Innovative Observation of MDA-MB-231 Cell Vitality in Mice Upon 266-nm Laser Irradiation
Article Information
Ray-Ling Hsiao1*, Ying-Chun Chen2, Chiang-Yun Chen3, Yu-Wei Lin3, Chung-Yi Wu1, 3*
1Program for Cancer Biology and Drug Discovery, China Medical University and Academia Sinica, Taiwan
2Institute of Cellular and Organismic Biology, Academia Sinica, Taiwan
3Genomics Research Center, Academia Sinica, Taiwan
*Corresponding Author: Ray-Ling Hsiao, Program for Cancer Biology and Drug Discovery, China Medical University and Academia Sinica, Taiwan; Chung-Yi Wu, Genomics Research Center, Academia Sinica, Taiwan
Received: 24 October 2021; Accepted: 05 November 2021; Published: 12 November 2021
Citation:
Ray-Ling Hsiao, Ying-Chun Chen, Chiang-Yun Chen, Yu-Wei Lin, Chung-Yi Wu. Innovative Observation of MDA-MB-231 Cell Vitality in Mice Upon 266-nm Laser Irradiation. Journal of Biotechnology and Biomedicine 4 (2021): 159-168.
Share at FacebookAbstract
In this innovative experiment, MDA-MB-231 cells were irradiated with a 266-nm laser for 5s, and then injected into NOD/SCID mice. The survival rate of the experimental mice was significantly reduced compared to that of the control mice (p=0.029), which also indicated the vitality of the experimental cells in vivo. The enhancement effect of a 266-nm laser on cells in xenotransplantation after 5 s of exposure was studied for the first time in this study. The findings of this study provide potential for future live cell therapies.
Keywords
MDA-MB-231 Cell; Laser; Cell Vitality; Absorption Spectrum; Nucleotide
MDA-MB-231 Cell articles MDA-MB-231 Cell Research articles MDA-MB-231 Cell review articles MDA-MB-231 Cell PubMed articles MDA-MB-231 Cell PubMed Central articles MDA-MB-231 Cell 2023 articles MDA-MB-231 Cell 2024 articles MDA-MB-231 Cell Scopus articles MDA-MB-231 Cell impact factor journals MDA-MB-231 Cell Scopus journals MDA-MB-231 Cell PubMed journals MDA-MB-231 Cell medical journals MDA-MB-231 Cell free journals MDA-MB-231 Cell best journals MDA-MB-231 Cell top journals MDA-MB-231 Cell free medical journals MDA-MB-231 Cell famous journals MDA-MB-231 Cell Google Scholar indexed journals Laser articles Laser Research articles Laser review articles Laser PubMed articles Laser PubMed Central articles Laser 2023 articles Laser 2024 articles Laser Scopus articles Laser impact factor journals Laser Scopus journals Laser PubMed journals Laser medical journals Laser free journals Laser best journals Laser top journals Laser free medical journals Laser famous journals Laser Google Scholar indexed journals Cell Vitality articles Cell Vitality Research articles Cell Vitality review articles Cell Vitality PubMed articles Cell Vitality PubMed Central articles Cell Vitality 2023 articles Cell Vitality 2024 articles Cell Vitality Scopus articles Cell Vitality impact factor journals Cell Vitality Scopus journals Cell Vitality PubMed journals Cell Vitality medical journals Cell Vitality free journals Cell Vitality best journals Cell Vitality top journals Cell Vitality free medical journals Cell Vitality famous journals Cell Vitality Google Scholar indexed journals Absorption Spectrum articles Absorption Spectrum Research articles Absorption Spectrum review articles Absorption Spectrum PubMed articles Absorption Spectrum PubMed Central articles Absorption Spectrum 2023 articles Absorption Spectrum 2024 articles Absorption Spectrum Scopus articles Absorption Spectrum impact factor journals Absorption Spectrum Scopus journals Absorption Spectrum PubMed journals Absorption Spectrum medical journals Absorption Spectrum free journals Absorption Spectrum best journals Absorption Spectrum top journals Absorption Spectrum free medical journals Absorption Spectrum famous journals Absorption Spectrum Google Scholar indexed journals Nucleotide articles Nucleotide Research articles Nucleotide review articles Nucleotide PubMed articles Nucleotide PubMed Central articles Nucleotide 2023 articles Nucleotide 2024 articles Nucleotide Scopus articles Nucleotide impact factor journals Nucleotide Scopus journals Nucleotide PubMed journals Nucleotide medical journals Nucleotide free journals Nucleotide best journals Nucleotide top journals Nucleotide free medical journals Nucleotide famous journals Nucleotide Google Scholar indexed journals cell enhancement articles cell enhancement Research articles cell enhancement review articles cell enhancement PubMed articles cell enhancement PubMed Central articles cell enhancement 2023 articles cell enhancement 2024 articles cell enhancement Scopus articles cell enhancement impact factor journals cell enhancement Scopus journals cell enhancement PubMed journals cell enhancement medical journals cell enhancement free journals cell enhancement best journals cell enhancement top journals cell enhancement free medical journals cell enhancement famous journals cell enhancement Google Scholar indexed journals organism articles organism Research articles organism review articles organism PubMed articles organism PubMed Central articles organism 2023 articles organism 2024 articles organism Scopus articles organism impact factor journals organism Scopus journals organism PubMed journals organism medical journals organism free journals organism best journals organism top journals organism free medical journals organism famous journals organism Google Scholar indexed journals stem cell therapy articles stem cell therapy Research articles stem cell therapy review articles stem cell therapy PubMed articles stem cell therapy PubMed Central articles stem cell therapy 2023 articles stem cell therapy 2024 articles stem cell therapy Scopus articles stem cell therapy impact factor journals stem cell therapy Scopus journals stem cell therapy PubMed journals stem cell therapy medical journals stem cell therapy free journals stem cell therapy best journals stem cell therapy top journals stem cell therapy free medical journals stem cell therapy famous journals stem cell therapy Google Scholar indexed journals breast cancer articles breast cancer Research articles breast cancer review articles breast cancer PubMed articles breast cancer PubMed Central articles breast cancer 2023 articles breast cancer 2024 articles breast cancer Scopus articles breast cancer impact factor journals breast cancer Scopus journals breast cancer PubMed journals breast cancer medical journals breast cancer free journals breast cancer best journals breast cancer top journals breast cancer free medical journals breast cancer famous journals breast cancer Google Scholar indexed journals Ribonucleic acid articles Ribonucleic acid Research articles Ribonucleic acid review articles Ribonucleic acid PubMed articles Ribonucleic acid PubMed Central articles Ribonucleic acid 2023 articles Ribonucleic acid 2024 articles Ribonucleic acid Scopus articles Ribonucleic acid impact factor journals Ribonucleic acid Scopus journals Ribonucleic acid PubMed journals Ribonucleic acid medical journals Ribonucleic acid free journals Ribonucleic acid best journals Ribonucleic acid top journals Ribonucleic acid free medical journals Ribonucleic acid famous journals Ribonucleic acid Google Scholar indexed journals
Article Details
Abbreviations:
SDSC: Skin derivative stem cells; Nd: YAG laser- neodymium-doped yttrium aluminum garnet; Nd: Y3Al5O12 Laser; DNA: Deoxyribonucleic acid; CD90: Cluster of Differentiation 90; NOD/SCID mice: Non-Obese Diabetic/Severe Combined Immune Deficiency; PRP: Platelet-rich plasma; RNA: Ribonucleic acid; ATCC: American Type Culture Collection; FBS: Fetal Bovine Serum; EDTA: Ethylenediaminetetraacetic acid
1. Introduction
Our previous studies have reported that light can alter protein expression in SDSCs [1]. We used a Nd:YAG laser at a wavelength of 266-nm; this wavelength falls within the absorption spectrum of nucleotides. Thus, DNA can absorb this energy [1, 2, 3]. This presumably results in high-energy DNA, followed by the regulation of the expression of CD90, a cellular protein [1]. In the current study, we studied the effect on experimental MDA-MB-231 cells in NOD/SCID mice.
This study used the survival time of NOD/SCID mice to reflect the vitality of the inoculated cancer cells. In this study, Matrigel was not used, and the cells were allowed to grow freely inside the mice until natural death of the mice occurred [4]. Several in vivo studies have been conducted on breast cancers [4-6]. These studies focused on the modes of invasion of cancer cells; however, the current study aimed to determine the effect of light on experimental cancer cells. Although enhancement of a cancer cell seems unnecessary, a similar strategy can be used to enhance normal cells or stem cells. A viability experiment using MDA-MB-231 cells was performed in vitro, and it was found that an excimer laser at a wavelength of 248-nm could increase the viability of cancer cells, but the viability marginally decreased after irradiation with Nd:YAG at both 1064 and 532 nm [7]. Although the reason was unknown, we suggest that it could be due to the use of a 248-nm wavelength, which is closer to the 266-nm absorption maxima for nucleotides or DNA [1-3]. Thus, 248-nm could also have a similar energy transferring ability to nucleotides as the 266-nm laser used in our experiments (Figure 1).
Some studies have been published regarding the management of breast cancer using lasers, but most of them used lasers at high wavelengths such as 809-nm to kill MDA-MB-231 cells [8]. These studies were designed to use the thermos effect of infrared laser to kill cancer cells, which requires a longer exposure time (in hours) [9]. However, our study was designed to determine the effect of a 266-nm laser used to irradiate cells for a very short time, such as a few seconds (Figure 1) [1, 2, 3]. Many known therapies use live cells in the clinic, including blood transfusion, PRP injection, and cytotherapy. Cell performance and proliferation capability of long-term human dental pulp stem cells are important factors related to stem cell therapy [10], and enhanced cellular proliferation may be related to the lifespan of an organism [11]. However, to the best of our knowledge, no studies published that explored cell enhancement in vivo using laser irradiation. It is known that all biological processes of an organism follow the central dogma wherein DNA, RNA, and proteins function together to direct life activities [12], The traditional therapeutic concept in medical treatments is to use chemicals to modify the structure of proteins, resulting in altered protein performance and revised cellular activities [13]. In the past decade, therapeutic concepts based on protein molecular mechanisms have progressed, and many molecular recipes have been developed that show promising therapeutic effects in diseases that were earlier intractable [14]. Recently, therapeutic concepts have progressed to the mRNA level, wherein mRNA-based therapies have been used to treat the coronavirus disease 2019 pandemic. However, external mRNA is yet to be introduced into an organism [15]. There could be a promising strategy to use light to transfer energy to nucleotides within the genome. In general, this study proposes a method to induce changes in experimental cells. Further controllable methods based on this concept need to be investigated to confirm the effectiveness of this method in normal cells and in stem cell therapy. This can potentially enhance the anticancer ability of an organism, further aiding future cancer management or cytotherapy.
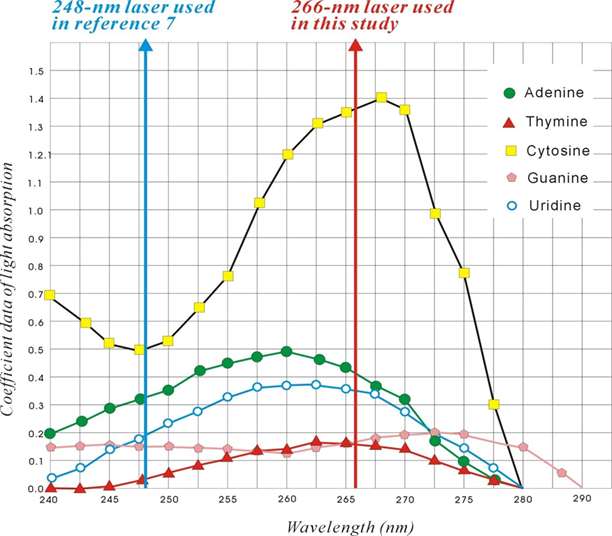
Figure 1: Absorption spectra of nucleotides at a laser wavelength of 266-nm and 248-nm. The figure was revised from reference 1.
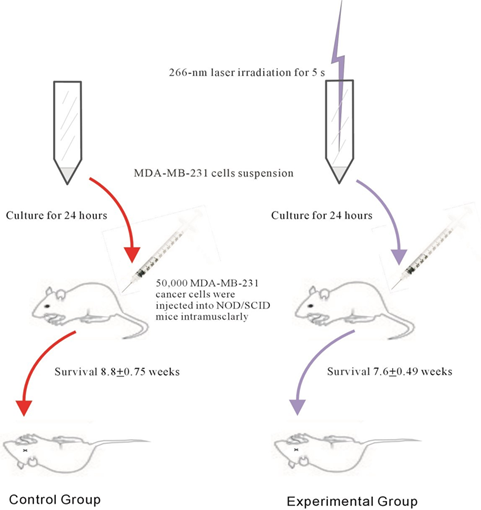
Figure 2: Experimental procedures followed in the current study, and the results obtained. The experimental MDA-MB-231 cells were prepared by irradiation at 266-nm for 5s. The cells were cultured for 24 h and the cell number was calibrated to 50,000 in 50 μl. These were then injected into the left hip of both groups of NOD/SCID mice. Mice were observed weekly until they died naturally. and their survival rate was determined.
2. Materials and Methods
2.1 Laser machine and laser light
The active Q-switched Nd:YAG laser was purchased from Continuum (Santa Clara, CA, USA). The Minilite laser produced horizontally polarized infrared light at 1,064 nm. The laser machine equipped with an optional nonlinear crystal in the beam can double, triple, and quadruple the frequency of this light at 532, 355, and 266 nm, respectively. A prism was used to refract the 266 nm laser directly to the target, and a fluorescent paper was used to guide the direction. The laser beam was directed to the central point of the orifice and the tip of the test tube; thus, the experimental cells at the bottom could be exposed to the laser beam. The laser pulse width was 5 ± 2 ns, the firing frequency was 10 Hz, and the pulse intensity was 30 μJ. The laser intensity was calibrated in μJ using a Micro Joule Meter of PEM 100 Display, which was procured from LTB Lasertechnik (Berlin, Germany).
2.2 Preparing MDA-MB-231 cells
The cell line MDA-MB-231, human breast cancer cells, were purchased from ATCC (cat#HTB-26); these cells are derived from the pleural effusion of human adenocarcinoma, with epithelial and adherent morphology. The cells were grown in L15 medium from ATCC, with 10 % FBS, 1 % non-essential amino acids, 1% sodium pyruvate, and 1% penicillin-streptomycin amphotericin liquid. Following this, the cells were kept at 37°C in an incubator with 5 % CO2 and sub-cultured until the harvested cell suspension reached a concentration of 106 cells/ml. The experimental cells were then irradiated using a 266-nm laser for 5s and cultured for 24 h. The cells were prepared for the experiment by washing out from the flasks using EDTA, neutralized using phosphate-buffered saline, washed three times, and finally 50,000 MDA-MB-231 cancer cells were injected into each mouse.
2.3 Generation NOD/SCID Mice and related procedures
Ten NOD/SCID mice were housed under sterile conditions in microisolator cages and bred in the Animal Room of the Institute of Cellular and Organismic Biology, Academia Sinica, Taiwan. The NOD/SCID mice were cultured and fertilized by the Institute and the age of the mice was 11 weeks. All procedures were performed according to approved protocols. Five mice were randomly divided into control and experimental groups. The cells for the experimental group were pre-treated with 266-nm laser irradiation for 5s, and then both groups of MDA-MB-231 cells were cultured for 24 h before injection. Both groups of mice were injected with 50 μl of a cell suspension of 106 cells/ml (equivalent to 50,000 cells) into their left thigh area intramuscularly. The mice were raised until they died naturally, and their survival times were calculated.
2.4 Longevity and survival time of mice
The birth week of the mice was considered to be zero, and their longevity was calculated a week before death. This is because the mice were checked weekly, and data for the exact birth and death days were not available. The survival week was calculated as zero weeks in the experimental performing week, and the survival weeks were calculated to the week before they were found dead in the cage.
2.5 Statistical analyses
Statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA, USA). The p-values were determined using Student’s t-tests, and results showing p < 0.5 were considered to be statistically significant.
2.6 Data availability
The data generated in this study are available upon request from the corresponding authors R. L. H. (rayling.hsiao@msa.hinet.net) and C.Y.W. (cyiwu@gate. sinica.edu.tw).
3. Results
3.1 Survival of experimental and control mice
The survival percentage of the mice in the experimental group was significantly lower than that of mice in the control group. A statistical difference in the area counts of the two groups was also observed when the survival percentages of the two groups were plotted as a ladder diagram (p=0.029; Figure 3A). The average survival time of the mice in the experimental group was 7.6 ± 0.49 weeks, and the control group was 8.8 ± 0.75 weeks (Table 1). The statistical difference in the survival weeks between the two groups was significant (p=0.028) (Figure 3B).
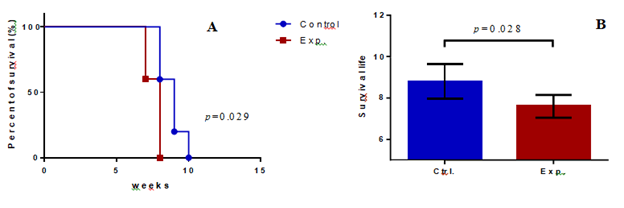
Figure 3: Comparison of the average longevities and survival time of the control and experimental NOD/SCID mice. (A) The survival times of the two groups of mice were plotted as a ladder diagram; X-axis represents survival weeks of the mice, Y-axis represents the percentage of survival, and a statistical difference was observed in the area of the two groups. (p=0.029). (B) Survival time for mice in the control group was 8.8 + 0.75 weeks (n = 5), whereas that in the experimental group was 7.6 + 0.49 weeks. (n = 5) (p= 0.028); the X-axis represents the experimental groups, and the Y-axis represents the survival of the mice.
|
Control group |
Experimental group |
|
|
1 |
8 |
7 |
|
2 |
8 |
7 |
|
3 |
9 |
8 |
|
4 |
9 |
8 |
|
5 |
10 |
8 |
|
Mean: 8.8 ± 0.75 wks |
Mean: 7.6 ± 0.49 wks |
Table 1: Survival time (weeks) of control and experimental mice.
4. Discussion
We previously reported that a cellular protein, CD90, could be regulated using 266-nm laser irradiation [1]. In this study, we determined that MDA-MB-231 cells were affected in vivo by light treatment. We observed that the experimental cells could be enhanced by a 5s exposure to 266-nm laser irradiation. Thus, this study reported the use of light as a tool to enhance the activity of MDA-MB-231 cells in NOD/SCID mice. Laser is a monochromatic source of light that has been used in biological studies for a long time. Many trials have used the thermal effect of lasers to kill cancer cells [7-9]; these successful applications have imparted considerable understanding regarding the role of such laser treatments in cancer therapy. The wavelengths of light used in the previous studies were in the range of 480-880 nm, and were different from the wavelengths used in our studies. The wavelength is very specific to light; moreover, the wavelength between 220 and 280 nm is in the range of the absorption spectrum of nucleotides (Figure 1) [1, 3, 4]. To the best of our knowledge, few studies have used light in this range. Tiina earlier used 266-nm laser light to kill HeLa cancer cells, but found an increase in DNA proliferation [16]. Badruzzamana found that laser at a wavelength of 248-nm could increase MDA-MB-231 cell viability in vitro [7]; however, they did not provide any reasonable explanation. We observed that light, at a wavelength of 220–280 nm, can transfer energy to the nucleotides of DNA (Figure 1) [1, 3, 4]. As shown in Figure 1, the light energy absorption of each nucleotide was quite different for the two lights with wavelengths of 248 and 266 nm: 266 nm was near the peak of cytosine, 248 nm was relatively equal to each nucleotide, and there would have different effects.
In our study, we found that MDA-MB-231 cells with and without a 5s exposure to light irradiation showed a significant difference in the survival time of NOD/SCID mice. Cell viability, proliferation, and performance were routinely examined during in vitro experiments. However, it is not as straightforward to examine cells in vivo, since the cells can be isolated only after sacrificing the host, and it is also difficult to isolate the metastasized cells in the host organism. Some experiments used 1:1 Matrigel mixed with cancer cells to form a relative cellular mass, but this would influence the actual behavior of cancer cells [4]. The current study did not use Matrigel and was designed to evaluate the actual cellular growth status of a living organism. We compared the difference in MDA-MB-231 cells between the two groups of mice; one group of cells received a 266-nm laser irradiation for 5s, whereas the other group (control) did not receive laser irradiation. In our previous study [1], we observed that the appearance and number of the experimental and control SDSC groups were approxi-mately the same after one day culture [1]. However, it is interesting to note that the effect of 5 s of 266-nm laser irradiation revealed a significant difference in survival, which lasted for 2 months until the longevity of mice (Table 1, Figure 3). The experimental MDA-MB-231 cells were found to be superior enhanced and led the NOD/SCID mice early dimes, indicating that the experimental cells were superior to the control cells. In general, the enhancement of cancer cells may involve cellular proliferation, performance, aviability, and/or toxicity, and the experimental cells were superior to the control cells in these fields. Although we did not examine the cells in the live or dead mice, the change in longevity of the mice reflects that the cells were different.
Cancer cells are immortal; hence, they can be inoculated into NOD/SCID mice for xenotransplantation experim-ents. Stem cells or normal tissue cells might have a life span shorter than mice, and thus might fail in xenotransplantation experiments. However, we obser-ved that cancer cells can be enhanced by this experi-ment; similar results can also be obtained for normal tissue or stem cells through the same mechanism; thus, we have provided an innovative method to treat normal cells in an organism, and even influence the longevity of the host organism [11]. Martin-Piedra et al. in 2014 reported that the cell viability and proliferation capability of long-term human dental pulp stem cell cultures is related to cytotherapy [10], and so this experiment may also be promising for dental pulp stem cell therapy and other live cell therapies such as blood transfusion, PRP, and even organ transplantation. In this experiment, MDA-MB-231 cell suspension was injected into both groups of NOD/SCID mice, the cancer cells could proliferate inside the body of the mice, and the cancer cells would terminate the life of the mice when the number and performance of cancer cells exceeded the threshold of mice tolerance. Thus, the survival time of the mice could be used as a monitoring assay for the vitality of the inoculated cancer cells inside the mice.
In this study, the survival time of the control group was 8.8 ± 1.5 weeks, and the experimental group was 7.6 ± 0.49 weeks (Table 1). The comparison between the two groups was statistically significant (p=0.028) (Figure. 3B). In summary, the cancer cells with 5 s 266-nm laser irradiation were superior to those without 266-nm laser irradiation. In our hypothesis, the light energy was absorbed by the nucleotides of DNA during laser irradiation, and DNA was likely to be charged, thus influencing DNA performance. Compared to our previous study on SDSCs [1], we used a longer exposure time than the highest data in the SDSC study (2s), since we assumed that a long exposure time may lead to decreased data sets [1]. We hypothesized that activation of a gene may require a discrepancy between different nucleotides; however, we obtained a relatively homogenous nucleotide-charged condition in this study. Thus, we fixed the exposure time to no longer than 5s; thus, we also avoided exposure for a longer time because it may lead to overcharged and damaged DNA. Initially, we assumed that DNA with high energy may be degraded after one or two generations of cells through mitosis; however, we found that the influence lasted for the final outcome.
In vitro studies help in determining of the cell count; dye staining is used to evaluate cellular viability. However, these data cannot replicate the natural conditions of a living organism. The viability of MDA-MB-231 breast cancer cells was studied in vitro using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay. Here, we designed an innovative method to evaluate breast cancer cells in vivo, which would reflect the real condition of the cells in mice, and that could provide a new model for the experiments of new drug discovery for triple-negative breast cancer [8]. Ten NOD/SCID mice were raised in relatively aseptic circumstances, with a close follow-up on the growth conditions, and excluded any possibility of infection or other diseases. Thus, the vitality of the inoculated cancer cells is a major factor influencing the survival time of the mice. The observed survival time of the mice reflects the true vitality of the cancer cells inside the organism. It appears that higher vitality of breast cancer cells would result in lower mouse survival time.
In this study, we did not use Matrigel to reflect true cancer cell behavior [4]. The MDA-MB-231 cells from human triple-negative breast cancer, could be aggressive and metastasize early [8]. Thus, it would be incorrect to weigh the cancer mass after autopsy, because the cancer cells might have metastasized everywhere inside the mouse body, and isolating every cell from the mice is impossible. However, we raised the mice until their longevity ended naturally; thus, the survival time reflected the vitalities of cancer cells in vivo. Although it is not helpful in the clinic to enhance cancer cells in vivo, this approach reveals the possibility that normal human cells in cytotherapy, such as blood transfusion, PRP therapy, and stem cell therapy, can be enhanced. Moreover, the light treatment can be from the outside, similar to this study, or from the inside by introducing light into the organism. The goal of this experiment was to provide an efficacious method to enhance a cell and to prove its effect in vivo. However, this study was a primitive experiment and needs more investigation for a detailed understanding before potential practice.
Author contributions
R.L.H. conceived the study; R.L.H. and C.Y.W. were the principal investigators; R.L.H. and C.Y.W. directed the overall study design; R.L.H. and Y. C. C. performed the experiments; R.L.H., C.Y.C., L.Y.W, and C.Y.W analyzed the data; R.L.H. wrote the manuscript. All authors discussed and interpreted the results.
Acknowledgments
We thank Institute of Cellular and Organismic Biology, Academia Sinica, Taiwan for the service of raising the mice. We thank Yi-Sheng Wang and Yu-Meng Ou at the Genomic Research Center, Academia Sinica, Taiwan, for technical assistance with laser setup.
Funding
Not Applicable
Conflict of Interest
The authors have no conflicts of interest to disclose, financial or otherwise.
References
- Hsiao RL, Chen Y-C, Huang M-Y, et al. Innovative finding of 266-nm laser regulating CD90 levels in SDSCs. Sci Rep (2021): 13932.
- Voet D, Gratzer WB, Cox RA, et al. Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the far ultraviolet. Biopolymers (1963): 193-285.
- Tataurov AV, You Y, Owczarzy R. Predicting ultraviolet spectrum of single stranded and double stranded deoxyribonucleic acids. Biophys Chem (2008): 66-70.
- Bao L, Matsumura Y, Baban D, et al. Effects of inoculation site and Matrigel on growth and metastasis of human breast cancer cells. Br J Cancer 292 (1994): 228-232.
- Price JE. Xenograft models in immunodeficient animals: I. Nude mice: spontaneous and experimental metastasis models. Methods Mol Med (2001): 205-213.
- Iorns E, Drews-Elger K, Ward TM, et al. A new mouse model for the study of human breast cancer metastasis. Plos One (2012): e47995.
- Badruzzamana A, Bidina N, Pauliena S, et al. The effect of laser irradiation on the viability of human breast cancer cell. J Teknol (2016): 315-320.
- Bozkulaka O, Yamaci RF, Tabakoglua O, et al. Photo-toxic effects of 809-nm diode laser and indocyanine green on MDA-MB231 breast cancer cells. Photodiagnosis Photodyn Ther (2009): 117-121
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin (2011): 250-281.
- Martin-Piedra MA, Garzon I, Oliveira AC, et al. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy (2014): 266-277.
- Li Y, Deeb B, Pendergrass W, et al. Cellular proliferative capacity and life span in small and large dogs. J Gerontol (1996): B403-B408.
- Crick F. Central dogma of molecular biology. Nature (1970): 561-563.
- Spicer CD, Davis BG. Selective chemical protein modification. Nat Commun (2014): 3214740.
- Dimitrov DS. Therapeutic proteins. Methods Mol Biol (2012): 1-26.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines-a new era in vaccinology. Nat Reviews Drug Discov (2018): 261-279.
- Karu T. Can cellular responses to continuous-wave and pulsed UV radiation differ? In: Young AR, editors. Environmental UV Photobiology. Plenum Press (1993): 203-232.
