Long-Term Outcomes of the Spinal Cord Injury Assessing Tolerability and Use of Combine Rehabilitation and NeuroAID (SATURN STUDY)
Article Information
Ramesh Kumara, Ohnmar Htweb, Azmi Baharudinb ,Shaharuddin Abdul Rhanic,
Kamalnizat Ibrahimd, Jagdeep Singh Nanrae, Muhindra Gsangayaf, Hezery Harung, Khairrudin Kandarh, Maatharasi Balanf, Shawn Peha, Yogesh Pokharkari, Mohammad Hisam Ariffinb
a Neurosurgery Unit, Department of Surgery, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre,Kuala Lumpur, Malaysia
b Department of Orthopaedics and Traumatology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia
c Department of Orthopaedic, KPJ Selangor Specialist Hospital, Selangor, Malaysia
d Department of Orthopaedic, KPJ Ampang Puteri Specialist Hospital, Selangor, Malaysia
e Prince Court Medical Centre, Kuala Lumpur, Malaysia
f Department of Orthopaedics and Traumatology, Hospital Serdang, Selangor, Malaysia
g Department of Orthopaedics and Traumatology, Hospital Pengajar Universiti Putra Malaysia, Selangor, Malaysia
h Department of Orthopaedic, Avisena Specialist Hospital, Selangor, Malaysia2+
i Singapore Clinical Research Institute
*Corresponding Author: Ohnmar Htwe, Department of Orthopaedics and Traumatology, Faculty of Medicine, University Kebangsaan Malaysia Medical Centre, 56000 Kuala Lumpur, Malaysia.
Received: 28 March 2023; Accepted: 03 April 2023; Published: 13 April 2023
Citation: Ramesh Kumar, Ohnmar Htwe, Azmi Baharudin ,Shaharuddin Abdul Rhani, Kamalnizat Ibrahim, Jagdeep Singh Nanra, Muhindra Gsangaya, Hezery Harun, Khairrudin Kandar, Maatharasi Balan, Shawn Peha, Yogesh Pokharkar, Mohammad Hisam Ariffin. Long-Term Outcomes of the Spinal Cord Injury Assessing Tolerability and Use of Combine Rehabilitation and NeuroAID (SATURN STUDY). Journal of Spine Research and Surgery . 5 (2023): 19-25
Share at FacebookAbstract
Background:
Spinal cord injury (SCI) is a devastating neurological disorder that affects thousands of individuals each year. Recent advances in research have given us greater understanding of the molecular and cellular events in SCI.The latest frontier in research involves neuroprotection, repair and regeneration. This paper aims to evaluate the effects of the initial 6-month treatment with MLC601/MLC901 on long term outcomes at 12 months,18 months and 24 months.
Methods:
The study was an open label, prospective, cohort trial of MLC601/MLC901 (NeuroAiD) in subjects with moderate to severe SCI. Patients age was 18 to 65 years old, and the SCI occurs within 3 days and 4 weeks. Each received MLC601/MLC901 for 6 months in addition to standard care and rehabilitation. Key endpoints were safety, American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade and AIS motor scores at month 6 (M6). The protocol and the primary results of the 6th month period were previously published. The primary result showed safety and potential role of MLC601/MLC901 in moderate to severe spinal cord Injury. Outcomes of the long-term follow up was assessed up to 24 months.
Results:
Long term follow-up after 6-month treatment showed durability of improvement in total motor, sensory and SCIM score .The improvement was maintained until 12, 18 and 24 months.
Conclusion:
The long-term outcomes further provided evidence in the safety and potential role of MLC601/MLC901 in SCI. This findings should help plan a study design for a randomized controlled trial.
Keywords
Spinal Cord Injury; NeuroAiD; MLC901; Neuroregeneration; ASIA motor score; ASIA Sensory score; Clinical trial
articles about Spinal Cord Injury Research articles about Spinal Cord Injury review articles about Spinal Cord InjurySpinal Cord Injury PubMed articles Spinal Cord Injury PubMed Central articles Spinal Cord Injury 2024 articles Spinal Cord Injury 2025 articles Spinal Cord Injury Scopus articles Spinal Cord Injury impact factor journals Spinal Cord Injury Scopus journals Spinal Cord Injury PubMed journals Spinal Cord Injury medical journals Spinal Cord Injury free journals Spinal Cord Injury best journals Spinal Cord Injury top journals Spinal Cord Injury free medical journals Spinal Cord Injury famous journals Spinal Cord Injury Google Scholar indexed journals articles about NeuroAiD Research articles about NeuroAiD review articles about NeuroAiDNeuroAiD PubMed articles NeuroAiD PubMed Central articles NeuroAiD 2025 articles NeuroAiD 2026 articles NeuroAiD Scopus articles NeuroAiD impact factor journals NeuroAiD Scopus journals NeuroAiD PubMed journals NeuroAiD medical journals NeuroAiD free journals NeuroAiD best journals NeuroAiD top journals NeuroAiD free medical journals NeuroAiD famous journals NeuroAiD Google Scholar indexed journals articles about MLC901 Research articles about MLC901 review articles about MLC901MLC901 PubMed articles MLC901 PubMed Central articles MLC901 2026 articles MLC901 2027 articles MLC901 Scopus articles MLC901 impact factor journals MLC901 Scopus journals MLC901 PubMed journals MLC901 medical journals MLC901 free journals MLC901 best journals MLC901 top journals MLC901 free medical journals MLC901 famous journals MLC901 Google Scholar indexed journals articles about Neuroregeneration Research articles about Neuroregeneration review articles about NeuroregenerationNeuroregeneration PubMed articles Neuroregeneration PubMed Central articles Neuroregeneration 2027 articles Neuroregeneration 2028 articles Neuroregeneration Scopus articles Neuroregeneration impact factor journals Neuroregeneration Scopus journals Neuroregeneration PubMed journals Neuroregeneration medical journals Neuroregeneration free journals Neuroregeneration best journals Neuroregeneration top journals Neuroregeneration free medical journals Neuroregeneration famous journals Neuroregeneration Google Scholar indexed journals articles about ASIA motor score Research articles about ASIA motor score review articles about ASIA motor scoreASIA motor score PubMed articles ASIA motor score PubMed Central articles ASIA motor score 2028 articles ASIA motor score 2029 articles ASIA motor score Scopus articles ASIA motor score impact factor journals ASIA motor score Scopus journals ASIA motor score PubMed journals ASIA motor score medical journals ASIA motor score free journals ASIA motor score best journals ASIA motor score top journals ASIA motor score free medical journals ASIA motor score famous journals ASIA motor score Google Scholar indexed journals articles about ASIA Sensory score Research articles about ASIA Sensory score review articles about ASIA Sensory scoreASIA Sensory score PubMed articles ASIA Sensory score PubMed Central articles ASIA Sensory score 2029 articles ASIA Sensory score 2030 articles ASIA Sensory score Scopus articles ASIA Sensory score impact factor journals ASIA Sensory score Scopus journals ASIA Sensory score PubMed journals ASIA Sensory score medical journals ASIA Sensory score free journals ASIA Sensory score best journals ASIA Sensory score top journals ASIA Sensory score free medical journals ASIA Sensory score famous journals ASIA Sensory score Google Scholar indexed journals articles about Clinical trial Research articles about Clinical trial review articles about Clinical trialClinical trial PubMed articles Clinical trial PubMed Central articles Clinical trial 2030 articles Clinical trial 2031 articles Clinical trial Scopus articles Clinical trial impact factor journals Clinical trial Scopus journals Clinical trial PubMed journals Clinical trial medical journals Clinical trial free journals Clinical trial best journals Clinical trial top journals Clinical trial free medical journals Clinical trial famous journals Clinical trial Google Scholar indexed journals articles about neurologic Research articles about neurologic review articles about neurologicneurologic PubMed articles neurologic PubMed Central articles neurologic 2031 articles neurologic 2032 articles neurologic Scopus articles neurologic impact factor journals neurologic Scopus journals neurologic PubMed journals neurologic medical journals neurologic free journals neurologic best journals neurologic top journals neurologic free medical journals neurologic famous journals neurologic Google Scholar indexed journals articles about AIS Motor Research articles about AIS Motor review articles about AIS MotorAIS Motor PubMed articles AIS Motor PubMed Central articles AIS Motor 2032 articles AIS Motor 2033 articles AIS Motor Scopus articles AIS Motor impact factor journals AIS Motor Scopus journals AIS Motor PubMed journals AIS Motor medical journals AIS Motor free journals AIS Motor best journals AIS Motor top journals AIS Motor free medical journals AIS Motor famous journals AIS Motor Google Scholar indexed journals
Article Details
Introduction
Spinal cord injuries remain a devastating disease with functional and neurologic sequalae requiring multimodal therapeutic approaches. Treatment remains supportive which mainly focuses on preventing further injury including surgery and medications[1]. Current understanding of SCI pathophysiology and neuronal recovery involving neuroprotective, immunomodulatory and neuro-regenerative are necessary to formulate appropriate treatment modalities to improve SCI recovery[2]. Currently, researchers are focussing on the development of effective neurorenegerative properties in moderate to severe SCI[3].
MLC901 (NeuroAiD II) is a Traditional Chinese Medicine (TCM) with 9 herbal components (Radix Astragali, Radix Salviae miltiorrhizae, Radix Paeoniae rubra, Rhizoma chuanxiong, Radix Angelicae sinensis, Carthamus tinctorius, Semen persica/Prunus persica, Radix Polygalae, Rhizoma acori tatarinowii). MLC901 is a simplified formulation of MLC601 (NeuroAiD I). MLC601 contains 9 herbal and 5 non-herbal components and has extensive historical use in human. The herbal components of MLC601 and MLC901 are the same. The similarity of the two products MLC601 and MLC901 is based upon preclinical work by Michel Ladzunski et al. [4,5]. The combination of the multiple herbal compounds produces the multimodal mechanism of action of neuroprotection and neurorepair. It demonstrated in vitro neuronal proliferation and neurite outgrowth and in vivo neurogenesis in animal study[6]. Treatment of MLC901 in ischemic mice model stimulates angiogenesis by significantly increasing the capillary density and BrdU-reactive endothelial cell in ischemic borders and increase in the Vascular Endothelial Growth Factor (VEGF) There was also improvement in neurological function in behavioural test performed among injured mice given MLC901[7]. Similar safety and benefit/risk profile observed for the 2 products in the extensive clinical experience with MLC601 and MLC901.
Both demonstrated to enhance neurological recovery and extensively studied in stroke [8,9,10] and traumatic brain injury with promising clinical and functional outcome[11].
MLC601/MLC901 has neuroprotective effects in neuronal and brain injuries as well as positive effects on functional recovery after stroke. We aimed to evaluate the safety and efficacy among subjects with moderate to severe SCI.
The Study protocol[12] and the primary result of an exploratory study[13] were previously published. Briefly, the study was an open label, prospective cohort trial of MLC601/MLC901 (NeuroAiD) in subjects with moderate to severe spinal cord injury (SCI). The results showed safe use and potential role of MLC601/MLC901 in moderate to severe spinal cord Injury.
Methodology :
The result of the primary outcome has been published and may refer to complete listing of the eligibility criteria and methodology. A total of 30 patients were enrolled. Patient received MLC601, 4 capsule 3x a day or MLC901, 2 capsule 3x a day for 6 months with the standard care and rehabilitation as prescribed by the physician. The AIS grade, AIS motor score, AIS sensory score, and occurrence medical events were ascertained at month 1,3,6,12,18 and 24 months. Compliance assessment was done at month 1,3, and 6.
Results
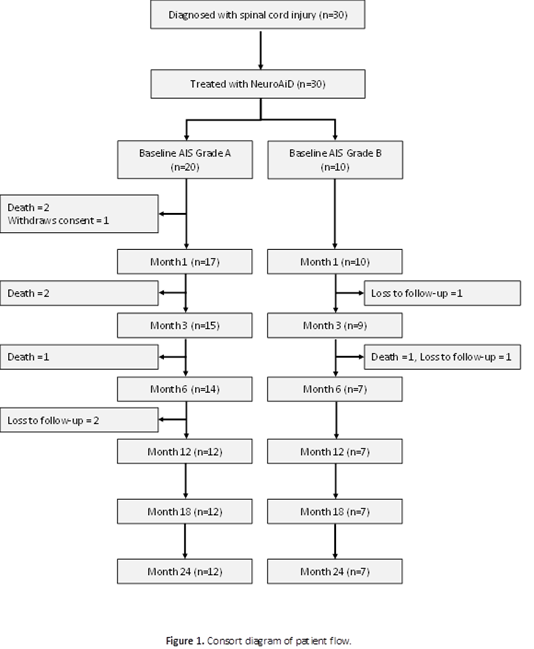
The long-term outcomes confirmed the potential benefit of MLC601/MLC901 in moderate to severe spinal cord injury. Baseline characteristics were detailed in the primary result publication. The 24th month study flow is shown in (Figure 1).
The MLC601/MLC901 was given for six months together with the standard care, and follow up to 24 months. On the long term follow up, secondary endpoints were AIS grade, AIS motor scores, AIS sensory scores, SCIM (Spinal Cord Independence Measure Outcomes) at 1, 3, 6, 12, 18, and 24-months including compliance to NeuroAiD at 1, 3, and 6 months.
At Month 6, conversion to a more functional grade was seen in 25 % (5/20) for AIS A patients and 50% (5/10) conversion for AIS grade B. This improvement was consistent and maintained up to 18 and 24 months for AIS grade A. For AIS grade B there was a higher percentage of conversion to a better grade at 24 months (Table 1; Figure 2).
|
Parameter |
NeuroAiD (N=30) |
95% CI $ |
P-value $ |
|
Baseline AIS Grade |
|||
|
A |
20 (66.7) |
||
|
B |
10 (33.3) |
||
|
Total |
30 (100.0) |
||
|
Patients achieving Better Grade Compared to Baseline |
|||
|
Month 1 (N=30) |
10 (33.3) |
(17.29, 52.81) |
0.068 |
|
Month 3 (N=30) |
10 (33.3) |
(17.29, 52.81) |
0.068 |
|
Month 6 (N=30) |
10 (33.3) |
(17.29, 52.81) |
0.068 |
|
Month 12 (N=30) |
10 (33.3) |
(17.29, 52.81) |
0.068 |
|
Month 18 (N=30) |
10 (33.3) |
(17.29, 52.81) |
0.068 |
|
Month 24 (N=30) |
11 (36.7) |
(19.93, 56.14) |
0.144 |
|
Patients with Baseline Grade A achieving Grade B or Better Compared to Baseline* |
NeuroAiD |
95 % CI $ |
p value $ |
|
Month 1 (N=20) |
6 (30.0) |
(11.89, 54.28) |
0.074 |
|
Month 3 (N=20) |
5 (25.0) |
(8.66, 49.10) |
0.025 |
|
Month 6 (N=20) |
5 (25.0) |
(8.66, 49.10) |
0.025 |
|
Month 12 (N=20) |
5 (25.0) |
(8.66, 49.10) |
0.025 |
|
Month 18 (N=20) |
5 (25.0) |
(8.66, 49.10) |
0.025 |
|
Month 24 (N=20) |
5 (25.0) |
(8.66, 49.10) |
0.025 |
|
Patients with Baseline Grade B achieving Grade C or Better Compared to Baseline& |
|||
|
Month 1 (N=10) |
4 (40.0) |
(12.16, 73.76) |
0.527 |
|
Month 3 (N=10) |
5 (50.0) |
(18.71, 81.29) |
> 0.999 |
|
Month 6 (N=10) |
5 (50.0) |
(18.71, 81.29) |
> 0.999 |
|
Month 12 (N=10) |
5 (50.0) |
(18.71, 81.29) |
> 0.999 |
|
Month 18 (N=10) |
5 (50.0) |
(18.71, 81.29) |
> 0.999 |
|
Month 24 (N=10) |
6 (60.0) |
(26.24, 87.84) |
0.527 |
AIS -American Spinal Injury Association(ASIA) Impairment Scale
CI- Confidence Interval
Missing values are imputed by LOCF (Last Observation Carried Forward) except for death
$ 95 % Confidence Interval and Two sided P values are derived from exact binomial test
Table 1: Improvement in AIS grade compared to baseline (Treated patients)
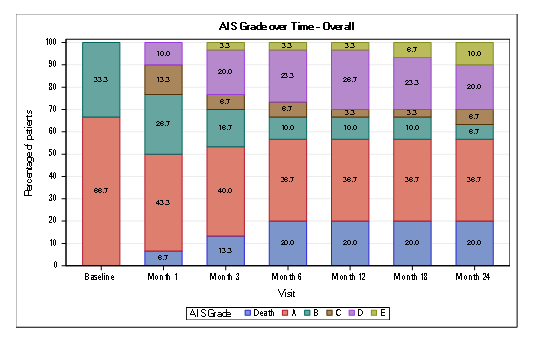
Figure 2: Conversion of AIS impairment grades
The median AIS total motor score improved significantly from 50 at baseline to 54 at M6, with the median change from baseline reported to be 9.0 (IQR: 0-44.5), P 0.00050 (Table 2).The change in motor score from baseline to Month 6 was statistically significant (P<0.005).The peak of improvement of the median motor score was maximal at 6 months, slight decline at 12 months then reached the plateau state at 18 and 24 months (Figure 3).There was no reversal of benefit after stopping the treatment at 6 months.
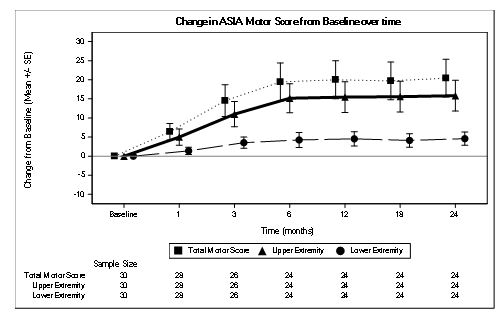
Figure 3 : Change in AIS Motor Score from Baseline over time
The most significant improvement in the total sensory score was at month 6.The total sensory score improved maximally at 6 months, slight decrease at 12 months then improved at 18 months then plateaus at 24 months (Table 2,Figure 4).
|
Time point |
Raw values |
Change from Baseline |
|||
|
Scale |
Mean (SD) |
Median (IQR) |
Median (IQR) |
p values$ |
|
|
Total Motor Score |
Baseline |
35.7 (20.0) |
50 (20,50) |
||
|
Month 1 |
43.4 (24.0) |
50 (29,50) |
0 (0,9) |
0.0007 |
|
|
Month 3 |
53.5 (27.2) |
50 (50,70) |
0.5(0,31) |
0.0004 |
|
|
Month 6 |
59.5 (26.7) |
54(50,81) |
9 (0,44) |
0.0005 |
|
|
Month 12 |
60.1 (26.3) |
50 (50,85) |
8(0,41) |
0.0001 |
|
|
Month 18 |
59.8 (26.9) |
50 (48,83) |
10(0,42) |
0.0003 |
|
|
Month 24 |
60.5 (27.2) |
50 (50,83) |
11(0,43) |
0.0002 |
|
|
AIS Sensory Subscore |
|||||
|
Baseline |
61(26) |
68(42,80) |
|||
|
Month 1 |
69 (24) |
74 (46,88) |
4(0,15) |
0.053 |
|
|
Month 3 |
72 (26) |
74 (48,94) |
3(0, 19) |
0.073 |
|
|
Month 6 |
78 (23) |
80(66,94) |
8(0,27) |
0.005 |
|
|
Month 12 |
74 (25) |
76 (48,98) |
6 (-4,25) |
0.006 |
|
|
Month 18 |
76 (29) |
80 (56,98) |
6 (0,24) |
0.022 |
|
|
Month 24 |
75 (24) |
80 (56,90) |
6 (-7,24) |
0.049 |
|
|
Total SCIM Score |
|||||
|
Baseline |
|||||
|
Month 1 |
34 (20) |
33 (15,49) |
|||
|
Month 3 |
43 (21) |
41 ( 24,60) |
4 (-0.5, 15) |
0.018 |
|
|
Month 6 |
47 (20) |
48(28,63) |
9 (2,20) |
0.006 |
|
|
Month 12 |
52 (18) |
53 (39,65) |
14 (2,23) |
0.001 |
|
|
Month 18 |
53 (23) |
56 (33,68) |
19 (1,29) |
0.003 |
|
|
Month 24 |
54 (25) |
50 (33,69) |
16 (1,32) |
0.002 |
|
AIS (ASIA Impairment Scale) ; SCIM ( Spinal Cord Independence Measure)
$ p values are calculated based on Wilcoxon signed rank test, missing values are imputed by LOCF except for death
Table 2: Summary of Mean and Median at All Time Points and Change from Baseline in American Spinal Injury Association (ASIA) International Standard for Neurological Classification of Spinal Cord Injury Total Motor and Sensory Score and Spinal Cord Independence Measure Outcomes.
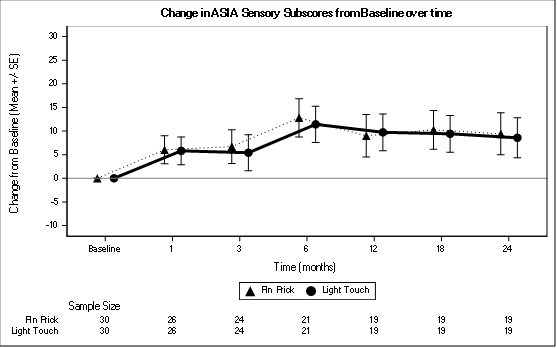
Figure 4: Change in AIS Sensory Subscore from Baseline over time
Functional recovery was assessed using the SCIM. (Figure 5).The score improved consistently across all timepoint and is clinically significant at month 3, month 12, and month 24.
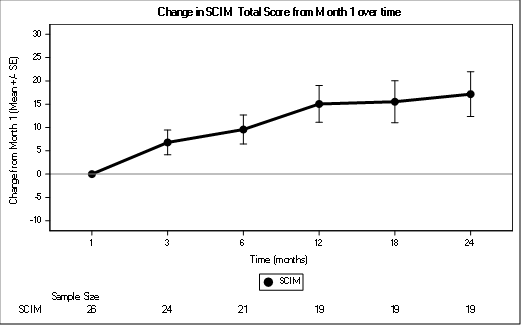
Figure 5: Change in SCIM Total Score from Month 1 over time
There were no delayed side effects noted up to 24 months.
The compliance was presented in terms of the missed doses. At month 1 the percentage of patient who never missed dose was (88%), at month 3 and month 6, 70 % and 46.47 % of patients never missed a dose respectively.
Discussion:
In the present study, favourable motor functional recovery was observed in patients who were given MLC601/MLC901 for 6 months. Furthermore, the higher motor scores were present at various timepoints, suggesting clinically meaningful improvement in neurological outcome of those treated with MLC601/MLC901.
The long-term follow up showed the consistency of findings between AIS conversion, motor, sensory and SCIM score. The median improvement of motor and sensory score peaked at 6 months and beyond 6 month the improvement was durable reaching the plateau state at 18 to 24 months. There was no reversal of benefit after stopping the treatment at month 6, meaning the recovery gained in 6 months continued to be seen in patients at 24 months. It may be possible that longer treatment duration could lead to further improvement. There were no delayed side effects noted at 24 months.
Spontaneous recovery in SCI of varying extent do occur even without treatment. According to published data, the rate of motor recovery after SCI is rapid during the first three months and motor improvement is almost complete by 9 months but ultimately plateaus at 12-18 months after SCI. In view of the likelihood that recovery of function following treatments will be concentrated close to the level of injury, it would be very useful to present some of the existing trial data in a format which shows recovery of function relative to distance below the neurologic level or whether recovery occurred within or beyond the Zone of partial preservation (ZPP) which may differentiate treatment effect and spontaneous recovery[14]. In one study by Waters et al, the change in AIS motor score for sensorimotor complete (AIS A) patients within initial two years after SCI showed that the most rapid improvement occurs within the first 6 months after SCI and is essentially maximal after 12 months[15]. In many studies AIS sensory examination often provided somewhat variable results within individual patients.
It is difficult to get a sufficient number of subjects to be able to analyse the effects of the new agent in SCI. The strength of this study is the 24 months follow up at a large health care facility with trained study investigators. This is longer as compared to the 12 months recommended follow up for SCI patients [16].
In the absence of control arm and the likelihood of spontaneous recovery, it is highly recommended to conduct a clinical trial with a six-month follow-up, but may be worth exploring the effect of treatment of longer than 6 months. We believe this study is important and will contribute the body of knowledge in treating SCI and does suggest a role for therapeutic neuromodulation in SCI patients.
Conclusion
The long-term outcomes further add evidence in the safety use and potential role of MLC601/MLC901 in SCI. This findings should help plan a study design for a randomized controlled trial.
Funding:
This research was supported by Moleac Pte Ltd. which provided the investigational product and grant for the conduct of the study
Conflict of Interest:
The authors have no conflict of interest
References
- Rabinstein AA. Traumatic Spinal Cord Injury. Continuum (Minneap Minn). 2018 Apr;24(2, Spinal Cord Disorders): 551-566.
- Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020 Oct 13;21(20): 7533.
- Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014 Apr 25;266:91-101.
- Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C. MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology. 2011 Sep;61(4):622-631.
- Moha Ou Maati H, Borsotto M, Chatelain F, Widmann C, Lazdunski M, Heurteaux C. Activation of ATP-sensitive potassium channels as an element of the neuroprotective effects of the Traditional Chinese Medicine MLC901 against oxygen glucose deprivation. Neuropharmacology. 2012 Sep;63(4): 692-700.
- Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, 'et al.' (2010). Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology. 58(7): 987-1001.
- Gandin C, Widmann C, Lazdunski M, Heurteaux C. MLC901 Favors Angiogenesis and Associated Recovery after Ischemic Stroke in Mice. Cerebrovasc Dis. 2016;42(1-2): 139-154.
- Venketasubramanian N, Lee CF, Young SH, Tay SS, Umapathi T, Lao AY,‘et al’.Prognostic Factors and Pattern of Long-Term Recovery with MLC601 (NeuroAiD™) in the Chinese Medicine NeuroAiD Efficacy on Stroke Recovery - Extension Study. Cerebrovasc Dis. 2017;43(1-2): 36-42.
- Suwanwela NC, Chen CLH, Lee CF, Young SH, Tay SS, Umapathi T,'et al.' Effect of Combined Treatment with MLC601 (NeuroAiDTM) and Rehabilitation on Post-Stroke Recovery: The CHIMES and CHIMES-E Studies. Cerebrovasc Dis. 2018;46(1-2): 82-88.
- Kong KH, Wee SK, Ng CY, Chua K, Chan KF, Venketasubramanian N, 'et al’. A double-blind, placebo-controlled, randomized phase II pilot study to investigate the potential efficacy of the traditional chinese medicine Neuroaid (MLC 601) in enhancing recovery after stroke (TIERS). Cerebrovasc Dis. 2009;28(5): 514-521.
- Theadom A, Barker-Collo S, Jones KM, Parmar P, Bhattacharjee R, Feigin VL. MLC901 (NeuroAiD II™) for cognition after traumatic brain injury: a pilot randomized clinical trial. Eur J Neurol. 2018 Aug;25(8):1055-e82.
- Kumar R, Htwe O, Baharudin A, Ariffin MH, Abdul Rhani S, Ibrahim K ,'et al.' Spinal Cord Injury-Assessing Tolerability and Use of Combined Rehabilitation and NeuroAiD (SATURN Study): Protocol of An Exploratory Study In Assessing the Safety and Efficacy of NeuroAiD Amongst People Who Sustain Severe Spinal Cord Injury. JMIR Res Protoc. 2016 Dec 5;5(4):e230.
- Kumar R, Htwe O, Baharudin A, Rhani SA, Ibrahim K, Nanra JS, 'et al.' Spinal cord injury - assessing tolerability and use of combined rehabilitation and NeuroAiD (SATURN) study - primary results of an exploratory study. J Spinal Cord Med. 2022 May 23: 1-5.
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007 Mar;45(3): 190-205.
- Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993 Mar;74(3): 242-247.
- Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, Jazayeri SB, Seyedpour S, Khodaei B, Hosseini M, Rahimi-Movaghar V. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019 Feb 15: 1-17.
