Is the Association of Platelet Rich Plasma and Hyaluronic Acid Useful for Severe Knee Arthrosis?
Article Information
Renato Luiz Bevilacqua de Castro, Breno Pazinatto Antonio*, Gustavo Concon de Castro, Maria Fernanda Concon de Castro
Tissue Regeneration Study Center (CERT), Avenida Barão de Itapura, 3378, Taquaral, Campinas, SP, Brazil
*Corresponding Author: Breno Pazinatto Antonio, Rua Paiquere, 530, Jardim Paiquere, Valinhos, SP, Brazil
Received: 22 October 2020; Accepted: 09 November 2020; Published: 24 November 2020
Citation:
Renato Luiz Bevilacqua de Castro, Breno Pazinatto Antonio, Gustavo Concon de Castro, Maria Fernanda Concon de Castro. Is the Association of Platelet Rich Plasma and Hyaluronic Acid Useful for Severe Knee Arthrosis?. Fortune Journal of Rheumatology 2 (2020): 090-106.
Share at FacebookAbstract
Background: Evaluate the effectiveness of intra-articular injections of platelet rich plasma associated with hyaluronic acid, in severe knee osteoarthritis in elderly patients. By comparing the results of mild and moderate osteoarthritis group (younger) with the severe osteoarthritis group (older).
Methods: The treatment was based on a technique which consisted of a superolateral knee injection of 2 mL of xylocaine (1% without constrictor vessel) followed by an injection of 2 mL of hyaluronic acid and an injection of 4 mL of platelet rich plasma. All injections were guided by ultrasound and given in the same site. Comparative analysis of pre and post treatment was performed.
Results: No significant difference were obtained between the groups (p value > 0.05), showing that despite having a different Kellgren and Lawrence classification, the two groups showed a similar improvement result.
Conclusion: The association of platelet rich plasma and hyaluronic acid in the treatment of knee arthrosis is useful for the improvement of the WOMAC total score, as well as of its subscores (pain, function, and stiffness). Similar results were obtained for patients with severe arthrosis and those with mild and moderate arthrosis.
Keywords
Osteoarthritis, Kellgren and Lawrence, Platelet-rich-plasma, Hyaluronic-acid
Osteoarthritis articles; Kellgren and Lawrence articles; Platelet-rich-plasma articles; Hyaluronic-acid articles
Osteoarthritis articles Osteoarthritis Research articles Osteoarthritis review articles Osteoarthritis PubMed articles Osteoarthritis PubMed Central articles Osteoarthritis 2023 articles Osteoarthritis 2024 articles Osteoarthritis Scopus articles Osteoarthritis impact factor journals Osteoarthritis Scopus journals Osteoarthritis PubMed journals Osteoarthritis medical journals Osteoarthritis free journals Osteoarthritis best journals Osteoarthritis top journals Osteoarthritis free medical journals Osteoarthritis famous journals Osteoarthritis Google Scholar indexed journals Kellgren and Lawrence articles Kellgren and Lawrence Research articles Kellgren and Lawrence review articles Kellgren and Lawrence PubMed articles Kellgren and Lawrence PubMed Central articles Kellgren and Lawrence 2023 articles Kellgren and Lawrence 2024 articles Kellgren and Lawrence Scopus articles Kellgren and Lawrence impact factor journals Kellgren and Lawrence Scopus journals Kellgren and Lawrence PubMed journals Kellgren and Lawrence medical journals Kellgren and Lawrence free journals Kellgren and Lawrence best journals Kellgren and Lawrence top journals Kellgren and Lawrence free medical journals Kellgren and Lawrence famous journals Kellgren and Lawrence Google Scholar indexed journals Platelet-rich-plasma articles Platelet-rich-plasma Research articles Platelet-rich-plasma review articles Platelet-rich-plasma PubMed articles Platelet-rich-plasma PubMed Central articles Platelet-rich-plasma 2023 articles Platelet-rich-plasma 2024 articles Platelet-rich-plasma Scopus articles Platelet-rich-plasma impact factor journals Platelet-rich-plasma Scopus journals Platelet-rich-plasma PubMed journals Platelet-rich-plasma medical journals Platelet-rich-plasma free journals Platelet-rich-plasma best journals Platelet-rich-plasma top journals Platelet-rich-plasma free medical journals Platelet-rich-plasma famous journals Platelet-rich-plasma Google Scholar indexed journals Hyaluronic-acid articles Hyaluronic-acid Research articles Hyaluronic-acid review articles Hyaluronic-acid PubMed articles Hyaluronic-acid PubMed Central articles Hyaluronic-acid 2023 articles Hyaluronic-acid 2024 articles Hyaluronic-acid Scopus articles Hyaluronic-acid impact factor journals Hyaluronic-acid Scopus journals Hyaluronic-acid PubMed journals Hyaluronic-acid medical journals Hyaluronic-acid free journals Hyaluronic-acid best journals Hyaluronic-acid top journals Hyaluronic-acid free medical journals Hyaluronic-acid famous journals Hyaluronic-acid Google Scholar indexed journals Arthrosis articles Arthrosis Research articles Arthrosis review articles Arthrosis PubMed articles Arthrosis PubMed Central articles Arthrosis 2023 articles Arthrosis 2024 articles Arthrosis Scopus articles Arthrosis impact factor journals Arthrosis Scopus journals Arthrosis PubMed journals Arthrosis medical journals Arthrosis free journals Arthrosis best journals Arthrosis top journals Arthrosis free medical journals Arthrosis famous journals Arthrosis Google Scholar indexed journals knee osteoarthritis articles knee osteoarthritis Research articles knee osteoarthritis review articles knee osteoarthritis PubMed articles knee osteoarthritis PubMed Central articles knee osteoarthritis 2023 articles knee osteoarthritis 2024 articles knee osteoarthritis Scopus articles knee osteoarthritis impact factor journals knee osteoarthritis Scopus journals knee osteoarthritis PubMed journals knee osteoarthritis medical journals knee osteoarthritis free journals knee osteoarthritis best journals knee osteoarthritis top journals knee osteoarthritis free medical journals knee osteoarthritis famous journals knee osteoarthritis Google Scholar indexed journals mechanical organ articles mechanical organ Research articles mechanical organ review articles mechanical organ PubMed articles mechanical organ PubMed Central articles mechanical organ 2023 articles mechanical organ 2024 articles mechanical organ Scopus articles mechanical organ impact factor journals mechanical organ Scopus journals mechanical organ PubMed journals mechanical organ medical journals mechanical organ free journals mechanical organ best journals mechanical organ top journals mechanical organ free medical journals mechanical organ famous journals mechanical organ Google Scholar indexed journals Synovial Membrane articles Synovial Membrane Research articles Synovial Membrane review articles Synovial Membrane PubMed articles Synovial Membrane PubMed Central articles Synovial Membrane 2023 articles Synovial Membrane 2024 articles Synovial Membrane Scopus articles Synovial Membrane impact factor journals Synovial Membrane Scopus journals Synovial Membrane PubMed journals Synovial Membrane medical journals Synovial Membrane free journals Synovial Membrane best journals Synovial Membrane top journals Synovial Membrane free medical journals Synovial Membrane famous journals Synovial Membrane Google Scholar indexed journals Synovial Fluid articles Synovial Fluid Research articles Synovial Fluid review articles Synovial Fluid PubMed articles Synovial Fluid PubMed Central articles Synovial Fluid 2023 articles Synovial Fluid 2024 articles Synovial Fluid Scopus articles Synovial Fluid impact factor journals Synovial Fluid Scopus journals Synovial Fluid PubMed journals Synovial Fluid medical journals Synovial Fluid free journals Synovial Fluid best journals Synovial Fluid top journals Synovial Fluid free medical journals Synovial Fluid famous journals Synovial Fluid Google Scholar indexed journals bone edema articles bone edema Research articles bone edema review articles bone edema PubMed articles bone edema PubMed Central articles bone edema 2023 articles bone edema 2024 articles bone edema Scopus articles bone edema impact factor journals bone edema Scopus journals bone edema PubMed journals bone edema medical journals bone edema free journals bone edema best journals bone edema top journals bone edema free medical journals bone edema famous journals bone edema Google Scholar indexed journals
Article Details
1. Introduction
Arthrosis is the most prevalent condition as a cause of disability in individuals over 65 years, affecting up to a third of the population in countries like the USA [1]. Knee arthrosis is a low-grade inflammatory disorder, induced by cytokines. Its origin can be mechanical, genetic, metabolic, among others. The pathology affects the whole joint and is always progressive [2]. The knee is a mechanical organ and includes ligaments, muscles, subchondral bone, and articular cartilage (AC). AC is an avascular and hydrated tissue, close to the Synovial Membrane (SM), which is highly vascularized, innervated and produces Synovial Fluid (SF). The main source of pain in knee osteoarthritis (OA) is synovitis and subchondral bone edema. The cellular elements of SM are an important source of SF components. These components contribute to the unique functional properties of joint surfaces and modulate chondrocyte activity. Two important molecules produced by the cells of the synovial lining are Lubricin and Hyaluronic Acid (HA), which help to protect and maintain the integrity of the surfaces of the articular cartilage [3]. Together, these two molecules reduce friction, providing lubrication to the joint surface. In addition, Lubricin also reduces the pathological deposition of proteins on the joint surface [4]. In the OA scenario or after a joint injury, the concentration and the average molecular weight of HA and the concentration of Lubricin in the SF are altered, which adversely affects the integrity of the cartilage. During OA progression, the SM is also a source of pro-inflammatory and catabolic products, including metalloproteinases and aggrecanases, which contribute to the degradation of the joint matrix. Therefore, changes in SM can result in decreased concentrations of cartilage protection factors and increased production of factors that contribute to cartilage degradation [5-7].
Traditional treatments for knee arthrosis are oral anti-inflammatory drugs and intra-articular injections of corticosteroids, which has frequent side effects and can lead to an acceleration of the decrease in cartilage volume [8-11]. The failure of conservative procedures, especially in severe knee arthrosis, leads to the indication of joint arthroplasty, which may not be indicated for some patients due to clinical conditions. Besides, the level of satisfaction with the results of the joint arthroplasty surgery is low, with dissatisfaction levels reaching more than 50%, in addition to possible complications in the perioperative period [12, 13]. An alternative non-operative intervention is the injection of HA or Platelet Rich Plasma (PRP).
PRP is a natural source of signaling molecules. With the activation of the platelets present in the PRP, the alpha granules are degranulated and Growth Factors (GF) and cytokines are released, modifying the pericellular microenvironment. Some of the most important GFs released by platelets in the PRP are Vascular Endothelial GF (VEGF), Fibroblast GF (FGF), Platelet Derived GF (PDGF), Epidermal GF (EGF), Hepatocyte GF (HGF), Insulin-like GF (IGF-1, IGF-2), Interleukin 8 (IL-8) and matrix metalloproteinases 2 and 9 [14]. Current literature on PRP has shown that the treatment method can provide relief from the pain and inflammation associated with knee arthrosis, making it an effective treatment. However, PRP seems more beneficial for mild or moderate knee arthrosis (Kellgren and Lawrence, stages 2 and 3), compared to more advanced and severe arthrosis (Kellgren and Lawrence stage 4). This may discourage the use of PRP treatment and lead to the indication of other, more aggressive therapeutic methods [12, 15, 16]. On the other hand, HA is a molecule that can bind to synovial and chondral CD44 receptors, inhibiting interleukin and leading to a decline in matrix metalloproteinases 1, 2, 3, 9 and 13, reducing apoptosis and improving pain and joint function, which is why it has also been indicated for the treatment of initial knee arthrosis, with a more discouraging effect for advanced and severe knee arthrosis (KL 4) [17, 18].
The aim of this study is to evaluate the effectiveness of intra-articular injections of PRP associated with HA, two substances with different mechanisms of action, in severe knee OA in elderly patients. By comparing the results of mild and moderate osteoarthritis group (younger) with the severe osteoarthritis group (older), the success rates in improving pain, function and stiffness of the knee are assessed.
2. Material And Methods
2.1 Study Design
This is a prospective, non-randomized study. The study was approved by the Ethical Committee of the Ifor Hospital and can be located on the Plataforma Brasil website by the Certificate of Presentation of Ethical Appreciation (CAAE) number: 63961416.8.0000.5625. Informed consent was given to all thirty-three patients (9 men and 24 women) with a mean age of 61 years (18-80) who underwent treatment and were classified by age, sex and knee arthrosis classification by the Kellgren and Lawrence (KL) score. Mild and moderate (stages 2 and 3), as well as advanced or severe (stage 4) [19] cases received one ultrasound-guided, intra-articular injection weekly, for three weeks, 4 ml of PRP and 2 ml of HA. Clinical evaluations were conducted using the Western Ontario and McMaster Universities (WOMAC) score, both before the intervention and in the follow-up, with an average of 527.9 days [20-22]. Another questionnaire was also applied, to evaluate the patient’s satisfaction regarding the evolution of the pain and the function of the treated knees. Two groups were compared regarding the outcomes, namely group A, formed by patients with mild or moderate knee arthrosis (KL2 and KL3), and group B, formed by patients with advanced or severe knee arthrosis (KL4).
All patients’ comorbidities are mentioned in Table 1. No patients were under anti-inflammatory or anti-platelet medications, before or during the treatment (inclusion criteria).
|
Group A |
Comorbidities |
|
1 |
None |
|
2 |
None |
|
3 |
None |
|
4 |
None |
|
5 |
Systemic arterial hypertension, cholesterol |
|
6 |
Meniscectomy |
|
7 |
Diabetes |
|
8 |
Hypothyroidism, systemic arterial hypertension, cholesterol |
|
9 |
Diabetes, cholesterol |
|
10 |
Diabetes |
|
11 |
None |
|
12 |
Osteoporosis |
|
13 |
None |
|
14 |
None |
|
15 |
None |
|
Group B |
Comorbidities |
|
1 |
None |
|
2 |
Diabetes |
|
3 |
Cholesterol |
|
4 |
Hypothyroidism |
|
5 |
Systemic arterial hypertension, cholesterol, diabetes |
|
6 |
None |
|
7 |
None |
|
8 |
Systemic arterial hypertension, cholesterol |
|
9 |
None |
|
10 |
Hypothyroidism, hyperuricemia, cholesterol, renal insufficiency |
|
11 |
None |
|
12 |
None |
|
13 |
None |
|
14 |
Systemic arterial hypertension |
|
15 |
None |
|
16 |
None |
|
17 |
Systemic arterial hypertension, cholesterol, arrhythmia |
|
18 |
Hypothyroidism |
Table 1: Comorbidities group A and B: Comorbidities of all patients that were included in the study.
2.2 Injury details
The diagnosis was made through anamnesis, physical examination, radiography and magnetic resonance images. All patients have weight-bearing radiographs and magnetic resonance images pre-treatment. Only one patient had a previous surgical treatment, close to the injury site and this procedure was a meniscectomy.
2.3 Intervention
The intervention was an intra-articular knee injection in the superolateral portal, guided by ultrasound. The ultrasound equipment used was a General Electronic Logic 9 (Healthcare, Milwaukee, USA), with a linear 13 MHz transducer. The needle used in the procedure was a 21 G × 1 ¼” (0.80 × 30 mm). An injection of Xylocaine (1% without constrictor vessel) is applied before the HA and PRP. Nonoperative findings were observed.
2.4 Blood collection
To obtain the PRP, 60 ml of peripheral blood was collected in vacuum tubes (BD Vacutainer ACD 8.5 mL) with anticoagulant ACD (citric acid, sodium citrate, dextrose). The whole blood was not exposed to light. Between collection and application, an average of 30 minutes was spent, during this period the sample remained in room temperature.
2.5 Blood characteristics
All samples had a normal blood test, platelet count greater than 150.000, all cells within normal limits and without infectious processes or atypia.
2.6 PRP preparation
The PRP was prepared from blood that was collected in 6 vacuum tubes (BD Vacutainer ACD 8.5 mL), containing anticoagulant ACD. The material collected in the ACD tubes was centrifuged at 1000G, for 10 minutes. After centrifugation, the upper portion (supernatant) was drawn off from the top of the plasma layer and was discarded. The remaining plasma, in the amount of 2 ml, named PRP (liquid format), was collected from just above the top of the red blood cell layer and transferred to a 10ml syringe to be used in the treatment [23]. According to the ACD (yellow top) tube method used by Theodore E et al. [23], the platelet recovery rate, or platelet yield of the method we used is 53%. After processing the PRP, the samples were storage in room temperature with no light exposure. The time between blood drawing and delivery was less than 30 minutes, and the PRP had no activation. All samples of PRP underwent an analysis of platelet, differential leukocyte and red cells.
2.7 Hyaluronic acid
HA was injected in the amount of 2 ml per week, with 10 mg of sodium hyaluronate with a molecular weight of 6 × 106 Daltons.
2.8 Delivery
The delivery point was the superolateral portal of the knee. All patients received a serial intra-articular injection weekly (4 ml of PRP and 2 ml of HA), for three weeks. Without concomitant use of stem cells, cytokines, carrier or scaffold.
2.9 Post-treatment care
- Two-day rest after each injection.
- 15 minutes of cryotherapy four times a day.
- Daily physiotherapy for 6 weeks, hamstring stretching and quadriceps strengthening with concentric and isometric exercise.
- After each session of physiotherapy, patients went through 15 minutes of cryotherapy in the anterior aspect of the knee.
2.10 Statistical analysis
Statistical comparisons between the different experimental groups were performed using the Wilcoxon non-parametric test, for related results and the Mann-Whitney non-parametric test, for unrelated results. Statistical analyses were performed using the GraphPad Prism 8.0 program (GraphPad Software, Inc., San Diego, CA, USA) and the differences were considered significant for a p-value < 0.05.
3. Results
Group A (KL 2 and 3) – After an average follow-up period of 527.9 days, 15 patients (5 men and 10 women), with an average age of 60.1 years, obtained an improvement in the WOMAC score: 57.9% in total; 59.2% in decreasing pain; 58.2% in improving function and 45.7% in improving stiffness (Table 2). All parameters showed significant statistical improvement, with a p-value less than 0.05, when compared to the use of the Wilcoxon non-parametric test (Figure 1). No relevant side effects were observed.
Group B (KL 4) – After the average follow-up period of 527.9 days, 18 patients (3 men and 15 women), with an average age of 67.8 years, obtained an improvement in the WOMAC score: 52.2% in total; 48.9% in decreasing pain; 53.9% in improving function and 47.1% in improving stiffness (Table 3). All parameters showed significant statistical improvement, with a p-value less than 0.05, when compared to the use of the Wilcoxon non-parametric test (Figure 2). No relevant side effects were observed. As for age, the 33 patients had an average age of 61.3 years. Group A (KL 2 and 3) had an average age of 60, 1 (18-74) years and group B (KL 4) 67.8 (51-80) years. Through the statistical analysis based on the Mann-Whitney non-parametric test, a p-value less than 0.05 was obtained, showing a significant difference between the groups, with group B being older (Figure 3).
When comparing the pre-treatment and post-treatment results obtained using the WOMAC questionnaire (groups A and B) through the Mann-Whitney statistical test, no significant difference between the groups (p value > 0.05) was obtained for the total score and its sub-items (pain, function and stiffness). These results show that, despite having a different Kellgren and Lawrence classification, the two groups showed similar improvement result (Figures 4, 5, 6, 7).
The patient satisfaction questionnaire regarding knee pain and function resulted in 67% (0-100%) of average improvement in group A and 64% (0-100%) of average improvement in group B (Tables 2 and 3). Through the Mann-Whitney statistical test, we did not obtain any significant difference between the groups (p value > 0.05), showing that despite having a different Kellgren and Lawrence classification, the two groups show a similar improvement result (Figure 8).
Table 2: Individual WOMAC results group A: Total and subscores of the WOMAC questionnaire, pre-treatment and post-treatment, for patients from group A. Percentage of improvement was assessed in the total score and in its sub-items (pain, function and stiffness). Answer of the free questionnaire regarding the improvement reported by the patient, is also present in the table as a percentage. The average of each score is found on the bottom line.
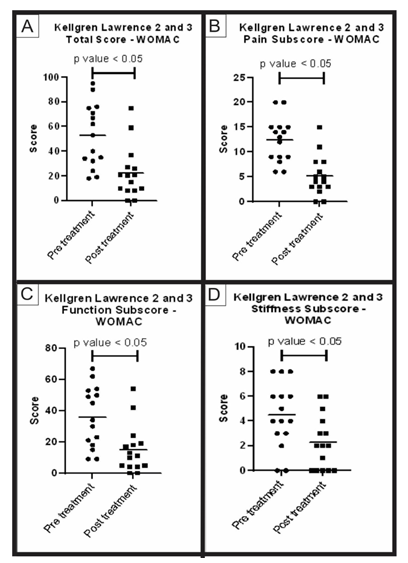
Figure 1: WOMAC score pre-treatment and post-treatment group A: Analysis of the WOMAC questionnaire total and subscores, pre-treatment and post-treatment of group A (KL 2 and 3). The figure presents four graphs with the individual values of the responses of each patient in relation to (A) the total score; (B) the pain sub-item; (C) the function sub-item; and (D) the stiffness sub-item of the WOMAC questionnaire. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses was performed using the Wilcoxon non-parametric test, with a significant difference in all cases, p-value < 0.05.
Table 3: Individual WOMAC results group B: Total and subscores of the WOMAC questionnaire, pre-treatment and post-treatment, for patients from group B. Percentage of improvement was assessed in the total score and in its sub-items (pain, function, and stiffness). Answer of the free questionnaire regarding the improvement reported by the patient, is also present in the table as a percentage. The average of each score is found on the bottom line.
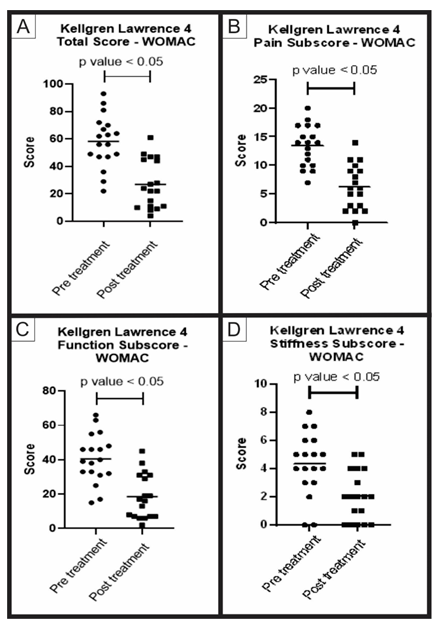
Figure 2: WOMAC score pre-treatment and post-treatment group B: Analysis of the WOMAC questionnaire total and subscores, pre-treatment and post-treatment of group B (KL 4). The figure presents four graphs with the individual values of the responses of each patient in relation to (A) the total score; (B) the pain sub-item; (C) the function sub-item; and (D) the stiffness sub-item of the WOMAC questionnaire. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses was performed using the Wilcoxon non-parametric test, with a significant difference in all cases, p-value < 0.05.
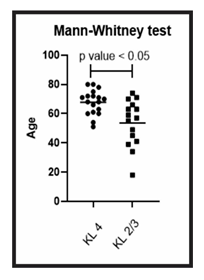
Figure 3: Age comparison between group A and B: Analysis of the ages of patients in group A (KL 2 and 3) and patients in group B (KL 4). The figure presents a graph with the individual values of the age of each patient. The graph also presents a bar with the mean value of the ages. The age comparison was performed using the Mann-Whitney non-parametric test, with a significant difference between the two groups, with group B (KL 4) being older, p-value < 0.05.
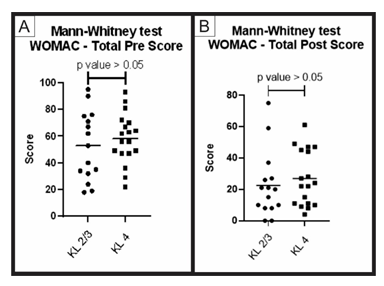
Figure 4: Pre-treatment and post-treatment, total score comparison between group A and B: Analysis of the total scores of the WOMAC questionnaire (pre-treatment and post-treatment) for groups A (KL 2 and 3) and B (KL 4). The figure presents two graphs with the individual values of the responses of each patient, in relation to (A) the total WOMAC score before; and (B) after treatment. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses between the two groups was performed using the Mann-Whitney non-parametric test, with no significant difference between groups, p-value > 0.05.
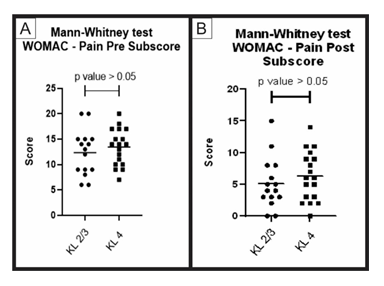
Figure 5: Pre-treatment and post-treatment, pain score comparison between group A and B: Analysis of sub scores referring to pain in the WOMAC questionnaire (pre-treatment and post-treatment) for groups A (KL 2 and 3) and B (KL 4). The figure presents two graphs with the individual values of the responses of each patient, in relation to (A) the pain WOMAC subscore before; and (B) after treatment. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses between the two groups was performed using the Mann-Whitney non-parametric test, with no significant difference between groups, p-value> 0.05.
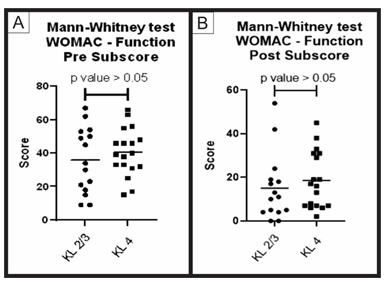
Figure 6: Pre-treatment and post-treatment, function score comparison between group A and B: Analysis of sub scores referring to function in the WOMAC questionnaire (pre-treatment and post-treatment) for groups A (KL 2 and 3) and B (KL 4). The figure presents two graphs with the individual values of the responses of each patient, in relation to (A) the function WOMAC subscore before; and (B) after treatment. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses between the two groups was performed using the Mann-Whitney non-parametric test, with no significant difference between groups, p-value> 0.05.
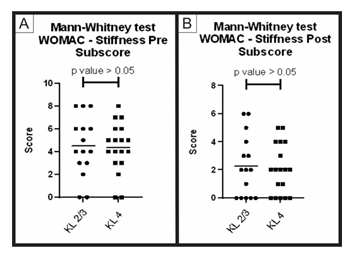
Figure 7: Pre-treatment and post-treatment, stiffness score comparison between group A and B: Analysis of sub scores referring to stiffness in the WOMAC questionnaire (pre-treatment and post-treatment) for groups A (KL 2 and 3) and B (KL 4). The figure presents two graphs with the individual values of the responses of each patient, in relation to (A) the stiffness WOMAC subscore before; and (B) after treatment. The graphs also present a bar with the mean value of the responses. The comparison of pre-treatment and post-treatment responses between the two groups was performed using the Mann-Whitney non-parametric test, with no significant difference between groups, p-value> 0.05.
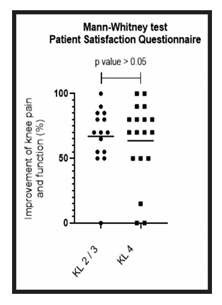
Figure 8: Comparison of patient satisfaction between group A and B: Analysis of the patient satisfaction questionnaire for groups A (KL 2 and 3) and B (KL 4). The figure presents a graph with the individual values of the responses of each patient, in relation to the improvement of knee pain and function. The graph also presents a bar with the mean value of the responses. The comparison was performed using the Mann-Whitney non-parametric test, with no significant difference between groups, p-value > 0.05.
4. Discussion
The aim of this study was to evaluate the effectiveness of intra-articular injections of PRP associated with HA in severe knee OA in elderly patients, comparing the results of mild and moderate osteoarthritis group (younger) with the severe osteoarthritis group (older). The current data showed that the benefits of this treatment in terms of clinically relevant scores improvement were similar and favorable in both groups, within an average follow-up of 527.9 days. Intra-articular injections treatments showed no relevant side effects, these injections have two main objectives in OA: to relieve pain and reduce functional disability. The use of PRP and HA alone for the treatment of pain and function improvement in knee arthrosis, is already known in the medical literature with solid evidence. Literature data indicate that intra-articular administration of HA can restore the viscoelastic properties of SF in the knee joint, with pain relief and improvement of joint mobility [24, 25].
Altman et al. [26], in a review of 17 articles (7 RTCs and 10 Cohort), evaluated the efficacy and safety of pain treatment in knee arthrosis with repeated injections of HA in a 6 to 25-month follow-up and concluded that the outcomes were significant in terms of pain improvement, however only four studies evaluated severe osteoarthritis (KL 4) [24]. Newberry et al. [27], evaluated the use of HA in patients with severe knee arthrosis in 13 studies, at the request of the Analysis and Coverage Group at the Centers for Medicare and Medicare Services (CMS), and concluded that there is no evidence for the use of HA in these cases, with the main restriction for elderly patients.
Intra-articular PRP injections can stimulate the production of endogenous HA and improve clinical scores. Filardo et al. reported that the clinical improvement of intra-articular therapy with PRP depends on time, with an average duration of 9 months, and that better and more lasting results have been achieved in younger patients with lower levels of degeneration [28, 29].
Smith et al. [30], in a randomized double-blind study sanctioned by the US Food and Drug Administration (FDA), evaluated 30 patients with knee OA who did not obtain satisfactory results with other non-operative treatments. These patients received weekly intra-articular injections of PRP or saline for 3 weeks and were evaluated for 1 year. This study revealed that patients who received PRP had a statistically significant improvement in the WOMAC score compared to the baseline, as well as the placebo group, starting at 2 weeks and continuing for 12 months. The results demonstrate that PRP is safe and effective for the treatment of knee OA, but the inclusion criteria selected only patients with mild and moderate knee arthrosis (KL 2 or 3). Bennell et al. [31], in a review on the use of PRP in knee and hip arthrosis, analyzed 25 RTCs and concluded that more severe arthrosis is less responsive to treatment.
In our study, we prospectively followed 33 patients, compared the ages of groups A and B using the Mann-Whitney test, and obtained a p-value less than 0.05, indicating a significant difference between groups, with group B being older (Figure 3).
Although group B is older than group A, the comparison between the total WOMAC score in the pre-treatment showed similar results, with a p-value > 0.05. The same happened with the total score in the post-treatment (Figure 4). The WOMAC score is divided into sub scores for pain, function, and stiffness. When comparing the results of the values obtained in the post-treatment for these sub scores, we found statistical similarity in the results of groups A and B (p-value > 0.05) (Figures 5, 6 and 7). Note that both groups had significant statistical improvement in these pre-treatment and post-treatment scores. Even though pain and function are already assessed on the WOMAC score, we conducted a patient satisfaction questionnaire regarding knee pain and function, so that he could freely answer how satisfied he/she was with the treatment. We obtained 67% (0-100%) improvement in group A and 64% (0-100%) in group B. This questionnaire was not validated but demonstrates a relevant level of satisfaction with the treatment, especially in the group of elder patients with severe arthrosis. Therefore, the treatment based on the association of PRP and HA has the same positive results, both in the younger group with mild and moderate osteoarthritis, as well as in the older group with severe osteoarthritis.
5. Limitations
Although we compared two groups, with statistically significant results, we did not perform a control group with placebo for ethical reasons, because the knowledge of the advantages of using PRP and HA in relation to placebo are already widely known in the literature.
6. Conclusion
The association of PRP and HA in the treatment of knee arthrosis is useful for the improvement of the WOMAC total score, as well as of its subscores, such as pain, function, and stiffness. Similar results were obtained for patients with severe arthrosis (KL 4) and those with mild and moderate arthrosis (KL 2 and 3). As for age, the improvement in all subscores was similar for younger and older patients. The treatment is safe and has no adverse effects.
Conflict of Interest
All authors have disclosed no conflicts of interest.
Acknowledgements
We would like to thank CERT staff for technical assistance. No support was received for conducting or reporting this study.
References
- Lawrence RC, Felson DT, Helmick CG, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58 (2008): 26-35.
- Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol 9 (2013): 485-497.
- Hui AY, McCarty WJ, Masuda K, et al. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med 4 (2012): 15-37.
- Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115 (2005): 622-631.
- Barker SA, Bayyuk SH, Brimacombe JS, et al. Fingerprinting the Hyaluronic Acid Component of Normal and Pathological Synovial Fluids. Clin Chim Acta 8 (1963): 902-909.
- Dahl LB, Dahl IM, Engstrom-Laurent A, et al. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis 44 (1985): 817-822.
- Stafford CT, Niedermeier W, Holley HL, et al. Studies on the Concentration and Intrinsic Viscosity of Hyaluronic Acid in Synovial Fluids of Patients with Rheumatic Diseases. Ann Rheum Dis 23 (1964): 152-157.
- Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 328 (2004): 869.
- Pham T, Maillefert JF, Hudry C, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis. A two-year prospective randomized controlled study. Osteoarthritis Cartilage 12 (2004): 46-55.
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16 (2008): 137-162.
- Ross A, Hauser MD. The Acceleration of Articular Cartilage Degeneration in Osteoarthritis by Nonsteroidal Anti-inflammatory Drugs. Journal of Prolotherapy 1 (2010): 305-322.
- Cole BJ, Karas V, Hussey K, et al. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med 45 (2017): 339-346.
- Castro RLB, Antonio BP, Castro CC, et al. Treatment of residual Pain After Total Knee Arthroplasty. Biomedical Journal of Scientific & Tecchnical Research 22 (2019): 16637-16644.
- Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 8 (2013): 645-658.
- Southworth TM, Naveen NB, Tauro TM, et al. The Use of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis. J Knee Surg 32 (2019): 37-45.
- Drengk A, Zapf A, Stürmer EK, et al. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs 189 (2009): 317-326.
- Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 16 (2015): 321
- Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis 9 (2017): 231-246.
- Kohn MD, Sassoon AA, Fernando ND. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res 474 (2016): 1886-1893.
- Bellamy N. Osteoarthritis: An evaluative index for clinical trials. M.Sc. Thesis. McMaster University, Hamilton, Ontario (1982).
- Bellamy N. Womac osteoarthritis index user guide. Version VII. Australia: Brisbane (2005).
- Fernandes MI. Translation and Validation of the Specific Quality of Life Questionnaire for Osteoarthritis WOMAC (Western Ontario McMaster Universities) for Portuguese Language. Universidade Federal de São Paulo (2002).
- Harrison TE, Bowler J, Levins TN, et al. Platelet yield and yield consistency for six single-spin methods of platelet rich plasma preparation. Platelets 31 (2020): 661-666.
- Giarratana LS, Marelli BM, Crapanzano C, et al. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee 21 (2014): 661-668.
- Guidolin D, Franceschi F. Viscosupplementation with high molecular weight native hyaluronan. Focus on a 1500-2000 KDa fraction (Hyalubrix®). Eur Rev Med Pharmacol Sci 18 (2014): 3326-3338.
- Altman R, Hackel J, Niazi F, et al. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: A systematic review. Semin Arthritis Rheum 48 (2018): 168-175.
- Newberry SJ, Fitzgerald JD, Maglione MA, et al. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee. Rockville (MD): Agency for Healthcare Research and Quality (US) (2015): 23.
- Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 19 (2011): 528-535.
- Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 13 (2012): 229.
- Smith PA. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-blind, Placebo-controlled Clinical Trial. Am J Sports Med 44 (2016): 884-891.
- Bennell KL, Hunter DJ, Paterson KL. Platelet-Rich Plasma for the Management of Hip and Knee Osteoarthritis. Curr Rheumatol Rep 19 (2017): 24.
