.
Significant weight difference at 95% CI and p ≤ 0.05
The effects of the crude extracts on the skin of the Newzealand albino rabbits are shown Table 3.
|
Crude Extract |
Time after removal of the patch |
0 minutes |
60 minutes |
24 hours |
48 hours |
72 hours |
|
TDLA |
Erythema |
0 |
0 |
0 |
0 |
0 |
|
Oedema |
0 |
0 |
0 |
0 |
0 |
|
|
TDFA |
Erythema |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
0 |
0 |
||
|
SDLA |
Erythema |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
0 |
0 |
ERYTHEMA: 0-No erythema; 1-Very slight erythema (barely perceptible); 2-Well defined erythema; 3-Moderate
to severe erythema; 4-Severe erythema (beef red); OEDEMA: 0-No oedema; 1-Very slight oedema, (barely perceptible); 2-Slight oedema, edges of the area well defined by definite rising; 3-Moderate oedema (raises approximately 1mm); 4-Severe oedema (raised more than 1mm and extending beyond the area of exposure).
Table 3: Dermal toxicity of crude aqueous extracts on Newzealand albino rabbit grading as per OECD 204.
3.3 Eye irritation test results for Tithonia diversifolia flowers aqueous crude extract, Tithonia diversifolia leaves aqueous crude extract, and Senna didymobotrya leave aqueous crude extract Results of eye irritation test are shown in Table 4.
|
Crude Extracts |
Cornea |
Iris |
Conjunctiva |
Chemosis |
|
TDFA |
0 |
0 |
0 |
0 |
|
TDLA |
0 |
0 |
0 |
0 |
|
SDLA |
0 |
0 |
0 |
0 |
CORNEA: 0-No ulceration or opacity was observed; 1-Scattered or diffuse areas of opacity; 2-Easily discernible
translucent area; 3-Nacreous area (no details of iris visible); 4-Opaque cornea; IRIS: 0-Normal; 1-Markedly deepened rugae, congestion, swelling, moderate circumcorneal hyperaemia; 2-Hemorrhage, gross destruction or no reaction to light; CONJUNCTIVA: 0-Normal; 1-Some blood vessels hyperaemic; 2-Diffuse crimson color, individual vessels not easily discernible; 3-Diffuse beefy red; CHEMOSIS: 0-Normal; 1-Some swelling above normal; 2-Obvious swelling with partial eversion of the lids; 3-Swelling with lids about half closed; 4-Swelling with lids more than half closed.
Table 4: T. diversifolia Flowers aqueous extract eye irritation test results.
NB: No significant irritation was observed over the entire period of the study.
3.4 Acute Oral Toxicity of Tithonia diversifolia Flowers and Leaves, and Senna Didymobotrya Crude Extracts in Wister Albino Rats
3.4.1 Effects of the crude extract on weight of the test animals: The effects aqueous crude extract on weight of
the Wister rats are shown in Tables 5, 6 and 7. The effects T. diversifolia leaves aqueous (TDLA) extract on weight of the Wister rats are shown in Tables 5.
|
RAT |
Weight in gram at the beginning |
Dose in mg |
Volume in ml |
Weight in gram on day7 |
Weight in gram on 14 |
|
TDLA1 |
99.58 |
199.16 |
0.50 |
107.16 |
136.10 |
|
TDLA2 |
110.11 |
220.22 |
0.55 |
124.36 |
133.27 |
|
TDLA3 |
104.20 |
208.40 |
0.52 |
116.45 |
135.57 |
|
TDLA4 |
107.52 |
215.04 |
0.54 |
119.59 |
121.61 |
|
98.22 |
Control |
Control |
101.67 |
108.03 |
Table 5: Effects of T. diversifolia leaves aqueous (TDLA) on rat weights before and after oral administration of the crude extract to Wister rats.
p = 0.01049 at day 7. The value indicates that there is a significant weight difference at 95% CI at the beginning and on day 7; p = 0.04384 at day 14. The value indicates that there is a significant weight difference on day 14 for Tithonia diversifolia Leaves Aqueous crude extract. The effects of Tithonia diversifolia Flowers aqueous crude extracts on weight of the Wister rats are shown in Table 6.
|
RAT |
Dose in mg |
Volume in ml |
Weight in gram on day 7 |
Weight in gram on day 14 |
|
|
TDFA1 |
100.00 |
200.00 |
0.50 |
122.07 |
134.44 |
|
TDFA2 |
100.27 |
200.54 |
0.51 |
115.07 |
118.34 |
|
TDFA3 |
98.90 |
197.80 |
0.49 |
113.54 |
124.03 |
|
TDFA4 |
102.29 |
204.58 |
0.511 |
115.92 |
126.13 |
|
TDFA5 |
97.34 |
Control |
Control |
105.13 |
108.85 |
Table 6: Effects of T. diversifolia Flowers Aqueous (TDFA) on rats’ weights before and after oral administration of the crude extract to Wister rats.
Weight beginning and after day 7, p = 0.02222 at day 7.
p = 0.02646 at day 14. The value indicates that there is a significant weight difference in the beginning and on day 14 for Tithonia diversifolia Flowers Aqueous crude extract. The effects of Senna didymobotrya Leaves Aqueous extract
(SDLA) on weights of the Wister rats are shown in Table 7.
|
RAT |
Weight in gram at (day 0) |
Dose in mg |
Volume in ml |
Weight in gram on day 7 |
Weight in gram on day 14 |
|
109.70 |
219.40 |
0.55 |
111.30 |
122.07 |
|
|
SDLA2 |
104.35 |
208.70 |
0.52 |
114.21 |
115.88 |
|
SDLA3 |
119.20 |
238.40 |
0.60 |
132.70 |
138.13 |
|
SDLA4 |
120.01 |
240.02 |
0.60 |
135.23 |
140.38 |
|
SDLA5 |
95.89 |
Control |
Control |
100.28 |
107.69 |
Table 7: Effects of Senna didymobotrya Leaves Aqueous extract (SDLA) on rat weights before and after oral administration of the crude extract.
Weight at the beginning and after 7 days for Senna didymobotrya Leaves Aqueous crude extract p = 0.1586 at day 7. The value indicates that there is no significant weight difference at 95 CI in the beginning. p = 0.1733 on day 14. The value indicates that there is no significant weight difference at 95% CI.
3.4.2 Acute LD50 results: The results of the limit test are summarized in Table 8. No rat died or was found moribund condition. One of the rats in Senna didymobotrya Leaves aqueous liter shown distress signs on the neck, but was stable up to the 14th day after drug administration. Another one in the same liter was wet with urine. The findings are shown in Table 8.
|
Extract Sample |
Means of initial weight (g) |
Means of weight (g) on day 7 |
Means weight (g) on day 14 |
mortality or moribund |
Toxicity signs |
|
TDFA |
100.37 ± 1.41 |
116.65 ± 3.75 |
125.74 ± 6.67 |
0/4 |
Nil |
|
TDLA |
105.35 ± 4.55 |
116.89 ± 7.23 |
131.64 ± 6.80 |
0/4 |
Nil |
|
SDLA |
113.32 ± 7.60 |
123.36 ± 12.35 |
129.12 ± 12.01 |
0/4 |
Nil |
|
Control ( H2O) |
97.15 ± 1.20 |
102.36 ± 2.50 |
108.19 ± 0.60 |
0/3 |
Nil |
TDLA- Tithonia diversifolia Leaves Aqueous; TDFA- Tithonia diversifolia Flowers Aqueous; SDLA-Senna
didymobotrya Leaves Aqueous.
Table 8: Acute Toxicity Results of Wister Rats.
One-Way Analysis of Variance (ANOVA) at 95 CI and level of significant being p ≤ 0.05, p = 0.2004 the value indicates that there is no significant weight difference at long run.
3.4.2.1 Effects of the extracts on blood profile of the Wister rats:
The effects of the extracts on the blood profile are shown in the Tables 9.
|
Blood profile |
Crude extract |
TDLA (4 rats) |
TDFA (4 rats) |
SDLA (4 rats) |
Control (3 rats) |
|
RBCs (1012) |
Means |
6.76 ± 1.26 |
6.21 ± 2.15 |
5.27 ± 3.56 |
6.62 ± 0.75 |
|
p Value |
0.261 |
0.3604 |
0.504 |
||
|
Hb (g/dl) |
Means |
15.83 ± 1.26 |
14.63 ± 2.15 |
13.68 ± 3.56 |
14.27 ± 0.75 |
|
p Value |
0.136 |
0.396 |
0.009 |
||
|
Hct (Percentage) |
Means |
40.58 ± 9.13 |
36.55 ± 13.2 |
46.45 ± 4.67 |
37.46 ± 4.76 |
|
p Value |
0.136 |
0.196 |
0.026 |
||
|
MCV(FI) |
Means |
59.73 ± 4.36 |
58.95 ± 3.99 |
64.18 ± 3.17 |
56.63 ± 1.29 |
|
p Value |
0.195 |
0.699 |
0.013 |
||
|
MCH (pg) |
Means |
21.73 ± 0.92 |
21.95 ± 0.54 |
21.98 ± 2.32 |
21.50 ± 0.79 |
|
p Value |
0.092 |
0.620 |
0.921 |
||
|
MCHC (g/dl) |
Means |
36.50 ± 2.72 |
37.40 ± 2.29 |
34.33 ± 3.41 |
37.97 ± 1.48 |
|
p Value |
0.892 |
0.831 |
0.054 |
||
|
RDW |
Means |
20.20 ± 0.71 |
19.08 ± 1.48 |
21.35 ± 1.02 |
20.70 ± 0.30 |
|
p Value |
0.109 |
0.115 |
0.161 |
||
|
PLTs (K/ µL) |
Means |
475.50 ± 295 |
471.00 ± 195 |
648.00 ± 299 |
381.67 ± 298.63 |
|
p Value |
0.059 |
0.416 |
0.393 |
||
|
WBCs (K/ µL) |
Means |
7.65 ± 3.84 |
10.12 ± 5.24 |
10.30 ± 2.11 |
10.58 ± 3.00 |
|
p Value |
0.855 |
0.917 |
0.042 |
||
|
N(Percentages) |
Means |
27.40 ± 19.20 |
38.48 ± 8.69 |
27.43 ± 18.04 |
49.93 ± 12.3 |
|
p Value |
0.027 |
0.843 |
0.020 |
||
|
L(×109) |
Means |
5.61 ± 3.63 |
6.03 ± 4.13 |
6.74 ± 0.37 |
4.78 ± 1.66 |
|
p Value |
0.228 |
0.862 |
0.016 |
||
|
M(×109) |
Means |
0.21 ± 0.13 |
0.26 ± 0.23 |
0.27 ± 0.03 |
0.28 ± 0.03 |
|
p Value |
0.301 |
0.751 |
0.205 |
||
|
E(×109) |
Means |
0.12 ± 0.05 |
0.17 ± 0.12 |
0.17 ± 0.07 |
0.20 ± 0.14 |
|
p Value |
0.119 |
0.053 |
0.082 |
||
|
Means p |
0.02 ± 0.01 0.391 |
0.015 ± 0.017 0.604 |
0.020 ± 0.01 0.18 |
0.10 ± 0.00 |
The extracts were compared with the control with level of significant being p ≤ 0.05 at 95% CI,
RBCs-Red Blood Cells; Hb (g/dl)-Haemoglobin; Hct - Hematocrit; MCV (FI)-Mean Corpuscular Volume; MCH- Mean
Cell Hemoglobin; MCHC-Mean Cell Hemoglobin Concentration; RDW-Red blood cells distribution width; PLT- Platelets; WBC-White Blood Cells; N-Neutrophils; L-Lymphocytes; M-Monocytes; E-Eosinophil; B-Basophils.
Table 9: Effects of the crude extracts on Red Blood Cells (RBCs) 14 days after oral administration to Wister rats.
4. Discussion
A discussion of antifleas efficacy and safety of the two plants is given below.
Tithonia diversifolia flowers aqueous extract (TFAE) was the most effective in anti- flea activity. At 100 mg/ml, TFAE killed 93.3% and at 10 mg/ml it killed 80.0 % of fleas in 24 hours, while pyrethrum flowers aqueous extract (PFAE) at the same concentrations killed 90.0% and 73.3% of the fleas respectfully in 24 hours. The Tithonia diversifolia leaves aqueous extract (TLAE) killed 86.7% and 53.3%, while Senna didymobotrya leaves aqueous extract (SLAE) killed 66.3% and 43.3% of the fleas within 24 hours at the same concentration. These findings agree with findings of Adayo et al., [11] and also its use for control of jiggers in Ikolomani Division of Kakamega County, Kenya [12]. However the findings reported were on the Tithonia diversifolia leaves only. The current study found flowers had more antifleas activity.
During the acute oral toxicity testing, Tithonia diversifolia flowers and leaves aqueous extracts were used at 2000 mg per kg. The LD50 of the extracts was above the dose used since there was no significant oral acute toxicity based on its effect on the weight and hematological profile except for Neutrophils, and there was no mortality within 24 hours. These findings are in agreement with reports of Ezeonwumelu et al., [20], and Kamatenesi-Mugisha et al., [21] who found that Tithonia diversifolia leaves aqueous extract had LD 50 of above 10000 mg/kg.
A study by Elufioye et al., [22], reported insignificant acute toxic effects as demonstrated by absence of hematological changes, when 400-1600 mg per kg of ethanoic extract was used. Funmilayo et al., [23], also found that there were no significant toxic effects, on hematological and biochemical parameters when Tithonia diversifolia meal was fed on cockerels for 98 days.
The current study found leaves of Tithonia diversifolia had a value of p = 0.0104 at day 7, and p = 0.044 at day 14, while flowers had a value of p = 0.0222 at day 7 and p = 0.0265 at day 14 and had an effect on weight gain in the Wister rats unlike S. didymobotrya. These findings support the use of T. diversifolia in animal feeds as reported previously by Mauricio et al., [24].
However, Oyewole et al., [25] found that the intra-peritoneal LD50 of Tithonia diversifolia aqueous extract was 120 mg per kg body weight, with the same dose (100 mg/ kg) repeated daily for 14 days. Unlike in this study, he reported severe acute toxic effects as portrayed by the significant weight changes, hematological changes and high mortality. This difference is likely due to multiple dosing and use of intra-peritoneal route of administration. The acute dermal irritation and eye irritation of the crude aqueous extracts of both Tithonia diversifolia leaves and flowers were insignificant.
A total of 2000 mg per kg body weight of Senna didymobotrya leaf aqueous extract was used in the study for acute oral toxicity test, the LD50 was found to be above 2000 mg per kg body weight. However, significant acute toxic effects were observed being indicated by the hematological changes on the Hemoglobin (p = 0.009), Hematocrit (p = 0.026), Mean Cell Volume (p = 0.013), White Blood Cells (p = 0.042), Neutrophils (p = 0.0204) and Lymphocytes (p = 0.016) although there were no mortalities reported or significant weight loss in the animals. Moreover, there was no significant acute dermal toxicity/corrosion, or eye irritation/ corrosion. Korir et al., [26] found that the LD50 of the Senna didymobotrya DCM extract was between 1000 mg and 5000 mg per kg body weight. There were no significant acute toxic effects at low doses, but the toxic effects increased significantly as the dose increased above 3000 mg per kg body weight and with continuous daily dosing as illustrated by the weight loss. Nyamwamu et al., [27], found that the LD50 of the methanoic and Dichloromethane Senna didymobotrya crude root extracts was 1927 mg per kg body weight while that of the hexane and water extracts were more than 5000 mg per kg body weight in mice, and this is in agreement with the findings of the present study. Aqueous extract is thus only slightly toxic if accidentally ingested. Nyamwamu et al., [27] and Njoroge et al., [28] also reported topical use of Senna didymobotrya extracts in the management of both human and livestock dermal conditions and ectoparasites, and this is in agreement with results on the safety of extracts for topical use as in this study.
The study found that Tithonia diversifolia flowers aqueous (TDFA) were safer than the other extracts used in the study, as it did not affect the blood profile significantly. It was also found to be the most effective on fleas, with very close resemblance with the positive control (pyrethrum flowers aqueous extract), in its antiflea activity. Tithonia diversifolia (TDLA) leaves aqueous extract had better activity than Senna didymobotrya (SDLA) leave aqueous extract but had activity level slightly below that which was observed in the flowers. It was also found that it was not very toxic to the blood cells as only neutrophils (p = 0.027) showed significantly decline as compared with the control Wister rats.
5. Conclusion
The following conclusions were made from the studies. The study findings show that there was no significant difference in the In vitro anti-flea activity of C. cinerariifolium (pyrethrum) flowers aqueous extract compared with Tithonia diversifolia aqueous leaves and flowers (p-value of 0.8321) extracts as well as Senna didymobotrya leaves aqueous extract.
Flowers of Tithonia diversifolia needs to be investigated further for pesticide activities.
6. Recommendations
The following recommendations were made from the studies.
Further studies should be conducted on Tithonia diversifolia and Senna didymobotrya extracts in order to improve on purification of usable pesticides, which are cost effective especially in control of the jigger menace in Kenya and other parts of the world. More emphasis should be placed on the flowers of Tithonia diversifolia having demonstrated both effectiveness and safety. However, further studies should be conducted in order to purify the extracts of the flowers in order to provide safe, cost effective and efficient pesticides. Lastly, Kakamega herbalists who use boiled Tithonia diversifolia concoction to clean jigger infested areas of the body, should be encouraged and the people of Murang’a and the rest of the country can consider using the concoction.
Acknowledgements
The authors wish to thank Mrs. Dorcas Nduati for data analysis, Mr. Mathias Mbale for plant identifications, Mr. Joseph Nderitu, Mr. Kenneth Maloba and Lucy Mwangi for assistance in the laboratory, Mr. Jared Onyancha for assistance in the field study, Dr. Ali Koech for assistance in fleas harvesting, and Mr. Richard Otieno for professional handling of specimens in parasitology laboratory.
References
- Beugnet F, Franc M. Results of a Europeanmulticentric Field Efficacy Study of Fipronil-(S) Methoprene Combination on Flea Infestation of Dogs and Cats During 2009 Summer. Parasite 17 (2010): 337-342.
- Awoke A, Kassa L. Vector and Rodents Control. Haramaya University (2006): 165-175.
- Hawlena H, Rynkiewicz E, Toh H, et al., The Arthropod, but not the Vertebrate Host or Its Environment, Dictates Bacterial Community Composition of Fleas and Ticks. ISME J 7 (2013): 221-223.
- Bitam I, Dittmar K, Parola P, et al., Fleas and Flea Borne Diseases. International Journal of Infectious diseases 14 (2010): 667-676.
- Laudisoit A, Leirs H, Makundi RH, et al., Plague and the Human Flea, Tanzania. Emerg Infect Dis 13 (2007): 687-693.
- Prentice MB. Plgue. The Lancet 369 (2007): 1196-1207.
- Gage KL, Burkot TR, Eisen RJ, et al., Climate and Vector borne Diseases. American Journal of Preventive Medicine 35 (2008): 436-450.
- Traub R, Wisseman CL, Farhard-Azad A. The Ecology of Murine Typhus-A Critical Review. Trop Dis Bull 75 (1978): 237-317.
- Zabron W. Tungiasis Risk Factors in Rural Community in Murang’a County, Kenya. Ir-library.ku.ac.ke (2017).
- Mwangi JN, Ozwara HS, Gicheru MM. Epidemiology of Tunga penetrans Infection in Selected Areas in Kiharu Constituency, Murang’a, Kenya. Trop Dis Travel Med vaccines 1 (2015): 13.
- Adayo F, Mukalama JB, Enyola M. Using Tithonia concoctions for Termite Control. ILEIA Newsletter 13 (1997): 24.
- Shisanya CA. Determinants of Sustainable Utilization of Plant Resources in the Former Kakamega District, Kenya. Organization for Social Science Research in Eastern and Southern Addis Ababa (2011): 62-63.
- OECD. Guideline for Testing of Chemicals. Acute Oral Toxicity-Acute Toxic Class Method. 423 adopted 17th December, 2001.
- OECD. Guideline for Testing of Chemicals. Acute Oral Toxicity-Up-and-Down-Procedure (UDP). 425 adopted (2008).
- Bibi Y, Nisa S, Zia M, et al., In Vitro Cytotoxic Activity of Aesculusindica against Breast Adenocarcinoma Cell Line (MCF-7) and Phytochemical Analysis. Pak J Pharm Sci 25 (2012): 183-187.
- Sasidharan S, Chen Y, Saravanan D, et al., Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. African Journal of Traditional, Complementary, and Alternative Medicines 8 (2011): 1-10.
- Stanneck D, Ulrich EK, Schoenhense E, et al., The synergistic action of imidacloprid and flumethrin and their kinetics from collars applied for ectoparasite control in dogs and cats. Parasites and Vectors 5 (2012): 73.
- Dryden MW, Smith V, Bennett T, et al., Efficacy Of Fluralaner Flavored Chews (Bravecto) Administered to Dogs against the Adult Cat Flea, Ctenocephalides Felis and Egg Production. Parasites and Vectors 8 (2015): 364.
- OECD. Guideline for Testing of Chemicals. Acute Dermal Irritation/Corrosion (2015).
- Ezeonwumelu JOC, Omolo RG, Ajayi AM, et al., Studies of Phytochemical Screening, Acute Toxicity and Anti-diarrhoeal Effect of Aqueous Extract of Kenyan Tithonia diversifolia Leaves in Rats. British Journal of Pharmacology and Toxicology 3 (2012): 127-134.
- Kamatenesi-Mugisha M, Buyungo JP, Ogwang PE, et al., Oral Acute Toxicity Study of Selected Botanical Pesticide Plants Used By Subsistence Farmers around the Lake Victoria Basin. African Journal of Environmental Science and Technology 7 (2013): 93-101.
- Elufioye TO, Alatise IO, Fakoya FA, et al., Toxicity Studies of Tithonia diversifolia A. Gray (Asteraceae) in Rats. Journal of Ethnopharmacology 122 (2009): 410-415.
- Funmilayo SM, Ayodele AE. Hematological and Biochemical Changes in Cockerels Fed Rations Containing Graded Levels of Wild Sunflower (Tithonia diversifolia Helms. Gray) Meal. Sky Journal of Agriculture research 5 (2016): 91-96.
- Mauricio RM, Calsavara LHF, Ribeiro RS, et al., Feed Ruminants using Tithonia diversifolia as Forage. Journal of Dairy, Veterinary & Animal Research 5 (2017).
- Oyewole IO, Magaji ZJ, Awoyinka OA. Biochemical and Toxicological Studies of Aqueos Extracts of Tithonia diversifolia (Hemsl.) Leaves in Wister Albino Rats. Journal of Medicinal Plants Research 1 (2007): 30-33.
- Korir RK, Mutai C, Kiiyukia C et al., Antimicrobial Activity and Safety of two Medicinal Plants traditionally used in Bomet District of Kenya. Research Journal of Medicinal Plant 6 (2012): 370-382.
- Nyamwamu LB, Ngeiywa M, Mulaa M, et al., Cytotoxicity And In vitro Antiamoebic Activity Of Senna Didymobotrya Crude Root Extracts in Comparison With Metronidazole Against Entamoeba histolytica. International Journal of Pharmacy, 5 (2015): 1051-1057.
- Njoroge GN, Bussmann RW. Ethnotherapeutic Management of Skin Diseases among the Kikuyus of Central Kenya. J Ethnopharmacol 111 (2007): 303-307.
Citation: Githinji James Maina, Maitho Timothy, Mbaria James Muchunu. Antifleas Activity and Safety of Tithonia diversifolia and Senna didymobotrya Extracts. J Pharm Pharmacol Res 2 (2018): 078-092.
