MRS of the Brain in Patients with Severe Chronic Stenosis of Carotid Arteries
Article Information
Bartosz Mruk1,2, Micha? Fr?czek1,2, Marek Cia?1,2, Piotr Andziak3, Jerzy Walecki1,2, Katarzyna Sklinda1,2*
1Department of Diagnostic and Interventional Radiology, Central Clinical Hospital of the Ministry of Internal Affairs, Warsaw, Poland
2Department of Diagnostic and Interventional Radiology, Centre for Postgraduate Medical Education, Warsaw, Poland
3Department of General and Vascular Surgery, Central Clinical Hospital of the Ministry of Internal Affairs and Administration, Warsaw, Poland
*Corresponding author: Katarzyna Sklinda, Department of Diagnostic and Interventional Radiology, Central Clinical Hospital of the Ministry of Internal Affairs and Administra-tion, Wo?oska 137 Str., 02-507 Warsaw, Poland
Received: 30 June 2019; Accepted: 21 August 2019; Published: 04 September 2019
Citation: Bartosz Mruk, Michal Fraczek, Marek Cias, Piotr Andziak, Jerzy Walecki, Katarzyna Sklinda. MRS of the Brain in Patients with Severe Chronic Stenosis of Carotid Arteries. Cardiology and Cardiovascular Medicine 3 (2019): 322-328.
Share at FacebookAbstract
The risk of stroke in appropriately selected patients can be reduced through carotid endarterectomy. The main beneficent of operation are patients with at least 70% of ICA stenosis [1]. A stroke (or death) can be prevented with CEA in patients with asymptomatic ICA stenosis what has been reported in the Asymptomatic Carotid Artery Study [2].
Impairment of cognitive functions occurs in 10%-30% [4] of patients who underwent CEA although this kind of surgical intervention may also improve cognitive function [3]. It has been recently reported that 11% of patients who had CEA shown improvement in cognitive function after surgery, while another 11%, on the contrary, experienced a decline in cognitive function after surgery [5]. Along with these postoperative changes in cognitive function changes in cerebral metabolism have also been observed, but the relationship between these two factors remains unexplained.
Proton MR spectroscopy provides possibility of noninvasive chemical analysis of the brain in vivo as well as it allows estimation of relative changes in brain metabolites, including N-acetylaspartate (NAA), choline-containing compounds, and total creatine [6].
It is more sensitive than magnetic resonance imaging (MRI) in detection of changes related to decreased oxygen supply through the measurement of the metabolic changes that may occur before the morphologic changes. Studies have shown that the level of NAA in the brain may be interpreted as an index of neuronal viability [7] and that the choline level in the brain is associated with membrane synthesis or degeneration in neural tissues [8]. 1H-MRS may also provide information valuable for diagnosis and management of insufficient blood perfusion the brain at the primary stage.
These hypotheses were followed by studies in which 1H-MRS has
Keywords
Stenosis; Carotid Arteries
Article Details
Methods and materials
Fourteen noninfarcted patients with stenosis of unilateral ICA who were scheduled to undergo CEA were included in our prospective study. All patients were having ipsilateral cervical internal carotid artery stenosis (≥70%) on ultrasonography and/or CT-angiography and were having no infarction in the area of the brain perfused by the middle cerebral artery confirmed by MR imaging, including FLAIR sequence, which was performed +/-1 day before surgery.
After surgery no patient presented acute neurologic deficits lasting for 1 month after surgery nor ischemic lesions on MR imaging which have not been observed before. It was confirmed on FLAIR sequences, performed 1 month after surgery compared with preoperative MR imaging.
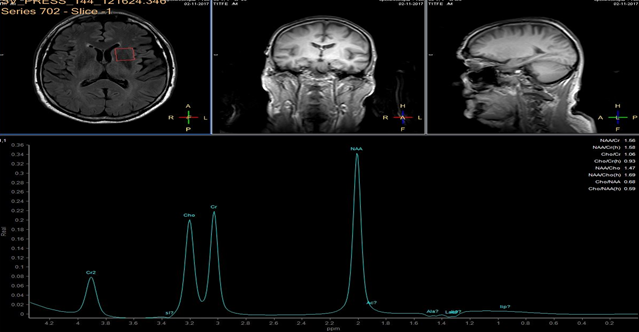
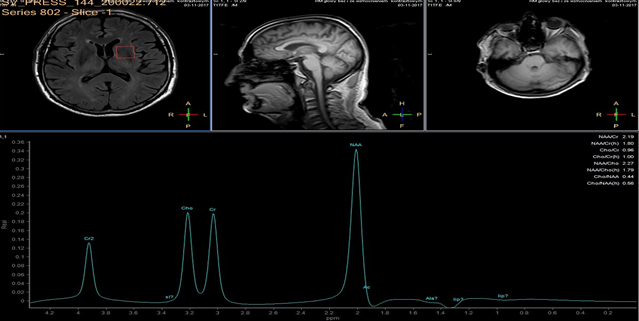
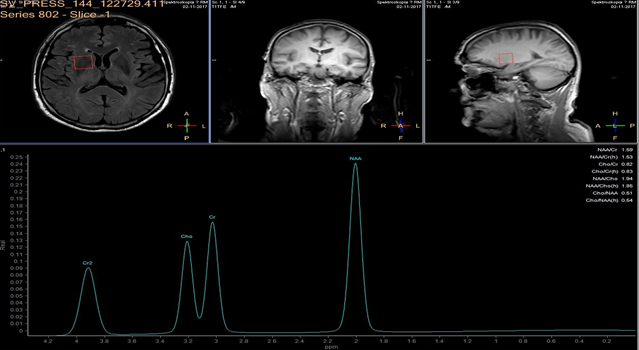
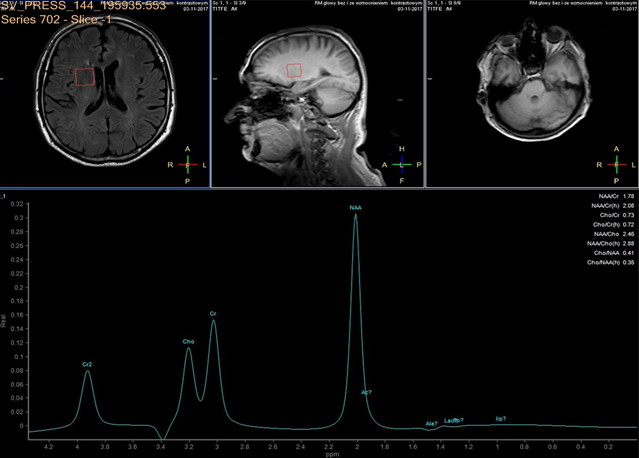
A 3 Tesla MRI scanner (Ingenia 3.0T, Philips, Amsterdam NL) imaging system was used for evaluation of ischemic lesions before and after surgery. Anatomic MR images were obtained with a FLAIR longTR sequence (repetition time 9s; echo time 125 ms). The slices were 4 mm thick with 1mm gap, and the matrix was 352 X 254. Proton MRS was performed using the double-spin echo sequence with a voxel size of 20 cm3. Volumes of interest were placed in the head of caudate nucleus, for which MR imaging confirmed the absence of infarction. Fluid-filled spaces such as ventricles and cerebral sulci were carefully omitted.
In each study one volume of interest was located on the side ipsilateral to stenotic artery and the other in the contralateral unaffected region in each patient (Figure 1). The areas of 3 major peaks, including choline (3.2 ppm), the sum of creatine and phosphocreatine (3.0 ppm), and NAA (2.0 ppm), were measured by the automated LCModel package.
As the peak intensity depends on the acquisition and processing parameters, no absolute metabolite quantification was attempted. Values relative to the peak of the sum of creatine and phosphocreatine at 3.0 ppm were given as results. This peak which is reflecting total creatine concentration is considered as constant value because of equilibrium between phosphocreatine and free creatine [14] within central nervous system and is known to be unchanged during the evolution of cerebral ischemia and under various pathologic conditions [15].
These were determined with a proton MR spectroscopy based on LC model and Naa/Cr ratios for deep nuclei of both cerebral hemispheres analysis. An asymmetry index, defined as (ipsilateral - contralateral)/(ipsilateral + contralateral) X 2 X 100%, was used to analyze the relationship between the relative MRS data and the stenosis of internal carotid artery.
Statistical significance was assessed with the Mann-Whitney test for comparing the patients' MRS data with that of the healthy volunteers. Linear regression analysis and the Pearson correlation coefficient were used to examine the relationship between the asymmetry index of MRS measurements and stenosis. P < 0.05 was considered significant.
Results
In healthy volunteers, the ratio of NAA to the sum of creatine and phosphocreatine (NAA/Cr) was 1.67 ±0.11 (mean ±SD) and the ratio of choline to the sum of creatine and phosphocreatine (Cho/Cr) was 0.85 ±0.04.
NAA/Cr and Cho/Cr of both hemispheres showed no significant difference. NAA/Cr in the affected hemisphere of patients decreased significantly compared with that of the healthy volunteers (P < 0.05), whereas that in the unaffected hemisphere of patients did not.
Cho/Cr did not differ significantly between healthy volunteers and patients in either the affected or the unaffected hemisphere. Neither the asymmetry index of NAA/Cr nor the asymmetry index of Cho/Cr shown significant correlation with stenosis of ICE (r=0,12, P<0.05). Sufficient circulation through the circle of Willis on the contralateral side is a probable explanation of this fact.
Prior to surgery, in all patients the ratio of NAA to the sum of creatine and phosphocreatine (NAA/Cr) decreased and the ratio of choline to the sum of creatine and phosphocreatine (Cho/Cr) increased in the hemisphere ipsilateral to stenotic ICA compared with the contralateral side. Lactate peaks were observed in 2 patients. All patients underwent endarterectomy and follow up MR study within 24 hours after surgery. In all patients subtle increase of NAA/Cr level was seen (delta 0,38 ±0.73 (mean ±SD)) and decrease of lactates on the recanalised side was observed.
The metabolic changes were relatively subtle but were associated with surgical procedure. No relationship between metabolic abnormalities and neurological status of the patients has been observed.
Conclusions
1H-MRS confirms metabolic changes within the brain which may help in guiding treatment decisions and be useful for preoperative evaluation.
Metabolic changes revealed in the head of caudate nucleus can indicate a hemodynamically compromised state in patients with unilateral steno-occlusive carotid artery disease. It may help predict which patients will benefit from endarterectomy through improvement of neurological status. The anatomic and blood flow changes caused by hypoperfusion and stroke can be additionally evaluated with proton MRS in clinical settings, adding relevant metabolic information.
Our study was based on 3T proton MR spectroscopy. It was shown that postoperative changes in cerebral metabolites were not associated with changes in cognitive function after TEA or CAS confirmed in standard neurologic evaluation. Further investigations using different imaging modalities, such as positron-emission tomography, would be worth doing to confirm the relationship between changes in cerebral metabolism and change in cognitive function after surgical intervention.
Moreover an evaluation of cognitive function before and after treatment could reveal whether the change in brain metabolism has impact on cognitive functions. A large group prospective study would be needed to establish whether a postoperative changes in cerebral metabolites measured by 1H-MRS is associated with impairment or improvement in cognitive function after TEA or CAS.
Compliance with Ethical Standards
None of the authors declared any conflict of interest.
The study has been approved by the local Bioethics Committee (Approval of the Ethics and Surveillance Committee for Research in Human and Animal Sciences at the Central Clinical Hospital of the Ministry of Internal Affairs; No. 54/2016 of 01.06.2016).
The written informed consent for surgical intervention as well as for performing MRI has been obtained from all patients participating in this study.
Funding
This work was supported by grant Nr 501-9-999-94-18 from the Centre of Postgraduate Medical Education.
References
- Chaturvedi S, Bruno A, Feasby T, et al. Carotid endarterectomy: an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 65 (2005): 794–801.
- The Executive Committee fort he Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 273 (1995): 1421-1428.
- De Rango P, Caso V, Leys D, et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke 39 (2008): 3116–3127.
- Heyer EJ, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol 59 (2002): 217–222.
- Yoshida K, Ogasawara K, Kobayashi M, et al. Improvement and impairment in cognitive function after carotid endarterectomy: comparison of objective and subjective assessments. Neurol Med Chir (Tokyo) 52 (2012): 154–160.
- Di Costanzo AD, Trojsi F, Tosetti M, et al. Proton MR spectroscopy of the brain at 3T: an update. Eur Radiol 17 (2017): 1651–1662.
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 13 (1989): 23–31.
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 4 (1991): 47–52.
- Gonzalez-Toledo E, Kelley RE, Minagar A. Role of magnetic resonance spectroscopy in diagnosis and management of multiple sclerosis. Neurol Res 28 (2006): 280–283.
- Ben Salem D, Walker PM, Bejot Y, et al. N-acetylaspartate/creatine and choline/creatine ratios in the thalami, insular cortex and white matter as markers of hypertension and cognitive impairment in the elderly. Hypertens Res 31 (2008): 1851–1857.
- Waldman AD, Rai GS. The relationship between cognitive impairment and in vivo metabolite ratios in patients with clinical Alzheimer's disease and vascular dementia: a proton magnetic resonance spectroscopy study. Neuroradiology 45 (2003): 507–512.
- Kim GE, Lee JH, Cho YP. Can carotid endarterectomy improve metabolic status in patients with asymptomatic internal carotid artery flow lesion? Studies with localized in vivo proton magnetic resonance spectroscopy. J Vasc Surg 36 (2002): 559–564.
- Uno M, Harada M, Nagahiro S. Quantitative evaluation of cerebral metabolites and cerebral blood flow in patients with carotid stenosis. Neurol Res 23 (2001): 573–580.
- Lewis LD, Ljunggren B, Ratcheson RA, Siesjo BK. Cerebral energy state in insulin-induced hypoglycemia, related to blood glucose and to EEC. J Neurochem 23 (1974): 673-679.
- Sappey-Marinier D, Calabrese G, Hetherington HP, et al. Proton magnetic resonance spectroscopy of human brain: applications to normal white matter, chronic infarction, and MRI white matter signal hyperintensities. Magn Reson Med 26 (1992): 313-327.