Noninvasive, Wearable, Pulsed Shortwave (Radiofrequency) Therapy for Analgesia following Knee and Hip Arthroplasty: A Case Series
Article Information
Engy T Said1, John J Finneran IV2, Rodney A Gabriel3, Jacklynn F Sztain4, Scott T Ball5, Krishna R Cidambi6, Baharin Abdullah7, Brian M Ilfeld8*
1-8University of California San Diego, San Diego, California, USA
2, 3,8The Outcomes Research consortium, Cleveland, Ohio, USA
*Corresponding Author: Brian M Ilfeld, University of California San Diego, San Diego, California, USA.
Received: 31 October 2022; Accepted: 18 November 2022; Published: 30 November 2022
Citation: Engy T Said, John J Finneran IV, Rodney A Gabriel, Jacklynn F Sztain, Scott T Ball, Krishna R Cidambi, Baharin Abdullah, Brian M Ilfeld. Noninvasive, Wearable, Pulsed Shortwave (Radiofrequency) Therapy for Analgesia following Knee and Hip Arthroplasty: A Case Series. Archives of Clinical and Medical Case Reports 6 (2022): 758-762.
Share at FacebookAbstract
Background: Minimizing pain and opioid requirements following major orthopedic surgery can be challenging. Nonthermal, pulsed, shortwave (radiofrequency) therapy is a noninvasive treatment described previously as a possible analgesic modality following minor surgical procedures. The devices may be applied in a few minutes or less, are simply taped in place over the area of pain, are less expensive than a large bottle of acetaminophen, function for 30 days without any required intervention, may be applied to any part of the body, and produce no side effects-patients cannot detect any sensations from the devices-or substantial risks. Here we present a case series to explore the use of pulsed shortwave therapy for major joint arthroplasty.
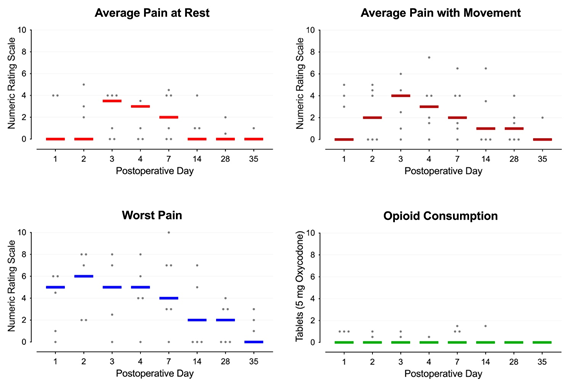
Case Report: Following unilateral total knee (n=2) and hip (n=5) arthroplasty, patients had 1-2 noninvasive, wearable, disposable, pulsed shortwave devices (Model 088, BioElectronics, Frederick, Maryland) affixed over the surgical incision (functioning continuously for 30 days). Average daily pain at rest and with movement was a median of 0-4 as measured using a 0-10 numeric rating scale for the entire follow-up period. Maximum pain each day was a median of 5 and 6 for the first two days, respectively, and over the subsequent weeks fell to 0 by Day 35. Three patients avoided opioid use entirely, while the remaining individuals required 0-7.5 mg of oxycodone daily. No device-related localized irritation, side effects, or complications were identified.
Conclusions: Pulsed shortwave devices may be an effective analgesic, possibly obviating opioid requirements in some cases following knee and hip arthroplasty.
Keywords
Arthroplasty, Replacement, Knee; Arthroplasty, Replacement Hip; Postoperative Analgesia, Acute; Pulsed Radiofrequency Treatment
Article Details
1. Background
Minimizing pain and opioid requirements following major orthopedic surgery can be challenging. While continuous peripheral nerve blocks are an option, they induce proprioception, sensory, and motor deficits which can interfere with physical therapy, and are associated with an increased risk of falling [1]. Considering the millions of knee and hip replacements performed annually worldwide, an analgesic that decreases pain and opioid requirements for multiple weeks without the side effects could have a significant positive impact on health outcomes.
One possible alternative is the use of nonthermal, pulsed, shortwave (radiofrequency) therapy. Originally described in the mid-1950s as pulsed electromagnetic fields, nonthermal shortwave therapy has been described to treat both acute and chronic pain, hasten bone regeneration and wound healing, and decrease edema and inflammation [2,3]. The explanation for treatment effects is only partially understood [4]. As we described in our previous work, “The most generally accepted biochemical-based theory involves the promotion of calcium binding to calmodulin which activates endothelial and neuronal nitric oxide synthase isoforms, producing nitric oxide which has anti-inflammatory and analgesic effects, among other consequences such as decreasing edema while increasing blood and lymph flow” [5]. A biophysical-based theory for the mechanism of action involves stochastic resonance: the radiofrequency stimulation modulation of Aa and Aβ nerve fibers results in a barrage of nonpainful stimuli that essentially raise the perioperative pain threshold [4]. If accurate, this approach to modulate the activity of the sensory nervous system to modulate the effect of the central nervous system by increasing “afferent noise” may mitigate the onset of central sensitization and development of persistent post-surgical pain [6].
Pulsed shortwave fields have been used to treat pain following orthopedic surgery with various degrees of success, primarily for minor procedures involving soft tissue [3]. Three studies with negative findings used 15-45 minute treatments 1-3 times/day for 3 days [3]. In contrast, 3 of the 4 studies with positive results increased the cumulative treatment duration by prolonging sessions to over 1 hour each, providing 7-14 sessions/day, and/or continuing treatment 7-14 days [3]. This suggests the possibility that increased treatment duration may improve outcomes. Short treatments were the norm for decades because the original pulsed electromagnetic field machines available were large, heavy, and required an external power supply [7]. But in the last few decades, small, light, battery-powered, disposable, wearable, nonthermal devices “have been developed and are now cleared by the United States Food and Drug Administration, with indications including the treatment of musculoskeletal and postoperative pain as well as edema” [5]. These wearable devices now allow for continuous treatment for a full month.
It remains unexamined whether pulsed shortwave therapy will provide analgesia following knee and hip arthroplasty both during hospitalization and following discharge. Consequently, we now report seven patients who used pulsed shortwave therapy for 30 days after knee and hip arthroplasty.
2. Case Report
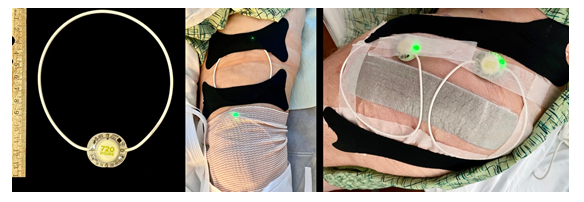
We consented 7 patients undergoing unilateral, primary, total knee (n=2) and hip (n=5) arthroplasty for the use of nonthermal, pulsed shortwave (radiofrequency) therapy after their surgical procedures. All provided written, informed consent for unidentifiable inclusion in this publication. The University of California San Diego Institutional Review Board (San Diego, California, USA) waives review requirements for case reports and short series. The 4 female and 3 male patients had a mean (SD) age of 64 (20) years, height of 171 (7) cm, weight of 79 (16) kg, and body mass index of 26.8 (4.5). Patients having knee arthroplasty received preoperative ipsilateral single-injection adductor canal blocks with ropivacaine 0.5% and epinephrine (20 mL). All patients received a bupivacaine spinal anesthetic intraoperatively and a mixture of bupivacaine 0.25% (50 mL), ketorolac (30 mg), and epinephrine (250 mg) infiltrated into the surgical area prior to closure. Within the recovery room, patients had affixed over their surgical incision 1-2 noninvasive, wearable, pulsed shortwave devices (Model 088, BioElectronics Corporation, Frederick, Maryland) with included kinesiology tape, paper tape, and/or circumferential bandages (Figure 1).
Figure 1: A wearable, pulsed shortwave (radiofrequency) electromagnetic field device with a pulse generator and flexible 12 cm-diameter antenna. The units are secured with included cotton-based kinesiology tape (black bandages in image) and either a circumferential bandage (knee, middle) or paper tape (hip, right). The single control is an on/off button on the back of the pulse generator, and the green light emitting diode indicates the unit is functioning.
Postoperatively, patients received acetaminophen 975 mg qid, celecoxib 200 mg bid, and, if needed, the synthetic oral opioid oxycodone (5 mg tablets). Upon discharge home, patients continued these medications and were provided with the contact phone numbers of the administering physician and acute pain service. Patients were instructed to wear their device(s) continuously through postoperative day 30 when they could discard the disposable, single-use units. In case of dislodgement or if repositioning was desired, the devices could be reaffixed with either additional included kinesiology tape, another type of tape, or any bandage/clothing that would hold the device in place. The electromagnetic pulses pass through bandages and clothing, so adhering the device to the skin was not required. Showering with the device(s) in place was acceptable, but not submerging the rings in water. Patients were asked to check daily that the light emitting diode on the front of the device was green, indicating a functioning unit, but no other device care or adjustment was required. Patients were contacted by telephone at intervals standard for our acute pain service on postoperative days 1, 2, 3, 4, 7, 14, 28 and 35. Average daily pain at rest and with movement was a median of 0-4 as measured using a 0-10 numeric rating scale for the entire follow-up period (Figure 2, below). Maximum pain each day was a median of 5 and 6 for the first two days, respectively, and over the subsequent weeks fell to 0 by Day 35 (Figure 2). Three patients used no opioids at all, and four required 0-7.5 mg of oxycodone daily (Figure 2). The disposable devices were discarded at home 30 after surgery.
No patient contacted a healthcare provider with a question or concern during the follow-up period; and no complications or side effects related to the pulsed shortwave fields devices were identified during the telephone follow-up. Because this was an uncontrolled proof-of-concept series, no primary outcome measure was designated, and no statistics were applied to the data.
3. Discussion
These cases demonstrate that following knee and hip arthroplasty, the use of pulsed shortwave therapy devices is feasible in both the in- and out-patient settings and may improve analgesia while decreasing—perhaps sometimes obviating—opioid consumption. Bearing in mind the danger of opioid misuse, dependence, and diversion, decreasing postoperative opioid requirements is not an insignificant proposition since the greater the number of prescribed opioid pills, the higher the likelihood of continuing their use [8]; and 6% of opioid-naïve patients within the United States continue to use opioids a year after surgery [9]. Additionally, pulsed shortwave therapy induces no muscle weakness or sensory/proprioception deficits and therefore should not increase the risk of falls, unlike continuous peripheral nerve blocks. Finally, nonthermal pulsed shortwave therapy may bring benefits beyond analgesia following major joint arthroplasty by decreasing inflammation and edema, as well as hastening wound healing and bone regeneration [2,3].
To validate and quantify the benefits (and risks) following knee and hip arthroplasty requires a randomized, placebo-controlled study; however, the characteristics of pulsed shortwave therapy suggest significant potential to treat postoperative pain: the devices produce no perceptible sensations, have no side effects, are noninvasive, weigh less than 10 g, function through surgical bandages and clothing, require no intervention by provider or patient once initiated, have a 30-day duration, are relatively inexpensive compared with most medical devices, and have no potential for misuse, dependence, or diversion. The safety of nonthermal, pulsed, electromagnetic fields has been investigated and confirmed by multiple government agencies and independent societies [10-12]. For example, the Institute for Electrical and Electronics Engineers Standards for Radio Frequency Electromagnetic Field Exposure concluded that, “there are no adverse health effects that are not thermally related” [10]. Within the past 25 years, over 3-million treatments with pulsed electromagnetic field devices have been delivered without reports significant side effects or adverse events [13]. Contraindications are few, but important to note, including placement within 6” of an existing implanted pulse generator (e.g., cardiac pacer), pregnancy, use in an area of preexisting malignancy, and use in children less than 17 years of age [12].
A favorable risk-benefit ratio is possible for pulsed radiofrequency therapy with even a small analgesic and/or opioid-sparing effect due to its low cost, ease of application/use, and lack of complications. Acetaminophen may be an appropriate comparator: although its analgesic benefits are modest compared with alternatives, it “is a core component of multimodal analgesia in all exemplar hip and knee ERAS pathways [enhanced recovery after surgery]” due to its relatively benign risk profile, lack of side effects, and ease of administration [14]. Compared with acetaminophen, the device used in the present report requires less cumulative time for administration (one-minute application for 30 days of analgesia vs. taking oral medication every 6 hours); has an equivalent lack of side effects; and possesses a superior safety profile (no known significant complications vs. the most common cause of acute liver failure in the United States) [15]. Nevertheless, clinical adoption is limited by a lack of systematic evidence, historically a limited understanding of the mechanism of action, and “a wide variety of unsubstantiated claims that are used for marketing purposes” [13].
These cases demonstrate that following knee and hip arthroplasty, the use of pulsed shortwave therapy devices is feasible in both the in- and out-patient settings and may improve analgesia while decreasing—perhaps sometimes obviating—opioid consumption. Considering their ease of placement, few contraindications, low provider/patient burden, lack of systemic side effects and serious adverse events as well as any misuse/dependence/diversion potential, further study with a randomized, controlled trial appears warranted to document and quantify potential analgesic and opioid-sparing benefits of these wearable, noninvasive devices and use in children less than 17 years of age [9].
In conclusion, this case series suggests that pulsed shortwave therapy may decrease opioid requirements and effectively treat postoperative pain after total hip and knee arthroplasty. Further investigation with a randomized, controlled trial appears justified due to the relatively few contraindications, lack of side effects, low risk profile, and low patient burden of these noninvasive devices.
Acknowledgements
The authors would like to thank Sree Koneru, PhD (BioElectronics Corporation, Frederick, MD; and Meraqui Medical, Fremont, CA) and Kenneth J. McLeod, PhD (Department of Systems Science and Industrial Engineering and the Clinical Science and Engineering Research Laboratory, Binghamton University, Binghamton, NY), for reviewing much of the information in this manuscript for technical accuracy.
Financial Support
Funding for this project provided by the University California San Diego Department of Anesthesiology (San Diego, California). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding entity.
Conflict of Interest
The University of California San Diego has received funding and/or product from the following companies for other research studies of Drs. Finneran, Said, Gabriel, Sztain, Abdullah and Ilfeld: Epimed International (Farmers Branch, Texas, USA), SPR Therapeutics (Cleveland, Ohio, USA), Infutronix (Natick, Massachusetts, USA), and Avanos Medical (Irvine, California, USA). None of the authors has a personal financial interest in this research.
References
- Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg 111 (2010): 1552-1554.
- Guo L, Kubat NJ, Nelson TR, et al. Meta-analysis of clinical efficacy of pulsed radio frequency energy treatment. Ann Surg 255 (2012): 457-467.
- Kumaran B, Watson T. Radiofrequency-based treatment in therapy-related clinical practice—a narrative review. Part I: acute conditions. Physical Therapy Reviews 20 (2015): 241-254.
- Sam J, Catapano M, Sahni S, et al. Pulsed Radiofrequency in Interventional Pain Management: Cellular and Molecular Mechanisms of Action - An Update and Review. Pain Physician 24 (2021): 525-532.
- Ilfeld BM, Said ET, Abdullah B, et al. Treating intractable postamputation pain with noninvasive, wearable, nonthermal, pulsed shortwave (radiofrequency) therapy: A 12-patient case series. Pain Ther (2022).
- Lin FY, Huang KF, Chen JC, et al. The Clinical Application of Pulsed Radiofrequency Induces Inflammatory Pain via MAPKs Activation: A Novel Hint for Pulsed Radiofrequency Treatment. Int J Mol Sci 22 (2021).
- Fenn JE. Effect of pulsed electromagnetic energy (Diapulse) on experimental hematomas. Can Med Assoc J 100 (1969): 251-254.
- Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 66 (2017): 265-269.
- Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152 (2017): e170504.
- Committee on M, Radiation. COMAR technical information statement: expert reviews on potential health effects of radiofrequency electromagnetic fields and comments on the bioinitiative report. Health Phys 97 (2009): 348-356.
- Ahlbom A, Green A, Kheifets L, et al. Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect 112 (2004): 1741-1754.
- Guo L, Kubat NJ, Isenberg RA. Pulsed radio frequency energy (PRFE) use in human medical applications. Electromagn Biol Med 30 (2011): 21-45.
- Gaynor JS, Hagberg S, Gurfein BT. Veterinary applications of pulsed electromagnetic field therapy. Res Vet Sci 119 (2018): 1-8.
- Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. Acta Orthop 91 (2020): 3-19.
- Lee WM. Acute liver failure. Semin Respir Crit Care Med 33 (2012): 36-45.