Multidisciplinary Approach In Advanced Perivascular Epithelioid Cell Tumour (PEComa): A Case Report
Article Information
Martina Panebianco1*, Debora Ierinò1, Annalisa Milano2, Chiara D’Antonio2, Anna Maria Aschelter2, Paolo Marchetti1
1Department of Clinical and Molecular Medicine, Sapienza University, Sant’Andrea Hospital, Italy
2Oncological Sciences Department, Sant’Andrea Hospital, Italy
*Corresponding Author: Martina Panebianco, Department of Clinical and Molecular Medicine, Sapienza University, Sant’Andrea Hospital, Italy
Received: 27 April 2021; Accepted: 19 May 2021; Published: 09 June 2021
Citation: Martina Panebianco, Debora Ierinò, Annalisa Milano, Chiara D’Antonio, Anna Maria Aschelter, Paolo Marchetti. Multidisciplinary Approach In Advanced Perivascular Epithelioid Cell Tumour (PEComa): A Case Report. Archives of Clinical and Medical Case Reports 5 (2021): 495-502.
Share at FacebookAbstract
Background: Perivascular epithelioid cell tumours (PEComas) are mesenchymal neoplasms with variable biological behaviour, ranging from benign to extremely aggressive diseases able to metastasize. No prospective studies are available to define a validate approach to this type of tumours.
Case presentation: We present a case report of a 53-year-old woman affected by advanced retroperitoneal PEComa who developed an abdominal disease progression after 40 months of therapy with mTOR inhibitor. The patient underwent cyto-reductive surgery followed by radiotherapy on the residual disease and started chemotherapy with olaratumab plus doxorubicin for seven cycles achieving a partial response so she continued monotherapy with olaratumab for one year with stable disease to quarterly CT evaluation. On September 2019 treatment was definitely interrupted because of marketing authorisation of olaratumab was revoked due to insufficient proof of its medical advantage in phase III trial ANNOUNCE. Currently the patient is still in follow up after 18 months from the interruption of therapy, without a disease progression.
Discussion and Conclusion: In our PEComa clinical case, we highlight how a multidisciplinary approach can lead to disease control and a long clinical benefit.
Keywords
Retroperitoneal PEComa; mTOR inhibitor; Olaratumab
Article Details
1. Introduction
Perivascular epithelioid cell tumours (PEComas) are mesenchymal neoplasms [1] with variable biological behaviour, ranging from benign to extremely aggressive diseases able to metastasize; a classification based on pathological and histological data has been proposed to predict the biological behaviour of the PEComas, but not yet validated [2]. In the literature we have no prospective data about the treatment of this type of cancer [3-6]; in certain patients with PEComas was identified the genetic alterations of the tuberous sclerosis complex which lead to the hyperactivity of the mTOR complex, so the use of mTor inhibitors has been endorsed [4-12].
Response to mTOR inhibitors in PEComas was first published by Wagner et al. in 2010 reporting a series of 3 patients with advanced disease [10]. Two other small PEComa case series have since been published confirming the activity of sirolimus in this disease [9, 11]. These results were confirmed by Benson et al., that recommended mTOR inhibitors as first-line treatment in this rare disease [6]. Another recent retrospective study, that evaluated different treatment approaches to metastatic PEComas, confirmed the limited role of chemotherapy in first line setting and defined mTOR inhibitors as the most active agents in this histologic subtype, with an ORR of 41% and a PFS of 9 months [12]. The only prospective study published is the phase 2 AMPECT trial, that evaluated the efficacy and safety of nab-sirolimus in 34 advanced malignant PEComas. The study demonstrated substantial and durable responses (ORR was 42% PR, 35% SD) with manageable toxicities [13]. In the second line setting, after a mTOR inhibitor, the response to standard chemotherapeutic regimens [14] and anti VEGFR [15-16] was evaluated in single case reports.
2. Case Presentation
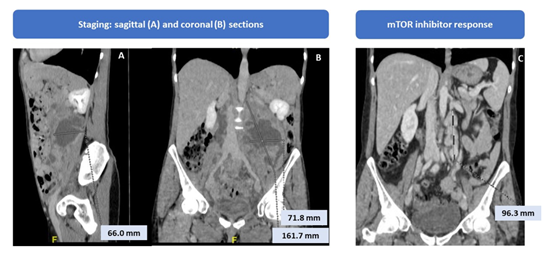
We report the case of a 53-years-old woman who performed a total body computerized tomography (CT) for the appearance of a sore abdominal swelling, that showed the presence of multiple peritoneal and retroperitoneal tumour masses with necrotic hypodense component and hyperdense peripheral solid portion. The largest mass at the left of the retroperitoneum was 13 cm x 9 cm x 7 cm extended upwards to left kidney, renal artery and vein, abdominal aorta and psoas muscle and downwards to descending colon and reached pelvis where it appeared in contiguity with the homolateral external iliac artery. In pelvis were observed several expansive lesions, the greater ones of about 4 cm, that was extended to the aortic carrefour, the right common and external iliac regions, and to presacral adipose tissue, close to the periosteum. Discrete retroperitoneal and pelvic effusion, and multiple abdominal lymphadenopathies were observed. Moreover, she presented increased uterus in size with irregular endometrium (Figure 1).
On August 2013, the patient was subjected to retroperitoneal eco-guided biopsy with a histological diagnosis of perivascular retroperitoneal epithelioid cell tumor (PEComa): the neoplastic cells were positive for smooth muscle actin and CD56, have focal positivity for HMB-45 and negativity for CKAE1/CKAE3, desmin, MyoD-1, CD34, S100, DOG1, synaptophysin, CD31, chromogranin A. The proliferative index ki67 was 1%. An additional histological review in Italian Rare Cancer Network confirmed the diagnosis. Considering the few experiences reported in the literature on the treatment of this rare neoplasm and the moderate activity demonstrated by mTOR inhibitors, on May 2014 she started treatment with temsirolimus according to a planned schedule of 25 mg i.v. given on a weekly basis [11].
The first CT re-evaluation showed a partial response based on RECIST criteria because of 30% decrease in the sum of the longest diameter of target lesions represented by mass with necrotic component extended to the left lumboaortic area and the pelvic lesions. The CT on December 2015 showed the complete resolution of the known retroperitoneal expansive formation and numerous partially necrosed lymphadenopathic formations in the same site (the largest in the lumboaortic area of about 1.6 x 1.3 centimeters) (Figure 1).
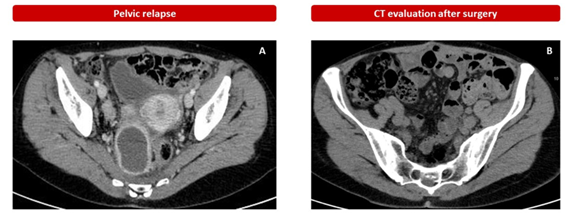
Given the good treatment response, the patient continued therapy until July 2017 when the re-evaluation CT showed disease progression for bilateral pleural and pelvic effusion and increase in the retroperitoneal mass (7.5 x 5.5 cm) with dislocation of the rectum (Figure 2).
In view of the small sample size of the studies in the literature, the limited availability of further systemic treatments as effective as that used on the front line [16], as well as the diagnostic doubt on endometrium, the patient’s young age and good clinical condition, after multidisciplinary discussion, we decided to proposed patient a surgical approach.
On August 2017 she undergone surgery on hysteroannexectomy, excision of pathological retroperitoneal tissue, sigma-rectum, para-aortocaval lymph nodes resections and omentectomy. The histological examination confirmed the diagnosis of PEComa, with a low mitotic index of 0 mitosis/50 HPF and a Ki67 proliferative index of less than 1%. Uterine leiomyoma with right ovarian haemorrhagic luteum were histological diagnosed.
The CT of December 2017 (Figure 2), after surgery, evidenced a residual left paraaortic and multiple bilateral lumboaortic lymphadenopathies so she started II-line therapy with olaratumab (15 mg/kg) intravenously on day 1 and day 8 plus doxorubicin (75 mg/m2 on day 1 of each 21-day cycle for seven cycles, based on phase II clinical trial that evaluated efficacy of olaratumab plus doxorubicin in patients with advanced or metastatic soft-tissue sarcoma [17]. The CT re-evaluation after six cycles showed stable disease so she continued a maintenance therapy with olaratumab for one year with a partial response evidence by centimeter-size lymph nodes in the abdominal area. In view of the response obtained and the withdrawal from the market of olaratumab for insufficient proof of its medical advantage in phase III trial [18, 19], treatment was discontinued in August 2019.
Moreover, the patient received radiotherapy with on the retroperitoneal lymph nodes for a total dose of 50 Gy in 25 fractions from December 2019 to Jenuary 2020. Then, she began clinical and instrumental follow-up (FU). She is continuing the FU and the last CT of February 2021 showed no recovery of the disease with an overall survival (OS) of 7 years and 6 months.
3. Discussion
The family of PEComas is characterized by the presence of mesenchymal neoplastic cells in the context of the vasal walls which are immunoreactive for both smooth muscle and melanocytic markers [1, 20]. This group of neoplasms includes clear cell tumors "sugar like" of the lung, angiomyolipomas (AMLs), lymphangioleiomyomatosis (LAM), clear cell myomelanocytic tumour of the falciform ligament / ligamentum teres (CCMMT) and a group of rare, morphologically and immunophenotypically similar tumors arising at a variety of visceral and soft tissue sites [4, 21-23].
Four main hypotheses have correlated the origin of PEComas from: a) undifferentiated cells of the neural crest, since they express several melanocytic markers [21]; b) smooth muscle cells which have developed alterations leading to the expression of melanocytic markers [25]; c) telocytes, from which gastrointestinal stromal tumours (GISTs) would also originate, considering the expression of markers typical of these cells (e.g., S-100, SMA, VEGF) that are also expressed in PEComas [26]; d) or melanocytic markers could be acquired during tumour development and related to chromosomal translocations or mutations affecting the pathway of melanosomal proteins [20]. Their biological behavior is extremely heterogeneous, ranging from indolent and benign forms to aggressive tumors with malignant transformation and metastatic potential. With the aim to define the biological behavior of PEComas, a stratification system, based on the following clinical and histopathological features, was proposed: 1) Tumour size > 5 cm; (2) Mitotic rate > 1/50 HPF; (3) High nuclear grade and cellularity; (4) Presence of necrosis; (5) Vascular invasion; and (6) An infiltrative growth pattern. These characteristics define three groups: (1) benign tumors ( < 5 cm with one risk feature; (2) having uncertain malignant potential (tumours > 5 cm with no other risk features), and (3) malignant tumors (with two or more risk features) [2].
According to European guidelines ESMO [30], surgical treatment may be sufficient for the treatment of localized tumors [3, 5, 31-33], but for metastatic disease or non-surgically treatable lesions an effective treatment may be mammalian target of the rapamycin (mTOR) inhibitors [4, 6, 8, 9, 11, 12, 21]. In our case the patient presented at the diagnosis a widespread pathology of the retroperitoneum and the pelvis, involving multiple abdominal lymph node stations, in the absence of a primitive organ involved, and therefore not susceptible to surgery. For this reason, she performed therapy with temsirolimus, an mTOR inhibitor, reaching a PFS of 40 months. At disease progression a second line treatment with oralatumab, a human IgG1 monoclonal antibody selective for human platelet-derived growth factor (PDGF) receptor “a” (PDGFRa), was administrated [19, 34-36]. In fact, overexpression or aberrant activation of PDGFRa has been observed in several types of cancer, including sarcomas [37, 38]. In a phase II trial (JGDG trial; NCT01185964), olaratumab in combination with doxorubicin improve progression free survival (PFS) and OS (26.5 months, compared with 14.7 months) compared with doxorubicin alone in patients with advanced soft tissue sarcoma, reducing the risk of death by 53.7% (hazard ratio = 0.463; p = 0.0003) [17]. These results lead to approval of olaratumab by Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2016 [34, 35]. In June 2019, the confirmatory phase III study ANNOUNCE, did not reach the primary end-points of OS in the full study population [18]. Due to insufficient proof of its medical advantage, it was removed from the United States and European Union markets, so our patient was forced to stop treatment.
4. Conclusion
We decided to report this clinical case because it is an example of how a malignant biological behaviour soft tissue sarcoma, treated with cyto-reductive surgery and subsequent chemo-radiotherapy consolidation, has reached a partial response maintained for a long time. In conclusion, we proposed a combination approach as a possible solution in selected cases, even if at an advanced or locally advanced stage. Therefore, considering the lack of prospective studies and the possibility to draw conclusions only on retrospective studies or case reports, a multidisciplinary approach becomes fundamental in order to select patients susceptible to treatments with curative intent, such as in our case.
References
- WHO Pathology and Genetics of Tumours of Soft Tissue and Bone.
- Folpe AL, Mentzel T, Lehr H-A, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 29 (2005): 1558-1575.
- Rutkowski PL, Mullen JT. Management of the “Other” retroperitoneal sarcomas. J Surg Oncol 117 (2018): 79-86.
- Touloumis Z, Giannakou N, Sioros C, et al. Retroperitoneal perivascular epithelioid cell tumours: A case report and review of literature. World J Clin cases 7 (2019): 3524-3534.
- Liang W, Xu C, Chen F. Primary retroperitoneal perivascular epithelioid cell neoplasm: A case report. Oncol Lett 10 (2015): 469-472.
- Benson C, Vitfell-Rasmussen J, Maruzzo M, et al. A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: The Royal Marsden Hospital experience. Anticancer Res 34 (2014): 3663-3668.
- Kenerson H, Folpe AL, Takayama TK, et al. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol 38 (2007): 1361-1371.
- Gennatas C, Michalaki V, Kairi PV, et al. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J Surg Oncol 10 (2012): 181.
- Dickson MA, Schwartz GK, Antonescu CR, et al. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J cancer 132 (2013): 1711-1717.
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol Off J Am Soc Clin Oncol 28 (2010): 835-840.
- Italiano A, Delcambre C, Hostein I, et al. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol Off J Eur Soc Med Oncol 21 (2010): 1135-1137.
- Sanfilippo R, Jones RL, Blay J-Y, et al. Role of Chemotherapy, VEGFR Inhibitors, and mTOR Inhibitors in Advanced Perivascular Epithelioid Cell Tumors (PEComas). Clin cancer Res an Off J Am Assoc Cancer Res 25 (2019): 5295-5300.
- Wagner AJ, Ravi V, Ganjoo KN, et al. ABI-009 (nab-sirolimus) in advanced malignant perivascular epithelioid cell tumors (PEComa): Preliminary efficacy, safety, and mutational status from AMPECT, an open label phase II registration trial. J Clin Oncol 37 (2019): 11005.
- Scheppach W, Reissmann N, Sprinz T, et al. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol 19 (2013): 1657-1660.
- Liapi A, Mathevet P, Herrera FG, et al. VEGFR Inhibitors for Uterine Metastatic Perivascular Epithelioid Tumors (PEComa) Resistant to mTOR Inhibitors. A Case Report and Review of Literature. Front Oncol 11 (2021): 641376.
- Hindi N, Sanfilippo R, Stacchiotti S, et al. Systemic Therapy in Perivascular Epithelioid Cell Tumors (Pecoma). Ann Oncol 25 (2014): iv506.
- Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet (London, England) 388 (2016): 488-497.
- Tap WD, Wagner AJ, Papai Z, et al. ANNOUNCE: A randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS). J Clin Oncol 37 (2019): LBA3-LBA3.
- Italiano A. Olaratumab failure in sarcomas: what are the lessons learned? Eur J Cancer 117 (2019): 69-70.
- Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol 19 (2015): 359-368.
- Martignoni G, Pea M, Reghellin D, et al. PEComas: the past, the present and the future. Virchows Arch 452 (2008): 119-132.
- Hornick JL, Fletcher CDM. PEComa: what do we know so far? Histopathology 48 (2006): 75-82.
- Armah HB, Parwani A V. Perivascular epithelioid cell tumor. Arch Pathol Lab Med 133 (2009): 648-654.
- Theegarten D, Hager T. [Pulmonary lymphangioleiomyomatosis (LAM)]. Pathologe 42 (2021): 35-39.
- Stone CH, Lee MW, Amin MB, et al. Renal angiomyolipoma: further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med 125 (2001): 751-758.
- Ardeleanu C, Bussolati G. Telocytes are the common cell of origin of both PEComas and GISTs: an evidence-supported hypothesis. J Cell Mol Med 15 (2011): 2569-2574.
- Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 68 (2001): 64-80.
- Au KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 9 (2007): 88-100.
- Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol 34 (2010): 1395-1406.
- Casali PG, Abecassis N, Aro HT, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 29 (2018): iv51-iv67.
- Lans TE, van Ramshorst GH, Hermans JJ, et al. Perivascular epithelioid cell tumor of the retroperitoneum in a young woman resulting in an abdominal chyloma. J Gastrointest Surg Off J Soc Surg Aliment Tract 13 (2009): 389-392.
- de León DC, Pérez-Montiel D, Bandera A, et al. Perivascular epithelioid cell tumor of abdominal origin. Ann Diagn Pathol 14 (2010): 173-177.
- Gunia S, Awwadeh L, May M, et al. Perivascular epithelioid cell tumor (PEComa) with perirenal manifestation. Int J Urol Off J Japanese Urol Assoc 12 (2005): 489-492.
- US FDA. Lartruvo (olaratumab) injection: US prescribing information (2016).
- European Medicines Agency. Lartruvo (olaratumab): summary of product characteristics.
- Loizos N, Xu Y, Huber J, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther 4 (2005): 369-379.
- Shah GD, Loizos N, Youssoufian H, et al. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer 116 (2010): 1018-1026.
- Heldin C-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 11 (2013): 97.