Inhibition of Methicillin Resistant Staphylococcus aureus (MRSA) Biofilm Proliferation by Chitosan Isolated from Portunus Pelagicus (Linnaeus 1758)
Article Information
Therese Baller Guanzon, Brian Mortejo Denney*, Berley Jane Diaz Aurestila, Angelica Mae Evardo Manliguez, Kristiane Fortun Codera, Joseph Pilones Cabrera, Glydenne Glaire Poncardas Gayam, Joanna Compay Cabriles, Michel Marilouis Lozada
Cebu Institute of Medicine, Ramos Cebu, Philippines
*Corresponding author: Brian Mortejo Denney, Cebu Institute of Medicine, Ramos Cebu, Philippines.
Received: 20 January 2022; Accepted: 28 January 2022; Published: 23 February 2022
Citation: Therese Baller Guanzon, Brian Mortejo Denney, Berley Jane Diaz Aurestila, Angelica Mae Evardo Manliguez, Kristiane Fortun Codera, Joseph Pilones Cabrera, Glydenne Glaire Poncardas Gayam, Joanna Compay Cabriles, Michel Marilouis Lozada. Inhibition of Methicillin Resistant Staphylococcus aureus (MRSA) Biofilm Proliferation by Chitosan Isolated from Portunus pelagicus (Linnaeus 1758). Archives of Microbiology and Immunology 6 (2022): 101-114.
Share at FacebookAbstract
The dramatic increase of microorganisms capable of producing structures which render them drug resistant calls for novel antimicrobial agents. MRSA, a nosocomial drug-resistant pathogen, is capable of adhering to surfaces of medical devices and produce biofilm which render it antibiotic resistant. This study investigated the potential antibiofilm activity of chitosan extracted from Portunus pelagicus (Linnaeus, 1758) against MRSA biofilms. Chitosan was extracted using a 3-step process: demineralization with an acid solution, deproteination with a low concentration of alkaline and deacetylation with concentrated alkaline solution. Preformed biofilms of MRSA were then treated with the extracted chitosan (treatment group) and 0.1M acetic acid (control group). The chitosan extracted had antibiofilm activity against MRSA. Films treated with chitosan showed optical density values of 0.5392±0.0454, 0.4897±0.0638, and 0.4284±0.0500 (control group with 0.6951±0.0659, 0.7373±0.0618, and 0.8064±0.0418) at 24, 48, and 72 hours respectively. On the average, optical density of group treated with 0.1M acetic acid (M=0.746,SE=0.012,95% CI[0.720,0.772]) was significantly higher than group treated with chitosan (M=0.486,SE=0.009,95% CI[0.466,0.506]). The ability of chitosan to inhibit biofilm proliferation was demonstrated as an increase in the calculated percent biofilm inhibition values as incubation time was lengthened with mean percent (%) inhibition of biofilms of 22.06±6.71, 33.27±9.63, and 46.92±5.03 at 24, 48, and 72 hours correspondingly. Viewed on scanning electron microscope, significant morphologic membrane changes were noted at Chitosan treatment group after 72 hours seen as misshapen shrunken deformities in the cell membrane, however, no gross membrane disruption or pore formation were noted.
Keywords
Methicillin-resistant Staphylococcus aureus (MRSA); NaOH; ATR-FTIR; optical density (OD)
Methicillin-resistant Staphylococcus aureus (MRSA) articles; NaOH articles; ATR-FTIR articles; optical density (OD) articles
Article Details
1. Introduction
Recent studies pointed out that there has been dramatic increase in the number of drug-resistant microorganisms which poses a serious health problem worldwide. These microorganisms are known to produce structures that render them antibiotic resistant thus making the treatment process difficult [1]. In the United States, at least 2 million people become infected with these antibiotic resistant pathogens [2]. Among the emerging drug resistant bacteria is Staphylococcus aureus - a normal flora of the body which can be an opportunistic pathogen causing a wide range of diseases ranging from skin infections to severe bacteremia. In the 1990’s, there has been increasing reports of S. aureus being resistant to on hand treatments which led to the development of Methicillin-resistant Staphylococcus aureus (MRSA) [3]. MRSA is one of the many pathogens which causes nosocomial infections since it has the ability to attach to medical devices and produce biofilms - making them antibiotic resistant [4].
According to a study conducted by Zhang et al. (2013), chitosan, a polysaccharide, has the ability to destroy biofilms formed by resistant bacteria [5]. The idea that led to studies about the anti-biofilm formation of chitosan was brought about by the fact that it has been known to have antimicrobial activity against many different types of microorganisms including bacteria, fungi, and yeasts [6]. These rising problems against antibiotic resistance instigated the conduct of this research which will be focused on MRSA - one of the most common causes of nosocomial infection in the Philippine hospital setting. This research paper shows how chitosan extract act as anti-biofilm agent against MRSA biofilm.
2. Materials and Methods
2.1 Sample Size
A sample size of at least 17 replicates per treatment group was determined using R software for two-sample comparison of means designed to detect an effect size of 99%, with 80% certainty and no more than 5% chance of erroneously concluding that a difference exists.
2.2 Collection of Samples
Identification and authentication of the crab samples were done by an expert at the Bureau of Fisheries and Aquatic Resources. Results showed the genus and species of the crab as Portunus pelagicus (Linnaeus, 1758). The crab samples were cooked and the shell wastes were collected for the extraction of chitosan.
2.3 Extraction of Chitosan
Chitosan extraction from crab shells were done in three consecutive processes: demineralization, deproteination, and deacetylation [7].
The shells were washed then sun dried until all the moisture was gone. After which, the shells were crushed into small pieces. Demineralization was done by soaking the crab shells in 9% HCl in the ratio 1:14 for 40 hours at room temperature. After which, deproteinization was done by subjecting the shells to a solution of 5% NaOH in room temperature for 24 hours in a ratio of 1:14. The shells was then sun-dried for 2 days and then grinded. For the deacetylation of chitin to chitosan, the powdered chitin was treated with 70% NaOH solution in a ratio of 1:15 (w/v) for 72 hours at room temperature. The resulting mixture was then drained and filtered. After which, it was oven-dried at 80°C to obtain the powdered chitosan [8].
2.4 Identity Tests for Chitosan
The extract was then characterized with ATR-FTIR spectroscopy analysis. The dried chitosan was grounded into very fine powder with potassium bromide. The dried mixture was pressed under vacuum to form a disc containing the sample. The FTIR spectra was read over a frequency of 600-4,000 cm−1. The characteristic peaks were compared with the spectra of chitosan standard recorded in literature.
2.5 Preparation of Chitosan Stock Solution
The extracted chitosan powder was treated with 0.1M acetic acid to produce a chitosan solution. Then, the chitosan solution was stirred for 8 hours using a magnetic stirrer set at 50°C, 100 rpm to completely dissolve the chitosan. Acetic acid was added drop wise to the chitosan solution to reach a pH range of about 5.6 to 5.8. Then, the chitosan solution was stored in a refrigerator with a temperature of 4°C.
2.5 Preparation of Methicillin-Resistant Staphylococcus aureus Biofilm on Microtiter Plates
MRSA was isolated from an admitted patient in Cebu Velez General Hospital. Biofilms were cultured in a 96-well micro plate. MRSA was suspended in TSB and standardized using McFarland Barium Sulfate Standard. The wells were then filled with one hundred microliter (100μL) of the diluted culture of MRSA and incubated in a 96-well flat-bottomed polystyrene micro plate at 37°C for 48 hours without shaking. A baseline OD was measured at 630 nm using a micro plate reader.
2.6 Application of Chitosan Treatment
One hundred microliter of the chitosan solution was introduced into seventeen microplate wells with the MRSA biofilms. These served as the test groups of the study. One hundred microliter of 0.1M acetic acid was introduced into seventeen microplate wells containing MRSA biofilms which served as the control group of the study. Then, the microplate was incubated for 3 days at 37 °C. OD was measured at 24, 48 and 72 hours post treatment.
2.7 Biofilm Inhibition
For the visualization of adhesion, contents of the well were discarded and the microplate was washed with deionized water. This was done to remove non-adherent cells in the wells. Then, the wells with the microorganisms were treated with 200 µL of ethanol for 15 minutes. The ethanol was discarded and air dried. Two hundred microliter of crystal violet solution was added to the wells for 5 minutes and the excess stain was discarded and the wells were rinsed under water and air dried. Adherence was quantified through measuring the OD at 630 nm with the use of micro plate reader [9].
A control of 1% (v/v) acetic acid for each organism was used. The results obtained from this test were computed as percentage of biofilm formation inhibition by using the formula by Costa et al, 2014: [10]
% biofilm formation inhibition = 100 − (ODassay/ODcontrol) × 100
2.8 Scanning Electron Microscopy
Biofilms were fixed n 2.5% buffered formalin solution then rinsed with phosphate buffered saline, and then dehydrated through an ethanol series [11]. Samples were critical point dried and gold-palladium coated. Examinations were made on Field Emission Scanning Electron Microscope (ZEISS GEMINISEM).
3. Results
3.1 FTIR characterization of extracted chitosan from crab shells
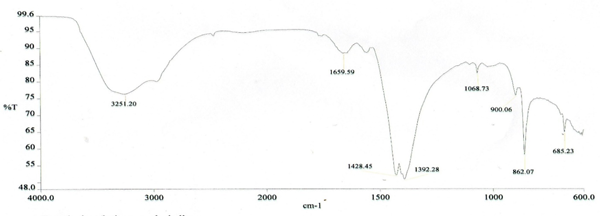
The functional groups of the molecules comprising the extracted chitosan from crab shells were characterized using ATR-FTIR. The IR spectra were recorded on an ATR-FTIR spectrometer in the 4000-600 cm-1 spectral region. Comparing the basic characteristic peaks of the extracted chitosan depicted in Figure 1 to that of IR spectra of standard chitosan reported in literature, confirmed a successful extraction of chitosan from crab shells consequently elucidating the impression that optimal chitosan extraction can be carried out by three consecutive processes: demineralization, deproteination, and deacetylation. A comparison with standard chitosan spectra is shown in Table 1.
Table 1: The FT – IR bands (cm-1) of chitosan isolated from crab (P. pelagicus) shells
|
FUNCTIONAL GROUPS AND VIBRATION MODES |
CHITOSAN FROM CRAB SHELL |
CHITOSAN FROM STANDARD |
|
v(NH2) associated with primary amines and v(OH) associated with pyranose ring |
3251 |
3356 |
|
vas(CH2) in CH2OH group |
2954 |
2921 |
|
v(C=H) in pyranose ring |
2841 |
2871 |
|
v(C=O) in NHCOCH3 group (amide I band) |
1659 |
1652 |
|
v(NH2) in NHCOOCH3 group (amide II band) |
1557 |
1586 |
|
δ(CH2) in CH2OH group |
1428 |
1422 |
|
δs(CH3) in NHCOCH3 group |
1392 |
1380 |
|
vs(C-O-C) (glycosidic linkage) |
1142 |
1149 |
|
vas(C-O-C) (glycosidic linkage) |
1068 |
1062 |
|
v(C-O) in secondary OH group |
1034 |
1021 |
|
v(C-O) in primary OH group |
900 |
985 |
|
Pyranose ring skeletal vibrations |
862 |
892 |
|
δ(NH) out of plane |
685 |
665 |
|
References |
Current study |
Ibitoye et al 2018 [12]; Dilyanah 2010 [13]; Kaya et al 2013 [14] |
3.2 Mean optical density and percent inhibition in each of the control and experimental group 24, 48, to 72-hours post-treatment
Data processing and analysis were carried out with the aid of IBM SPSS version 22. Results were expressed as mean with the standard deviation of the optical density and percent inhibition of the acetic acid and chitosan treatment groups. From Table 2, mean optical density (OD) readings of control (acetic acid) group were 0.6951±0.0659, 0.7373±0.0618, and 0.8064±0.0418 at 24, 48, and 72 hours respectively. Mean OD readings of treatment group with chitosan were 0.5392±0.0454, 0.4897±0.0638, and 0.4284±0.0500 at 24, 48, and 72 hours respectively.
Table 2: Optical Density at 24, 48, and 72 Hours of the Treatment Group
|
Treatment Group and Time |
n |
Mean±SD |
|
0.1M Acetic acid at 24 hours |
17 |
0.6951±0.0659 |
|
0.1M Acetic acid at 48 hours |
17 |
0.7373±0.0618 |
|
0.1M Acetic acid at 72 hours |
17 |
0.8064±0.0418 |
|
Chitosan at 24 hours |
17 |
0.5392±0.0454 |
|
Chitosan at 48 hours |
17 |
0.4897±0.0638 |
|
Chitosan at 72 hours |
17 |
0.4284±0.0500 |
From the OD readings of both treatment and control group, extent of deterrence of biofilm formation expressed as percent (%) biofilm inhibition may be computed [10]. As shown in Table 3, the mean percent (%) inhibitions of biofilms formed were 22.06±6.71, 33.27±9.63, and 46.92±5.03 at 24, 48, and 72 hours correspondingly.
Table 3: Percent Inhibition at 24, 48, and 72 Hours of Chitosan Treatment Group
|
Time |
n |
Mean±SD |
|
24 hours |
17 |
22.06±6.71 |
|
48 hours |
17 |
33.27±9.63 |
|
72 hours |
17 |
46.92±5.03 |
3.3 Mean optical density difference across time in each of the control and experimental group
Factorial repeated-measures ANOVA were used to determine the significant difference in the mean optical density at 24, 48, and 72 hours among the control and experimental treatment groups shown in Table 4. All effects were reported as significant at .
Table 4: Repeated-Measure ANOVA for Optical Density across Time of the Treatment Groups
|
Source |
Type III Sum of Squares |
df |
Mean Square |
F- value |
p-value |
Interpretation |
|
Treatment |
1.730 |
1 |
1.730 |
1064.100 |
0.000 |
Significant |
|
Error |
0.026 |
16 |
0.002 |
|||
|
Time |
0.000 |
2 |
0.000 |
0.120 |
0.887 |
Not significant |
|
Error |
0.043 |
32 |
0.001 |
|||
|
Treatment*Time |
0.212 |
2 |
0.106 |
60.739 |
0.000 |
Significant |
|
Error |
0.056 |
32 |
0.002 |
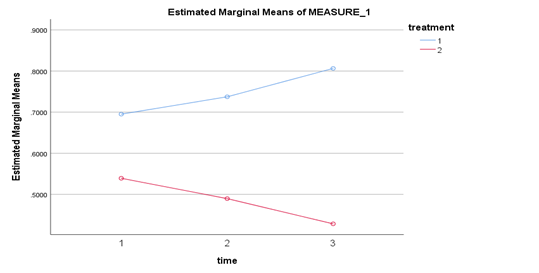
Mauchly's test indicated that sphericity assumption had been met for the main effects of time, . However, sphericity assumption could not be checked for the main effects of treatment. Repeated-Measures ANOVA showed that there was a significant main effect of type of treatment on optical density, . It also showed that there was no significant main effect of time on optical density, . Further, there was a significant interaction effect between the type of treatment and time, . This indicates that time had different effects on optical density depending on which type of treatment was used. Optical density difference between the type of treatment becomes greater as the time increases as shown in Figure 2.
Table 5: Repeated-Measures ANOVA for Optical Density across Time of the 0.1M Acetic Acid
|
Source |
Type III Sum of Squares |
df |
Mean Square |
F- value |
p-value |
Interpretation |
|
Time |
0.107 |
2 |
0.054 |
50.135 |
0.000 |
Significant |
|
Error |
0.034 |
32 |
0.001 |
Mauchly's test indicated that sphericity assumption had been met for the interaction effects of time and 0.1M acetic acid treatment group, . Repeated-Measures ANOVA shown in Table 5 revealed that there was a significant interaction effect of time and 0.1M acetic acid on optical density, .
Table 6: Bonferroni Multiple Comparisons Test Optical Density across Time of the 0.1M Acetic Acid Treatment Group
|
Group |
Mean Difference |
p-value |
Interpretation |
|
24 hours & 48 hours |
0.042 |
0.001 |
Significant |
|
24 hours & 72 hours |
0.111 |
0.000 |
Significant |
|
48 hours & 72 hours |
0.069 |
0.000 |
Significant |
As shown in Table 6, optical density of 0.1M acetic acid treatment group significantly increased from 24 hours to 48 hours by 0.042. Further, optical density of 0.1M acetic acid treatment group significantly increased form 48 hours to 72 hours by 0.069. An average total of 0.111 increased optical density of 0.1M acetic acid treatment group from 24 hours to 72 hours.
Table 7: Repeated-Measures ANOVA for Optical Density across Time of the Chitosan Treatment Group
|
Source |
Type III Sum of Squares |
df |
Mean Square |
F- value |
p-value |
Interpretation |
|
Time |
0.105 |
1.476 |
0.071 |
25.865 |
0.000 |
Significant |
|
Error |
0.065 |
23.615 |
0.003 |
Mauchly's test indicated that sphericity assumption had been violated for the interaction effects of time and chitosan treatment group, . Therefore, degrees of freedom were corrected using Huynh-Feldt estimates of sphericity . As shown in Table 7, Repeated-Measures ANOVA revealed that there was a significant interaction effect of time and chitosan treatment group on optical density, .
Table 8: Bonferroni Multiple Comparisons Test Optical Density across Time of the Chitosan Treatment Group
|
Group |
Mean Difference |
p-value |
Interpretation |
|
24 hours & 48 hours |
0.050 |
0.027 |
Significant |
|
24 hours & 72 hours |
0.111 |
0.000 |
Significant |
|
48 hours & 72 hours |
0.061 |
0.014 |
Significant |
Depicted in Table 8, on the average, optical density of chitosan treatment group significantly decreased from 24 hours to 48hours (M=0.490,SE=0.015,95% CI[0.457,0.522]) by 0.050. Further, optical density of chitosan treatment group significantly decreased form 48 hours to 72 hours by 0.061. An average total of 0.111 increased optical density of chitosan treatment group from 24 hours to 72 hours.
3.4 Mean percent inhibition difference across time in each of the control and experimental group
One-way repeated-measures ANOVA was used to determine the significant difference in the mean percent inhibition treated with chitosan from 24, 48, to 72 hours. All effects were reported as significant at .
Table 9: Repeated-Measures ANOVA for Percent Inhibition of Chitosan Treatment Group
|
Source |
Type III Sum of Squares |
df |
Mean Square |
F- value |
p-value |
Interpretation |
|
Time |
5269.392 |
2 |
2634.696 |
44.015 |
0.000 |
Significant |
|
Error |
1915.500 |
32 |
59.859 |
Presented in Table 9, Mauchly's test indicated that sphericity assumption had been met for the effects of time, . Repeated-Measure ANOVA showed that there was a significant effect of time on percent inhibition, .
Table 10: Bonferroni Multiple Comparisons Test for Percent Inhibition of Chitosan Treatment Group
|
Group |
Mean Difference |
p-value |
Interpretation |
|
24 hours & 48 hours |
11.22 |
0.004 |
Significant |
|
24 hours & 72 hours |
24.86 |
0.000 |
Significant |
|
48 hours & 72 hours |
13.64 |
0.001 |
Significant |
Depicted on Table 10, percent inhibition of chitosan significantly increased from 24 hours to 48 hours by 11.22%. Further, percent inhibition significantly increased form 48 hours to 72 hours (M=46.92, SE=1.22, 95% CI [44.33, 49.50]) by 13.64%. An average total of 24.86% increased of percent inhibition from 24 hours to 72 hours.
3.5 FESEM Evaluation
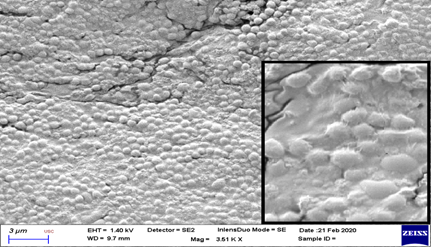
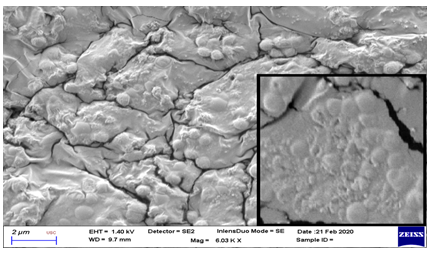
On evaluation with scanning electron microscope, significant membrane changes were noted at Chitosan treatment group after 72 hours (Figure 3 and 4). These were seen as cell surface alterations appearing like a misshapen deformed or shrunken membrane [11]. No pore formation or gross membrane disruption was noted from the electron micrographs.
* (Inset shows close-up view. Note the intact cell membrane with no deformation, pore formation or disruption)
* (Inset shows close-up view. Note the irregularities in the deformed and shrunken cell membrane.)
4. Discussion
FTIR spectral analysis of the chitosan extracted from P. pelagicus shells displayed a broad band at 3251 cm-1 conforming to the stretching or extension vibration of N-H and intermolecular O-H of polysaccharide [12]. The stretching vibrations at absorption range 2954 – 2841 cm-1 indicate that the extract also contains the functional groups methylene in CH2OH and methyne in pyranose ring. A noticeable larger intensity band seen at 1557 cm-1 suggested effective N-deacetylation or removal of acetyl group in the chitosan. Asymmetrical C-H bending vibrations of the CH2 group were evident in the absorption bands at 1428 and 1392 cm-1. Absorption band at wave number 1142 cm-1 confrmed the presence of a bridge – O – stretching vibration. A C-O stretching vibration from secondary hydroxyl group in the alcohol was seen at 1034 cm-1 and 900 cm-1 O stretch from primary hydroxyl group. The band 862 cm-1 was ascribed to the C-H out-of-plane vibration of the ring of monosaccharide. The characteristic spectrum in this study was comparable to the standard chitosan spectra in previous reports [12-14].
These noteworthy findings clearly revealed that chitosan was effectively extracted from crab shells following demineralization with an acid solution – 9% HCl at 1:14 ratio, deproteination with low concentration of alkaline solution – 5% NaOH at 1:14 ratio and deacytelation with concentrated alkaline solution – 70% NaOH at 1:15 ratio. Quantitative determination of the amount of chitosan present in the extract was beyond the scope of this study.
In this study, OD measurements were used to correlate microbial growth. OD value is proportional to bacterial growth/cell number provided necessary instrumentation calibrations were performed prior to measurements [15]. A direct association was evident between OD readings in the control group (0.1M acetic acid) across time. That is, absorbance readings tend to increase as duration of incubation (24, 48, to 72 hours) was lengthened. This implies that increasing incubation period favors continued increase in viable bacterial colony counts provided optimal conditions for growth were met. This undoubtedly confirms findings of previous studies that increasing duration of incubation favored growth and isolation of bacteria promoting cell adhesion leading to biofilm formation [16-19]. Conversely, an opposite trend was apparent between optical density readings in the treatment group (chitosan from crab shells) across time. Specifically, as incubation time was lengthened (from 24, 48, to 72 hours), a pattern of decreasing absorbance was discernable. To be precise, an inverse relationship was apparent. These noteworthy findings strongly supported outcomes of previous studies highlighting the bacterial growth inhibitory properties of chitosan [20-26].
As depicted in table 3, percent inhibition of biofilm formation at 24, 48, and 72 hours of Chitosan treatment group showed a direct correlation with the length of incubation time. So as to say, evidence of the ability of chitosan to inhibit biofilm formation was demonstrated as an increase in the calculated percent biofilm inhibition values as incubation time was lengthened. Although the exact mechanism explaining the bacteriostatic properties of chitosan are not fully understood [24] several models have been proposed which may elucidate as to why a pattern of increasing percent inhibition was evident in this study. Previous studies revealed that electrostatic interactions between the protonated NH3+ group in the polycationic chitosan and negatively charged microbial cell wall polymers (e.g. teichoic acids) induce changes in the cell membrane permeability causing osmotic imbalances in the microbial cell consequently inhibiting growth of the microorganism [24, 27-30]. Moreover, an earlier study revealed significant electrostatic and hydrophobic interactions as well as hydrogen bonds between chitosan and lipids on the bacterial membrane [31] acting as a membrane perturbant [32-34].
Another plausible mechanism was chitosan’s chelating properties. By binding to metals through its amine groups, chitosan cause suppression of spore elements and removing essential ions for microbial growth [24]. Previous studies had proven that chitosan caused down regulation of macromolecular biosynthesis as well as interference in bacterial metabolism of carbohydrates, amino acids, nucleotides and nucleic acids [24, 29], interruption of the electron transport chain [35], and triggered oxidative stress [36].
On the average, optical density of group treated with 0.1M acetic acid (M=0.746, SE=0.012, 95% CI [0.720, 0.772]) was significantly higher than group treated with chitosan (M=0.486, SE=0.009, 95% CI [0.466, 0.506]). These findings clearly revealed the bacteriostatic effect of chitosan on the MRSA isolate, since a lower OD after incubation in a suitable medium signifies bacterial growth inhibition. Furthermore, gram positive bacteria are more markedly inhibited than gram negative bacteria through the electrostatic interaction of chitosan on teichoic acid which appear to extend to the peptidoglycan layer [26, 29, and 37]. Generally, mean optical densities at 24 hours (M=0.617,SE=0.012,95% CI[0.592,0.643]) 48 hours (M=0.614,SE=0.012,95% CI[0.588,0.640]) and 72 hours (M=0.617,SE=0.010,95% CI[0.596,0.639]) were the same. Direct correlation of incubation time with OD values as depicted in Figure 2 showed an increasing OD values in the control group as incubation time was lengthened. On the other hand, an inverse relationship was evident with decreasing OD values in the treatment group as incubation time was lengthened. As cited in previous studies, duration of incubation considerably affects growth of microorganism and biofilm formation [16-19].
Repeated-Measure ANOVA showed that there was a significant effect of time on percent inhibition, . These findings supported the claims of previous studies that chitosan has inhibitory effect causing reduction of viable bacterial cell growths [20-26, 28, 29, 38-40]. Chitosan being polycationic in nature was known to exhibit this bacteriostatic effect via interaction with negatively charged particles in the bacterial cell membrane [24, 28, 29, and 41]. By its membrane perturbing action, chitosan acts to disrupt the integrity of the inner and outer membrane of the bacteria thus increasing permeability and loss of cell membrane barrier function [32-34].
Viewed on scanning electron microscope, significant morphologic membrane changes were noted at Chitosan treatment group after 72 hours. These were seen as cell surface alterations appearing like a misshapen deformed or shrunken membrane [11]. One study showed that chitosan can cause extensive cell surface alterations by binding to the membrane and disrupting its integrity [32]. A similar result obtained from a previous study suggested that this shrunken membrane signified leakage of water and ions from the cell [29].
5. Conclusion
In a nutshell, extracted chitosan was able to demonstrate antibiofilm activity against Methicillin Resistant Staphylococcus aureus. This was supported by a decreased OD values and increased percent inhibition of biofilms treated with chitosan after 24, 48, and 72 hours of incubation. Furthermore, morphological changes noted during SEM evaluation supports previous literature findings that chitosan acts on membrane of bacterial cells to promote changes causing disruption and eventual loss of cell membrane barrier function.
Availability of Data and Materials
All datasets supporting the conclusion of this study are included within the article. A raw data may be provided by the corresponding author upon request.
Funding
None
Ethics Declarations
Ethics approval and Consent to Participate: Ethical Clearance was procured from the Ethical and Review Board of Cebu Institute of Medicine-Cebu Velez General Hospital in Cebu, Philippines.
Consent for publication: Not Applicable
Competing interest: The authors declare that they have no competing interests
Acknowledgement
The researchers would like to express their greatest appreciation to Cebu Institute of Medicine Biochemistry Department for granting us the permission to use the laboratory rooms to conduct this research. To Mr. Jonie Yee and Ms. Hannah Padayao of USC-TC Department of Biology for their aid and supervision during the treatment of preformed biofilm samples. And to Mr. Richard Capuyan for his input on the statistical analysis of the data.
References
- Todar K. Bacterial Mechanisms of Antibiotic Resistance. Todar’s Online Textbook of Bacteriology (2012).
- Centers for Disease Control and Prevention. Antibiotic / Antimicrobial Resistance (AR / AMR) (2013).
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7 (2009): 629-41.
- Otto M. Staphylococcal Biofilms. Current Topics in Microbiology and Immunology. 2008; 322:207-228.
- Zhang A, Mu H, Zhang W, Cui G, Zhu J, Duan J. Chitosan Coupling Makes Microbial Biofilms Susceptible to Antibiotics. Scientific Reports 3 (2013): 1-7.
- Dragland IS, Rukke HV, Stenhagen I, Lönn-Stensrud J, Kopperud, HM. Antibacterial and Antibiofilm Effect of Low Viscosity Chitosan against Staphylococcus epidermidis. International Journal of Microbiology (2016).
- Sujeetha M, Sharmila S, Jayanthi J, Ragunathan MG. Quantitative and Qualitative Analysis of Chitin and Chitosan from the Shell of the Mud Crab, Scylla Serrata (Forskal, 1775). International Journal of Pharmacy and Therapeutics 6 (2015): 69-72.
- Hussain MR, Iman M, Maji TK. Determination of Degree of Deacetylation of Chitosan and Their effect on the Release Behavior of Essential Oil from Chitosan and Chitosan-Gelatin Complex Microcapsules. Téc. Ing. Univ. Zulia 37 (2014): 69-77.
- Van Toan, Nguyen & Hanh Tran, Thi. Application of chitosan solutions for rice production in Vietnam. African Journal of Biotechnology 12 (2013): 382-384.
- Costa E, Silva S, Tavaria F, Pintado M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens 3 (2014): 908-919.
- Brian Mortejo Denney. Production of Rhamnolipidic biosurfactant by Pseudomonas libanensis strain IHB 17501 and its antibacterial and cytolytic effect on Streptococcus pneumoniae ATCC 49619. Int. Res. J. Pharm 10 (2019): 32-36
- Ibitoye, Emmanuel & Lokman IH & Mohd Noor, Mohd Hezmee & Goh, Yong & Abu Bakar Zuki & Jimoh, Akib. Extraction and Physicochemical Characterization of Chitin and Chitosan Isolated from House Cricket. Biomedical Materials 13 (2018).
- Zvezdova, Dilyana. Synthesis and characterization of chitosan from marine sources in Black Sea. Annual Proceedings 49 (2010): 65-69.
- Kaya M, Tozak KÖ, Baran T, Sezen G, Sargin I. Natural porous and nano fiber chitin structure from Gammarus argaeus (Gammaridae Crustacea). EXCLI J 12 (2013): 503-10.
- Stevenson K, McVey AF, Clark IBN, Swain PS, Pilizota T. General calibration of microbial growth in microplate readers. Sci Rep 6 (2016): 38828.
- Davis KE, Joseph SJ, Janssen PH. Effects of Growth Medium, Inoculum Size, and Incubation Time on Culturability and Isolation of Soil Bacteria. Appl Environ Microbiol 71 (2005): 826–34.
- Donlan RM. Biofilms: Microbial Life on Surfaces. Emerg Infect Dis 8 (2002): 881–90.
- Khelissa SO, Jama C, Abdallah M, Boukherroub R, Faille C, Chihib NE. Effect of incubatiaon duration, growth temperature, and abiotic surface type on cell surface properties, adhesion and pathogenicity of biofilm-detached Staphylococcus aureus cells. AMB Express 7 (2017): 191.
- Singh AK, Prakash P, Achra A, Singh GP, Das A, Singh RK. Standardization and Classification ofIn vitroBiofilm Formation by Clinical Isolates ofStaphylococcus aureus. J Glob Infect Dis 9 (2017): 93-101.
- Chen C-Y, Chung Y-C. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J Appl Oral Sci 20 (2012): 620–7.
- Coma V, Deschamps A, Martial-Gros A. Bioactive Packaging Materials from Edible Chitosan Polymer—Antimicrobial Activity Assessment on Dairy-Related Contaminants. J Food Sci 68 (2003): 2788–92.
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry 114 (2009): 1173–82.
- Eaton P, Fernandes JC, Pereira E, Pintado ME, Xavier Malcata F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108 (2008): 1128–34.
- Goy RC, Britto Dde, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros: Ciência e Tecnologia 19 (2009): 241–7.
- Kawakita ERH, Ré ACS, Peixoto MPG, Ferreira MP, Ricomini-Filho AP, Freitas O, et.al. Effect of Chitosan Dispersion and Microparticles on OlderStreptococcus mutansBiofilms. Molecules 24 (2019): 1808.
- No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74 (2002): 65-72.
- Lim JH, Song S-H, Park H-S, Lee JR, Lee S-M. Spontaneous detachment of Streptococcus mutans biofilm by synergistic effect between zwitterion and sugar alcohol. Sci Rep (2017).
- Muzzarelli R, Tarsi R, Filippini O, Giovanetti E, Biagini G, Varaldo PE. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother 34 (1990): 2019-23.
- Raafat D, von Bargen K, Haas A, Sahl HG. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74 (2008): 3764-73.
- Szymanska E, Winnicka K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar Drugs 13 (2015): 1819-46.
- Wydro P, Krajewska B, Hac-Wydro K. Chitosan as a lipid binder: a langmuir monolayer study of chitosan-lipid interactions. Biomacromolecules 8 (2007): 2611-7.
- Helander IM, Nurmiaho-Lassila E-L, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol 71 (2001): 235–44.
- Je J-Y, Kim S-K. Chitosan Derivatives Killed Bacteria by Disrupting the Outer and Inner Membrane. J Agric Food Chem 54 (2006): 6629–33.
- Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot Cell 4 (2005): 703-15.
- Kohler C, von Eiff C, Peters G, Proctor RA, Hecker M, Engelmann S. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J Bacteriol 185 (2003): 6928-37.
- Chang W, Small DA, Toghrol F, Bentley WE. Global Transcriptome Analysis of Staphylococcus aureus Response to Hydrogen Peroxide. J Bacteriol 188 (2006): 1648–59.
- Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274 (1999): 8405-10.
- Ardila N, Daigle F, Heuzey M-C, Ajji A. Effect of Chitosan Physical Form on Its Antibacterial Activity Against Pathogenic Bacteria. J Food Sci 82 (2017): 679–86.
- Jeon Y. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr Polym 44 (2001): 71–6.
- Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4 (2003): 1457-65.
- Vollmer W, Höltje JV. The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J Bacteriol 186 (2004): 5978-87.