Neonatal Ultrasound for Anorectal Malformations in Male Newborns Without Perineal Fistula: A Good Diagnostic Tool for A Safe One-Staged Surgery
Article Information
Carlos Giné 1, 2, Ana Coma 3, Laura García 1, Ana Laín 1, 2, Ignasi Barber 4, Rodrigo Maluje 1, Manuel López 1, 2
1Paediatric Surgery Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Barcelona Hospital Campus, Passeig de la Vall d’Hebron 119-129, 08035 Barcelona, Spain
2Universitat Autònoma de Barcelona, Departament de Cirurgia I Ciències Morfològiques, 08193 Bellaterra, Spain
3Paediatric Radiology Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Barcelona Hospital Campus, Passeig de la Vall d’Hebron 119-129, 08035 Barcelona, Spain
4Paediatric Radiology Department, Hospital Universitari Sant Joan de Deu, Passeig de Sant Joan de Déu 2, 08950 Barcelona, Spain
*Corresponding Author: Carlos Giné, MD PhD, Pediatric Surgery Department. Hospital Universitari Vall d’Hebron, Vall d’Hebron Barcelona Hospital Campus. Passeig de la Vall d´Hebron, 119-129, 08035 Barcelona, Spain
Received: 18 October 2021; Accepted: 02 November 2021; Published: 15 November 2021
Citation: Carlos Giné, Ana Coma, Laura García, Ana Laín, Ignasi Barber, Rodrigo Maluje, Manuel López. Neonatal Ultrasound for Anorectal Malformations in Male Newborns Without Perineal Fistula: A Good Diagnostic Tool for A Safe One-Staged Surgery. Archives of Clinical and Medical Case Reports 5 (2021): 811-820.
Share at FacebookAbstract
Background/Purpose: The standard neonatal approach for a male newborn with an anorectal malformation (ARM) and no perineal fistula remains the opening of a diverting colostomy. Here we describe radiological improvements in neonatal ultrasound (US) that characterize the recto-urinary fistula and allow a direct anorectal reconstruction.
Material and Methods: This is a descriptive report of radiological findings in neonatal ultrasound in all newborn male patients with an ARM, no perineal fistula and no colostomy, recruited over a 6-year period. A paediatric radiologist actively looked for recto-urinary fistulas and their relation to anatomical landmarks. These findings, along with the clinical status and associated anomalies, were considered to indicate a single-staged procedure.
Results: From 28 neonates with ARM only three met the inclusion criteria. The first 2 cases showed a bulbar and prostatic fistula in US but with associated malformations and benefited from a colostomy. The third baby presented a bulbar fistula in the US, normal sacrum and no lifethreatening malformations and benefited from a singlestaged procedure.
Conclusions: Neonatal perineal US can give the same anatomical information than the distal colostogram and leads to a safe single-staged procedure in expert teams, if there is a lack of associated life-threatening malformations.
Keywords
Anorectal malformations; Ultrasound; Newborn; Children; Diagnosis
Anorectal malformations articles; Ultrasound articles; Newborn articles; Children articles; Diagnosis articles
Article Details
1. Introduction
The current standard approach for male newborns with an anorectal malformation (ARM) and no evidence of perineal fistula is the so-called three-staged procedure, and the first step is the opening of a descending colostomy after the 20-24h period dedicated to discard other associated anomalies [1]. In the absence of a perineal fistula, these babies will most likely have a recto-urinary fistula, except for cases of Down Syndrome. This fistula is very difficult to characterize with standard neonatal radiologic tests and, therefore, an eventual single-staged procedure may become a blind exploratory act that risks harming important structures. Even with an invertogram where the rectum is seen below the coccyx, a direct pull-through may risk leaving the fistula untouched.
A distal colostogram through the mucous fistula shortly after the colostomy opening allows for an anatomical characterization of the recto-urinary fistula and the relation of the rectum to the sacrum and coccyx. Therefore, an accurate surgical repair can be planned [2]. Definitive treatment may depend on the type of malformation, allowing the surgeon to decide whether to employ the laparoscopic or open technique accordingly.
Among all the image studies used to rule out associated anomalies after birth, we include perineal ultrasound (US). This has normally been focused on determining the pouch-perineal distance [3, 4], which has little relevance for surgical decision-making, the search of the fistula to the urinary tract being seldom reported or achieved [5]. The aim of this study is to report three consecutive cases where a radiologist expert in ARM actively searched for the fistula, described the exact connection to the urinary tract and reported the relation of the rectum to the coccyx and skin. This information allowed the single-staged pull-through of the rectum in one of these newborns, avoiding colostomy.
2. Methods
This is a description of the perineal sonographic findings and clinical and surgical outcome of male patients, born in our institution, with an ARM and no evidence of a perineal fistula. Newborns referred from outside the centre with prior colostomy were excluded from the analysis. The period of recruitment was from May 2014 to May 2020.
Our standard protocol for ruling out associated anomalies was applied with a naso-gastric tube insertion, X-ray film of the entire spine and sacrum, cross-lateral X-ray film in prone position and cardiac, urinary tract, spine and perineal US. In addition to the standard search for well-known, potential associated anomalies, a radiologist expert in ARM actively searched for the eventual presence of a recto-urinary fistula trying to identify the anatomical level of the communication. They also described the anatomical relations of the rectum to the bladder neck, the coccyx and skin in order to consider an eventual single-staged procedure.
We name the “single-staged procedure” as the definitive rectal pull-through procedure in the neonatal period without colostomy in these patients, regardless of the technique that may be applied, such as posterior sagital anorectoplasty (PSARP) or laparoscopic assisted anorectoplasty (LAARP).
2.1 Perineal ultrasound
A high-frequency linear array transducer (12 MHz) is placed on the perineum at its midline between the baby’s anus and the scrotum or the labia, via longitudinal scanning. Supplemental transvers perineal views or complementary images through suprapubic access may be obtained in certain cases. The patient is positioned in supine, in modified lithotomy position. The linear transducer is covered with gel and sterile plastic wrap. Newborns are examined without specific preparations. Perineal ultrasound is a non-invasive method and does not require ionizing radiation or sedation, neither does the image require any specific software or reconstruction. A mid sagittal plane, by aligning the transducer with the pubic synchondrosis and coccyx, allows the depiction of the length of the urethra, the anterior wall of the rectum and the bladder neck. Sonography permits us to depict fistulas in anorectal malformations as a hypoechoic linear tract from the proximal side (rectal pouch) to distal side: involving the bladder in rectovesical fistulas, the prostatic urethra in prostatic-urethral fistula, the bulbar urethra in recto-bulbar urethral fistula and the perineum. The real-time scanning of sonography allows the detection of gas bowel bubbles moving within the urethra or the bladder neck, which confirms the presence of the fistula. In addition, it allows us to measure the length of the urethra, the distance between the bladder neck and the fistula and the distance between the fistula and the perineum. Measurements should be performed when the baby rests and not when the child is crying and care should be taken not to press the skin as the anatomy can be distorted by excessive pressure.
3. Results
During this 6-year period, we treated 28 neonates with ARM (13F/15M). Of the 15 male newborns, nine had no perineal fistula. Of these, five were referred from other institutions and already had a colostomy, one was affected by Down Syndrome and benefited from a colostomy, and only three male neonates met the study’s inclusion criteria.
3.1 Case 1
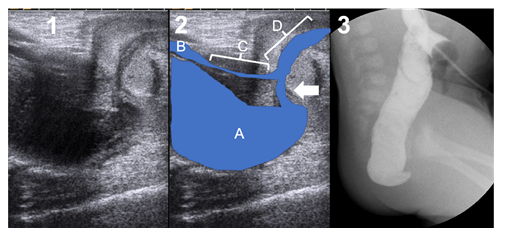
Neonate born at 38+1 weeks of gestation and 3940 g. Poly-malformative studies revealed a tetralogy of Fallot and a hemisacrum without presacral mass. The sonographic study of the perineum revealed a communication between the distal rectum and the bulbar urethra (Figure 1). However, due to cardiac and sacral malformations, single-stage surgery was not considered. The patient benefited from a two port laparoscopic descending colostomy as previously reported [6] on the second day of life. Two distal colostograms were required to weakly identify the fistula (Figure 1). He benefited from a PSARP at 4 months of age, confirming the bulbar fistula, and a colostomy closure at 7 months of age. The cardiac malformation required two surgical corrections.
3.2 Case 2
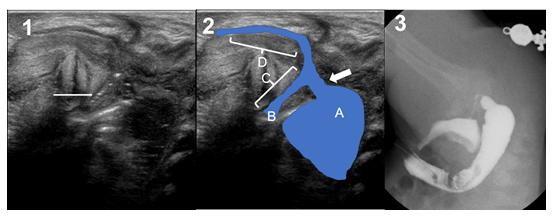
Neonate born at 38+5 weeks of gestation and 3120g with. Image exams showed a persistence of superior vena cava draining to a coronary sinus, tethered cord to the 3rd lumbar vertebra with thickness of the filum terminale and hypoplasia of the last sacral vertebrae and coccyx with a sacrum index of 0.5. Perineal US showed a recto-urethral fistula with gas passage at the level of the prostate (Figure 2). The sacral anomaly moved us to standard care for safety reasons, and the patient benefited from a two-port laparoscopic descending colostomy on day 2 of life. Distal colostogram confirmed the recto-prostatic fistula and LAARP was performed at 4 months of age (Figure 2). The colostomy was finally closed at 8 months of age.
3.3 Case 3
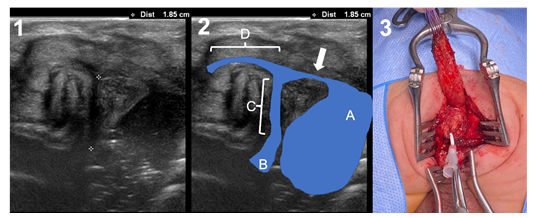
Neonate born at 37+6 weeks of gestation and 2720g. The patient was prenatally diagnosed with a left multicystic dysplasia of the kidney that was confirmed after birth, along with a cyst of the left seminal vesicle, probably expressing an ectopic ureter draining to it. No other associated malformations were found. Perineal US described a clear recto-bulbar urethral fistula, just in the “elbow” of the urethra (Figure 3). Distance of the rectum to the perineum was 13 mm and distance from the fistula to the bladder neck was 18 mm. The rectum was identified just below the coccyx. The absence of other major malformations and the normal sacrum, along with the good clinical status moved us to perform a single-staged procedure. A PSARP approach was performed 26 hours after birth. Surgery was uneventful, identifying the rectum just below the coccyx and the fistula in the lower part of the prostate (Figure 3). Reconstruction of the pelvic floor was in no way different from the standard procedure that we perform at 2-4 months of age in other patients with colostomy, and the quality of the tissues and contractility to electro-stimulation appeared normal. The patient was under total parenteral nutrition for 5 days and with antibiotics during 8 days, moment in which the urinary catheter was removed. No complications occurred. The dilatation program started normally two weeks after surgery. After 9 months of follow-up the patient had finished his dilatation program and had 2 to 3 bowel movements per day, with stool-clean diaper between them.
Figure 3: Case 3. 1) Transperineal Ultrasound. Crosses show the distance between the fistula and the bladder neck (1.85 cm). 2) Ultrasound landmarks: a = rectal pouch; B = bladder neck; C = prostatic urethra; D = bulbar urethra; arrow = fistula. 3) Surgical picture of the single-staged procedure, showing the level of the fistula.
4. Discussion
The most widely accepted strategy to treat ARM male newborns without perineal fistula is still the 3-staged procedure. This means a diverted colostomy in the neonatal period, anorectal reconstruction 2-4 months later and colostomy closure after a dilatation program of the anoplasty [1]. A single-staged procedure or a direct anorectal reconstruction in neonatal period is considered as a blind exploratory surgery and is not recommended for several reasons:
- Injuries to important anatomical structures, as the presence and characteristics of an eventual recto-urinary fistula are unknown.
- Higher risk of infection if not protected by a colostomy, as the extent of the surgical approach is large. That would jeopardize the long-term functional outcome.
- Less tolerance to stretching of the neonatal tissues that may suffer from neurological damage during surgery.
Ideally, a distal colostogram several weeks after the colostomy opening may provide sufficient information about the anatomy of the malformation in order to plan a surgical strategy [2, 7]. However, a recent paper reports poor agreement among experienced colorectal surgeons on preoperative colostograms [8]. The colostomy would play a role in preventing infection [9] and the surgeon would find better-quality tissue to work with in a procedure performed between 2 to 4 months of age. Nevertheless, the same authors recommend, in experienced hands, the neonatal single-staged procedure when the anatomy is well known, such as for recto-vestibular fistula in girls [1]. In this particular case, the extent of the dissection, that may reach beyond the levator muscle layer, and the neonatal soft tissue do not seem to be a major problem if the surgery is exquisite.
On the other hand, some other authors, mainly in Asia, advocate the single-staged procedure. The reasons for considering it advantageous are:
- The belief that early reconstruction benefits the development of neuronal networks and synapses responsible for sensation and normal function [10].
- It avoids colostomy and related complications that may reach 30-50% [11, 12], the need for resources related to colostomy management and the medical costs of a three-staged strategy, factors that increase in relevance in developing countries.
- Some consider that rectal identification and dissection is easier as it is not decompressed, tissues are not inflammatory and the dilatation protocol remains easier to perform afterwards in small babies [13].
Although some surgeons report no differences in functional outcome between these two strategies, we found a lack of studies providing high evidence in the literature favouring the single-staged repair.
Having stated the pros and cons, the authors believe that several criteria should be considered before indicating a single-staged repair for a male patient with ARM without perineal fistula:
- Good clinical condition of the patient.
- Lack of life-threatening associated anomalies or a hypoplastic sacrum.
- Experienced surgical team.
- Detailed radiological information about the anatomy of the ARM.
Regarding this final important point, a perineal US approach should always be considered in cases of anomalies of the lower pelvis, as it provides excellent documentation of the urethra, the periurethral soft tissue, the rectum and the distal genital tract. Perineal US has been successfully applied to the diagnosis of ARM and their associated fistulas in children [5]. It provides a precise anatomic view before surgery and can clearly show the presence, and the level, of the recto-urinary fistula in some newborns, as shown in the cases reported, preventing surgical complications. This fact represents a step forward in the surgical planning of these patients. It is true that this exam may be not reproducible in all centres as it requires an expert radiologist with in depth knowledge of ARM, but it can provide all the information required to safely perform the definitive pull-through, just as a good distal colostogram would do [2]. Perineal US requires patience, experience and a meticulous technique. There are special considerations and pitfalls when performing a perineal US [14]. The authors consider the following radiological criteria as the minimum information required to plan a single-staged surgery:
- The exact level of the fistula.
- The skin-to-rectum distance.
- The fistula-to-bladder neck distance.
- The situation of the rectum in relation to the sacrum and coccyx.
This new data from US is particularly interesting in male patients, as in females the exact position of the fistula can be assessed by the perineal exam, except in cloacal malformations, where a diverting colostomy is not usually avoidable for many reasons [15]. Furthermore, as shown in our first case, ultrasound could be more precise in diagnosing the fistula than the distal colostogram performed two weeks after the colostomy.
Other suggested neonatal radiological tests to clearly identify the anatomy and, therefore, justify a single-staged pull-through have been the voiding cystourethrogram with or without ultrasound, and neonatal magnetic resonance (MRI) [16, 17]. These strategies require invasive procedures for the neonate, sometimes with a tiny and fragile urethra, or, in the case of MRI, the eventual need for anaesthesia or sedation as natural sleep after breast-feeding may not be possible. MRI may not always be available within the period of time we have to perform this radiological exam before the need for surgery. Waiting too long may increase the bowel distension and, therefore, jeopardize the safety of the intervention, especially when performed in the prone position. Pre or intra-operative cystoscopy to identify the fistula in a newborn patient may also be an invasive procedure that could harm the urethra and requires sedation or intubation.
The role of minimally invasive repair can have a beneficial impact in the single-staged procedure because the extent of the perineal incision is minimal and, subsequently, the risk of infection less [18, 19]. Whether a bulbar fistula should be treated by laparoscopy remains a matter of debate for several reasons and is not the subject of the present report. The main limitations of this study are the small number of patients and the need for a radiologist expert in ARM that may make this exam non-reproducible everywhere.
Finally, we cannot recommend the single-stage repair in all male patients with ARM and no perineal fistula unless the perineal ultrasound is unequivocally clear and meets the radiological criteria. In some candidates for single-staged surgery, and in case of doubt, a pre-operative cystography or cystoscopy could be considered to confirm the US findings [16, 20] although in our cases we decided against this as they are invasive procedures and because the US findings were reliable enough. The low weight of the baby or prematurity, along with associated life threatening malformations or poor sacrum, should raise concerns in terms of safety from the surgical, anaesthesiologist and neonatologist point of view, and the authors do not recommend the single-staged pull-through procedure in these cases. Individual case analysis should be performed in order to offer the best option to each patient and their families.
In conclusion, neonatal perineal US remains a non-invasive technique that can provide sufficient information to undergo a safe single-staged repair of male patients with ARM without perineal fistula if the aforementioned clinical and radiological criteria are adhered to.
Declaration of Interest
The authors report no conflicts of interest.
References
- Levitt MA, Peña A. Anorectal malformations. Orphanet J Rare Dis. 2 (2007): 33.
- Kraus SJ, Levitt MA, Peña A. Augmented-pressure distal colostogram: the most important diagnostic tool for planning definitive surgical repair of anorectal malformations in boys. Pediatr Radiol. 48 (2018): 258-269.
- Riccabona M, Lobo M-L, Ording-Muller L-S, et al. European Society of Paediatric Radiology abdominal imaging task force recommendations in paediatric uroradiology, part IX: Imaging in anorectal and cloacal malformation, imaging in childhood ovarian torsion, and efforts in standardising paediatric uroradiology terminology. Pediatr Radiol. 47 (2017): 1369-1380.
- Hosokawa T, Takahashi H, Tanami Y, et al. Comparison Between the Pouch-Perineum Distance in Neonates With a Low-Type Anorectal Malformation With and Without an Opened Fistula: Pitfall of Measuring the Pouch-Perineum Distance on Sonography. J Ultrasound Med. 37 (2018): 2797-2802.
- Hosokawa T, Yamada Y, Tanami Y, et al. Diagnostic Accuracy of Sonography for Detection of a Fistula on the Birth Day in Neonates With an Imperforate Anus: Comparison of Diagnostic Performance Between Suprapubic and Perineal Approaches. J Ultrasound Med. 36 (2017): 1989-1995.
- Gine C, Santiago S, Lara A, et al. Two-Port Laparoscopic Descending Colostomy with Separated Stomas for Anorectal Malformations in Newborns. Eur J Pediatr Surg. 26 (2016): 462-464.
- Halleran DR, Ahmad H, Bates DG, et al. A call to ARMs: Accurate identification of the anatomy of the rectourethral fistula in anorectal malformations. J Pediatr Surg. 54 (2019): 1708-1710.
- Midrio P, van Rooij IALM, Brisighelli G, et al. Inter- and Intraobserver Variation in the Assessment of Preoperative Colostograms in Male Anorectal Malformations: An ARM-Net Consortium Survey. Front Pediatr. 8 (2020): 571.
- Tofft L, Salö M, Arnbjörnsson E, et al. Wound Dehiscence after Posterior Sagittal Anorectoplasty in Children with Anorectal Malformations. BioMed Res Int. (2018): 2930783.
- Gangopadhyay AN, Pandey V. Controversy of Single versus Staged Management of Anorectal Malformations. Indian J Pediatr. 84 (2017): 636-642.
- Patwardhan N, Kiely EM, Drake DP, et al. Colostomy for anorectal anomalies: high incidence of complications. J Pediatr Surg. 36 (2001): 795-798.
- Pena A, Migotto-Krieger M, Levitt MA. Colostomy in anorectal malformations: a procedure with serious but preventable complications. J Pediatr Surg. 41 (2006): 748-756.
- Gangopadhyay AN, Gopal SC, Sharma S, , et al. Management of anorectal malformations in Varanasi, India: a long-term review of single and three stage procedures. Pediatr Surg Int. 22 (2006): 169-172.
- Ekwunife OH, Umeh EO, Ugwu JO, et al. Comparison of trans-perineal ultrasound-guided pressure augmented saline colostomy distension study and conventional contrast radiographic colostography in children with anorectal malformation. Afr J Paediatr Surg AJPS. 13 (2016): 26-31.
- Levitt MA, Peña A. Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg. 19 (2010): 128-138.
- Hosokawa T, Yamada Y, Tanami Y, et al. Comparison of diagnostic accuracy for fistulae at ultrasound and voiding cystourethrogram in neonates with anorectal malformation. Pediatr Radiol. 49 (2019): 609-616.
- Diao M, Li L, Ye M, et al. Single-incision laparoscopic-assisted anorectoplasty using conventional instruments for children with anorectal malformations and rectourethral or rectovesical fistula. J Pediatr Surg. 49 (2014): 1689-1694.
- Diao M, Li L, Ye M, et al. Congenital anomaly rectified at birth: one-stage single-incision laparoscopic-assisted anorectoplasty for newborns with anorectal malformations and recto-urethral fistula. Surg Endosc. 30 (2016): 5156-5164.
- Diao M, Li L, Guan K-P, , et al. A novel laparoscopic technique for anorectal malformation with low recto-bulbar fistulae. Surg Endosc. 31 (2017): 4326-4330.
- Karsten K, Rothe K, Märzheuser S. Voiding Cystourethrography in the Diagnosis of Anorectal Malformations. Eur J Pediatr Surg. 26 (2016): 494-499.