Incidence, Severity and Reversibility of Acute Kidney Injury after Elective Hip and Knee Arthroplasty in Patients Receiving Celecoxib Perioperatively as One of the Standard Multimodal Analgesic Protocols
Article Information
Manson Tak Hei Chan1, Timmy Chi Wing Chan2*, Henry Chi Yeung Mak1, Will Shing Him Chan1, Stanley Sau Ching Wong2, Vincent Kai Chung Wong3, Lewis Ping Keung Chan4, Chi Wai Cheung2
1Department of Anaesthesia, Pain and Perioperative Medicine, Queen Mary Hospital, Hong Kong, China
2Department of Anaesthesiology, The University of Hong Kong, Hong Kong, China
3Department of Pharmacy, Queen Mary Hospital, Hong Kong, China
4Department of Orthopaedics and Traumatology, The University of Hong Kong, Hong Kong, China
*Corresponding Author: Timmy Chi Wing Chan; F2, Main Block, Anaesthesia, Pain and Perioperative Medicine, Queen Mary Hospital, 102 Pok Fu Lam Road, Hong Kong, China.
Received: 23 February 2023; Accepted: 10 April 2023; Published: 25 April 2023
Citation:
Manson Tak Hei Chan, Timmy Chi Wing Chan, Henry Chi Yeung Mak, Will Shing Him Chan, Stanley Sau Ching Wong, Vincent Kai Chung Wong, Lewis Ping Keung Chan, Chi Wai Cheung. Incidence, Severity and Reversibility of Acute Kidney Injury after Elective Hip and Knee Arthroplasty in Patients Receiving Celecoxib Perioperatively as One of the Standard Multimodal Analgesic Protocols. Journal of Orthopedics and Sports Medicine. 5 (2023): 199-206.
Share at FacebookAbstract
Background: Acute Kidney Injury (AKI) may complicate up to 10% of primary lower-extremity total joint arthroplasties. However, none of these previous studies evaluated the duration and reversibility of AKI. Moreover, none specifically evaluated the impact of perioperative celecoxib on the incidence and severity of AKI, especially for patients with preexisting renal impairment. This study was to retrospectively review the incidence, severity and duration of AKI with short term perioperative celecoxib. We also aimed to evaluate the impact of perioperative celecoxib on the incidence and severity of AKI in patients with and without preexisting renal impairment.
Methods: We retrospectively reviewed 1077 patients at Queen Mary Hospital, Hong Kong, from January 2018 to December 2021. Data were retrieved from the computerized medical records system.
Results: One hundred out of 1077 patients (9.3%) had postoperative AKI. Eight hundred eighty-eight patients (82.5%) were prescribed perioperative celecoxib, while 189 patients (17.5%) were not.
The overall incidence of AKI in those taking perioperative celecoxib was 9.2%, while it was 9.5% in those not taking perioperative celecoxib. There was no statistically significant difference. There was no association between perioperative celecoxib and postoperative AKI.
Among those who received perioperative celecoxib, the overall incidence of postoperative AKI in those with and without preexisting renal impairment (normal renal function test) was 9.3% and 9.2%, respectively. This was not statistically significant. The duration of AKI was 4 days for both groups. In both groups, most AKI cases were stage 1.
Conclusions: Short term perioperative celecoxib probably had no additional AKI risk even in patients with preexisting renal impairment.
Keywords
Acute Kidney Injury; Perioperative celecoxib; Renal impairment
Kidney injury articles Kidney injury Research articles Kidney injury review articles Kidney injury PubMed articles Kidney injury PubMed Central articles Kidney injury 2023 articles Kidney injury 2024 articles Kidney injury Scopus articles Kidney injury impact factor journals Kidney injury Scopus journals Kidney injury PubMed journals Kidney injury medical journals Kidney injury free journals Kidney injury best journals Kidney injury top journals Kidney injury free medical journals Kidney injury famous journals Kidney injury Google Scholar indexed journals Hip articles Hip Research articles Hip review articles Hip PubMed articles Hip PubMed Central articles Hip 2023 articles Hip 2024 articles Hip Scopus articles Hip impact factor journals Hip Scopus journals Hip PubMed journals Hip medical journals Hip free journals Hip best journals Hip top journals Hip free medical journals Hip famous journals Hip Google Scholar indexed journals Knee arthroplasty articles Knee arthroplasty Research articles Knee arthroplasty review articles Knee arthroplasty PubMed articles Knee arthroplasty PubMed Central articles Knee arthroplasty 2023 articles Knee arthroplasty 2024 articles Knee arthroplasty Scopus articles Knee arthroplasty impact factor journals Knee arthroplasty Scopus journals Knee arthroplasty PubMed journals Knee arthroplasty medical journals Knee arthroplasty free journals Knee arthroplasty best journals Knee arthroplasty top journals Knee arthroplasty free medical journals Knee arthroplasty famous journals Knee arthroplasty Google Scholar indexed journals Osteoarthritis articles Osteoarthritis Research articles Osteoarthritis review articles Osteoarthritis PubMed articles Osteoarthritis PubMed Central articles Osteoarthritis 2023 articles Osteoarthritis 2024 articles Osteoarthritis Scopus articles Osteoarthritis impact factor journals Osteoarthritis Scopus journals Osteoarthritis PubMed journals Osteoarthritis medical journals Osteoarthritis free journals Osteoarthritis best journals Osteoarthritis top journals Osteoarthritis free medical journals Osteoarthritis famous journals Osteoarthritis Google Scholar indexed journals Joint disease articles Joint disease Research articles Joint disease review articles Joint disease PubMed articles Joint disease PubMed Central articles Joint disease 2023 articles Joint disease 2024 articles Joint disease Scopus articles Joint disease impact factor journals Joint disease Scopus journals Joint disease PubMed journals Joint disease medical journals Joint disease free journals Joint disease best journals Joint disease top journals Joint disease free medical journals Joint disease famous journals Joint disease Google Scholar indexed journals Rehabilitative surgery articles Rehabilitative surgery Research articles Rehabilitative surgery review articles Rehabilitative surgery PubMed articles Rehabilitative surgery PubMed Central articles Rehabilitative surgery 2023 articles Rehabilitative surgery 2024 articles Rehabilitative surgery Scopus articles Rehabilitative surgery impact factor journals Rehabilitative surgery Scopus journals Rehabilitative surgery PubMed journals Rehabilitative surgery medical journals Rehabilitative surgery free journals Rehabilitative surgery best journals Rehabilitative surgery top journals Rehabilitative surgery free medical journals Rehabilitative surgery famous journals Rehabilitative surgery Google Scholar indexed journals Physiotherapists articles Physiotherapists Research articles Physiotherapists review articles Physiotherapists PubMed articles Physiotherapists PubMed Central articles Physiotherapists 2023 articles Physiotherapists 2024 articles Physiotherapists Scopus articles Physiotherapists impact factor journals Physiotherapists Scopus journals Physiotherapists PubMed journals Physiotherapists medical journals Physiotherapists free journals Physiotherapists best journals Physiotherapists top journals Physiotherapists free medical journals Physiotherapists famous journals Physiotherapists Google Scholar indexed journals Myocardial ischemia articles Myocardial ischemia Research articles Myocardial ischemia review articles Myocardial ischemia PubMed articles Myocardial ischemia PubMed Central articles Myocardial ischemia 2023 articles Myocardial ischemia 2024 articles Myocardial ischemia Scopus articles Myocardial ischemia impact factor journals Myocardial ischemia Scopus journals Myocardial ischemia PubMed journals Myocardial ischemia medical journals Myocardial ischemia free journals Myocardial ischemia best journals Myocardial ischemia top journals Myocardial ischemia free medical journals Myocardial ischemia famous journals Myocardial ischemia Google Scholar indexed journals Chronic pain articles Chronic pain Research articles Chronic pain review articles Chronic pain PubMed articles Chronic pain PubMed Central articles Chronic pain 2023 articles Chronic pain 2024 articles Chronic pain Scopus articles Chronic pain impact factor journals Chronic pain Scopus journals Chronic pain PubMed journals Chronic pain medical journals Chronic pain free journals Chronic pain best journals Chronic pain top journals Chronic pain free medical journals Chronic pain famous journals Chronic pain Google Scholar indexed journals Nephrotoxic drugs articles Nephrotoxic drugs Research articles Nephrotoxic drugs review articles Nephrotoxic drugs PubMed articles Nephrotoxic drugs PubMed Central articles Nephrotoxic drugs 2023 articles Nephrotoxic drugs 2024 articles Nephrotoxic drugs Scopus articles Nephrotoxic drugs impact factor journals Nephrotoxic drugs Scopus journals Nephrotoxic drugs PubMed journals Nephrotoxic drugs medical journals Nephrotoxic drugs free journals Nephrotoxic drugs best journals Nephrotoxic drugs top journals Nephrotoxic drugs free medical journals Nephrotoxic drugs famous journals Nephrotoxic drugs Google Scholar indexed journals Renal articles Renal Research articles Renal review articles Renal PubMed articles Renal PubMed Central articles Renal 2023 articles Renal 2024 articles Renal Scopus articles Renal impact factor journals Renal Scopus journals Renal PubMed journals Renal medical journals Renal free journals Renal best journals Renal top journals Renal free medical journals Renal famous journals Renal Google Scholar indexed journals Celecoxib articles Celecoxib Research articles Celecoxib review articles Celecoxib PubMed articles Celecoxib PubMed Central articles Celecoxib 2023 articles Celecoxib 2024 articles Celecoxib Scopus articles Celecoxib impact factor journals Celecoxib Scopus journals Celecoxib PubMed journals Celecoxib medical journals Celecoxib free journals Celecoxib best journals Celecoxib top journals Celecoxib free medical journals Celecoxib famous journals Celecoxib Google Scholar indexed journals Body mass index articles Body mass index Research articles Body mass index review articles Body mass index PubMed articles Body mass index PubMed Central articles Body mass index 2023 articles Body mass index 2024 articles Body mass index Scopus articles Body mass index impact factor journals Body mass index Scopus journals Body mass index PubMed journals Body mass index medical journals Body mass index free journals Body mass index best journals Body mass index top journals Body mass index free medical journals Body mass index famous journals Body mass index Google Scholar indexed journals Hypertension articles Hypertension Research articles Hypertension review articles Hypertension PubMed articles Hypertension PubMed Central articles Hypertension 2023 articles Hypertension 2024 articles Hypertension Scopus articles Hypertension impact factor journals Hypertension Scopus journals Hypertension PubMed journals Hypertension medical journals Hypertension free journals Hypertension best journals Hypertension top journals Hypertension free medical journals Hypertension famous journals Hypertension Google Scholar indexed journals Intraoperative fluids articles Intraoperative fluids Research articles Intraoperative fluids review articles Intraoperative fluids PubMed articles Intraoperative fluids PubMed Central articles Intraoperative fluids 2023 articles Intraoperative fluids 2024 articles Intraoperative fluids Scopus articles Intraoperative fluids impact factor journals Intraoperative fluids Scopus journals Intraoperative fluids PubMed journals Intraoperative fluids medical journals Intraoperative fluids free journals Intraoperative fluids best journals Intraoperative fluids top journals Intraoperative fluids free medical journals Intraoperative fluids famous journals Intraoperative fluids Google Scholar indexed journals
Article Details
List of Abbreviations:
ACEIs: Angiotensin Converting Enzyme Inhibitors; AKI: Acute kidney injury; AKIN: Acute Kidney Injury Network; ARBs: Angiotensin Receptor Blockers; ASA: American Society of Anesthesiologists; COX-2: Cyclooxygenase-2; ERAS: Enhanced Recovery After Surgery; LIA: Local Infiltration of Anesthetics; NSAIDs: Non-Steroid Anti-Inflammatory Drugs; OA: Osteoarthritis; sCr: Serum creatinine; RFT: Renal Function Test
Trial Registration:
ClinicalTrials.gov registration number NCT05595694
1. Introduction
Osteoarthritis (OA) is the most common joint disease, affecting more than 240 million people worldwide, with more than 32 million in the US. Osteoarthritis is the most frequent cause of limited activity in adults [1]. Hip and knee arthroplasty are common orthopedic procedures used to treat patients with end-stage knee arthritis. The demands for these procedures are increasing with time. Hip and knee arthroplasty is a rehabilitative surgery aimed at accelerating patient ambulation and reducing the length of hospital stay. To facilitate this process, multidisciplinary Enhanced Recovery After Surgery (ERAS) programs are implemented [2]. This involves surgeons, anesthetists, physiotherapists and nurses who follow an integrated care pathway to facilitate early patient mobilization, thus leading to early hospital discharge. The program is implemented to reduce the hospital length of stay, which can reduce the risks of complications and mortality [3,4]. Hip and knee arthroplasty is associated with significant perioperative pain, which can adversely affect recovery by increasing the risk of complications, length of stay, and cost [5]. Severe perioperative pain is associated with an increased risk of infection, myocardial ischemia, respiratory complications and the development of chronic pain [6]. Therefore, effective multimodal analgesia is an essential component in the ERAS program. It combines different oral analgesics to limit opioid use and its related side effects. Cyclooxygenase-2 (COX-2) inhibitors, such as celecoxib, have been shown to relieve pain and reduce opioid use after hip and knee arthroplasty. It is therefore recommended to be used routinely [7,8]. However, its potential nephrotoxic property has led to its judicious use, especially in patients with preoperative chronic renal impairment.
Postoperative Acute Kidney Injury (AKI) is an independent risk factor for mortality, cardiovascular complications, health care utilization and hospitalization [9-12]. Acute postoperative kidney injury was significantly correlated with increased length of hospital stay [10]. AKI may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure, including placement of an antibiotic-loaded cement spacer in the first stage [10,13-22]. None of the above studies showed the reversibility of AKI in the immediate postoperative period following hip and knee arthroplasty. Moreover, none of the above studies showed a specific evaluation of the impact of perioperative celecoxib as part of routine standard multimodal analgesic protocols on the incidence and severity of AKI, especially for patients with preexisting renal impairment.
Therefore, the aim of this study was to retrospectively review the incidence, severity and duration as well as the possible reversibility of AKI following elective hip and knee arthroplasty with perioperative celecoxib as part of the standard multimodal analgesic protocols. We also aimed to specifically evaluate the impact of perioperative celecoxib on the incidence and severity of AKI in patients with and without preexisting renal impairment.
2. Materials and Methods
This retrospective review was approved by the local research ethics committee and the requirement for informed consent was waived (UW22-230). We performed a retrospective review of 1077 patients in our database who underwent elective hip and knee arthroplasty at Queen Mary Hospital, Hong Kong, from January 2018 to December 2021. Relevant data were retrieved from the computerized medical records system. Patients who were scheduled to undergo hip and knee arthroplasty were routinely assessed by anesthetists in a preoperative assessment clinic. They were enrolled in an ERAS program, and a multimodal analgesic protocol was started. Oral analgesics were prescribed, including paracetamol 1 g three times per day for 1 week, pregabalin 50 mg or 75 mg at night for 1 week, depending on body weight, and oxycodone sustained release 5 mg twice per day for 2 days. If the patient’s preoperative renal serum creatinine was < 200 μmol/L, 200 mg celecoxib was administered at night immediately before operation day and for 5 days postoperatively. Local Infiltration of Anesthetics (LIA) using 300 mg ropivacaine with 30 mg ketorolac was performed intraoperatively for knee arthroplasty. Serum creatinine level was measured at hospital admission and daily for the first 2 postoperative days. AKI was defined using Acute Kidney Injury Network (AKIN) classification: Stage 1 = sCr increase greater than or equal to 26.4 mmol/L or an increase of 1.5-2.0 times from baseline. Stage 2 = sCr increase 2.0-3.0 times from baseline. Stage 3 = sCr increase >3.0 times or requiring dialysis. To assess the severity and duration of postoperative AKI, serial renal function tests were performed for the first 2 postoperative days and were further performed if there was AKI in postoperative day 2 until the baseline renal function test result was achieved. Celecoxib was stopped and adequate fluid hydration was initiated when there was AKI of Stage 2 using AKIN classification. Patients were discharged once they met the criteria for discharge according to the ERAS program protocol and without increasing serum creatinine levels compared with preoperative values.
We collected patients’ demographic data, including age, sex, body weight, and American Society of Anesthesiologists (ASA) status. The type of operation performed and the percentage of patients having preoperative nephrotoxic drugs - Angiotensin Converting Enzyme Inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARBs) - were retrieved. The incidences of postoperative AKI with and without celecoxib were measured according to AKIN classification. This was further analyzed for patients with and without “preexisting renal impairment” The incidence of AKI during each postoperative day and during the entire hospitalization period were measured.
2.1 Statistical analysis
Categorical variables were expressed as frequencies and percentages, which were compared using chi-squared tests. Continuous variables are summarized as the means ± SD and were compared using Student’s unpaired t test. The sample size was based on the existing patient number during the study period; thus, no sample size calculations were performed.
3. Results
From 2018-2021, our institution performed 1077 hip and knee arthroplasties, of which 215 patients (20%) had preexisting renal impairment. One hundred patients out of 1077 hip and knee arthroplasties (9.3%) had postoperative AKI. Eight hundred eighty-eight patients (82.5%) were prescribed perioperative celecoxib as part of the standard multimodal analgesic protocol, while 189 patients (17.5%) were not prescribed perioperative celecoxib as part of the standard multimodal analgesic protocol (Figure 1).
For those 888 patients with perioperative celecoxib, the percentage of preexisting renal impairment was 14.5%, while the percentage without preexisting renal impairment (normal renal function) was 85.5% (Figure 1). For those 189 patients not taking perioperative celecoxib, the percentage of preexisting renal impairment was 45.5%, while the percentage without preexisting renal impairment (normal renal function) was 54.5% (Figure 1).
Although there were significant differences in the type of arthroplasties performed for patients receiving perioperative celecoxib with preexisting renal impairment and normal renal function, there were no statistically significant differences in ASA status and percentage of preoperative ACEIs and ARBs (Table 1).
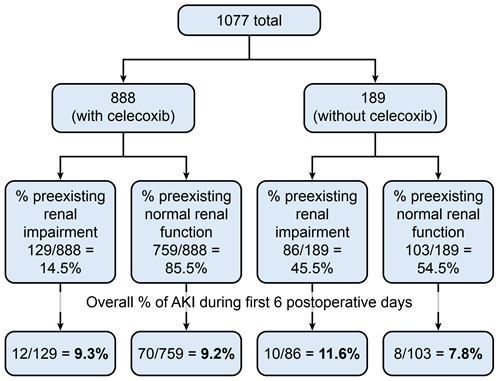
Figure 1: Overall percentage of AKI during first 6 postoperative days.
|
Variable |
Preexisting renal impairment |
Preexisting normal renal function |
p value |
|
With Celecoxib (n = 888) |
|||
|
N |
129 |
759 |
|
|
Age (years) |
69.6 ± 10.9 (25–92) |
68.7 ± 11.2 (17–97) |
0.379 |
|
Sex (%) |
|||
|
F |
61.2% (79/129) |
65.3% (496/759) |
0.366 |
|
M |
38.82% (50/129) |
34.7% (263/759) |
|
|
Body weight (kg) |
66.1 ± 14.5 (39.6–101) |
66.6 ± 13.8 (23.1–126) |
0.708 |
|
ASA status (%) |
|||
|
I |
0.8%a (1/129) |
9.9% (75/759) |
0.937a |
|
II |
45.0% (58/129) |
36.2% (275/759) |
|
|
III |
50.4% (65/129) |
49.0% (372/759) |
|
|
IV |
3.9% (5/129) |
4.9% (37/759) |
|
|
Operation type (%) |
|||
|
Knee |
82.9% (107/129) |
69.7% (529/759) |
0.002b |
|
Hip |
17.1% (22/129) |
30.3% (230/759) |
|
|
Preop ACEI/ARB (%) |
|||
|
Y |
48.1% (62/129) |
53.2% (404/759) |
0.277 |
|
N |
51.9% (67/129) |
46.8% (355/759) |
|
|
Without Celecoxib (n = 189) |
|||
|
N |
86 |
103 |
|
|
Age (years) |
68.2 ± 10.5 (42–94) |
71.8 ± 10.5 (42–92) |
0.019b |
|
Sex (%) |
|||
|
F |
62.8% (54/86) |
66.0% (68/103) |
0.794 |
|
M |
37.2% (32/86) |
34.0% (35/103) |
|
|
Body weight (kg) |
69.4 ± 14.5 (36.9–120) |
64.3 ± 14.0 (38–100.7) |
0.015b |
|
ASA status (%) |
|||
|
I |
1.2% (1/86) |
7.8% (8/103) |
0.415a |
|
II |
39.5% (34/86) |
38.8% (40/103) |
|
|
III |
51.2% (44/86) |
46.6% (48/103) |
|
|
IV |
8.1% (7/86) |
6.8% (7/103) |
|
|
Operation type (%) |
|||
|
Knee |
74.4% (64/86) |
68.9% (71/103) |
0.406 |
|
Hip |
25.6% (22/86) |
31.1% (32/103) |
|
|
Preop ACEI/ARB (%) |
|||
|
Y |
52.3% (45/86) |
46.6% (48/103) |
0.433 |
|
N |
47.7% (41/86) |
53.4% (55/103) |
|
|
Values are the mean ± SD (range) or %(n) |
|||
Table 1: Patient demographics, type of operations and percentage of patients taking preoperative nephrotoxic drugs - Angiotensin Converting Enzyme Inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARBs).
Although there were significant differences in age and body weight for patients not receiving perioperative celecoxib with preexisting renal impairment and normal renal function, there were no statistically significant differences in ASA status and percentage of preoperative ACEI and ARB (Table 1).
For the 888 patients taking perioperative celecoxib, the incidence of postoperative AKI (rise in sCr of >50% from baseline) was 5.6%, 5.5% and 1.8% from postoperative days 1-3, respectively (Table 2). For the 189 patients not taking perioperative celecoxib, the incidence of postoperative AKI (rise in sCr of >50% from baseline) was 4.8%, 5.5% and 5.9% from postoperative days 1–3, respectively (Table 2).
|
Postoperative day (POD) |
Percentage of postoperative AKI |
P value |
|
|
With celecoxib |
Without celecoxib |
||
|
POD1 (n = 1073) |
5.6% (50/886a) |
4.8% (9/187a) |
0.651 |
|
POD2 (n = 1035) |
5.5% (47/852) |
5.5% (10/183) |
0.978 |
|
POD3 (n = 430) |
1.8% (6/329) |
5.9% (6/101) |
0.039b |
|
POD4 (n = 335) |
1.2% (3/253) |
2.4% (2/82) |
0.599 |
|
POD5 (n = 175) |
0.0% (0/128) |
2.1% (1/47) |
0.269 |
|
POD6 (n = 122) |
0% (0/84) |
0% (0/38) |
1 |
|
Overall (n = 1077) |
9.2% (82/888) |
9.5% (18/189) |
0.901 |
|
Proportion (in %) (Number/total). |
|||
Table 2: Incidence of postoperative AKI in patients with and without perioperative celecoxib.
All were statistically insignificant except on postoperative day 3, showing an even lower incidence for those taking celecoxib. The overall incidence of AKI during hospitalization in those taking perioperative celecoxib was 9.2%, while it was 9.5% in those not taking perioperative celecoxib. There was no statistically significant difference (Table 2). The odds ratio was 0.97 [95% CI: 0.57–1.65]. There was no association between perioperative celecoxib and postoperative AKI.
Patients taking celecoxib (888 patients) were further divided into groups with and without preexisting renal impairment (normal renal function i.e.(RFT), and the incidence of postoperative AKI was compared. The results for postoperative AKI are summarized in Table 3.
|
Postop day (POD) |
Percentage of postoperative AKI |
P value (preexisting vs. normal RFT) |
||
|
Overall |
Preexisting renal impairment |
Preexisting Normal RFT |
||
|
POD1 (n = 886) |
5.6% (50/886) |
4.7% (6/129) |
5.8% (44/757) |
0.597 |
|
POD2 (n = 852) |
5.5% (47/852) |
7.1% (9/127) |
5.2% (38/725) |
0.401 |
|
POD3 (n = 329) |
1.8% (6/329) |
5.2% (3/58) |
1.1% (3/271) |
0.07 |
|
POD4 (n = 253) |
1.2% (3/253) |
2.0% (1/51) |
1.0% (2/202) |
0.493 |
|
POD5 (n = 128) |
0.0% (0/128) |
0% (0/35) |
0% (0/93) |
1 |
|
POD6 (n = 84) |
0% (0/84) |
0% (0/21) |
0% (0/63) |
1 |
|
Overall (n = 888) |
9.2% (82/888) |
9.3% (12/129) |
9.2% (70/759) |
0.977 |
|
Proportion (in %) of AKI (number/total). |
||||
Table 3: Incidence of postoperative AKI in patients taking celecoxib perioperatively.
The overall incidence of postoperative AKI for those with and without preexisting renal impairment was 9.3% and 9.2%, respectively (Figure 1, Table 3). This was not statistically significant. The duration for AKI was 4 days in both groups and peaked at postoperative day 2 in those with preexisting renal impairment and postoperative day 1 in those without preexisting renal impairment (Table 4). Most of the involved AKI cases were stage 1 for both groups (Tables 4).
|
With Preexisting Renal Impairment |
||||
|
Postop day |
AKI stage 1 |
AKI stage 2 |
AKI stage 3 |
AKI stages 1-3 |
|
POD1 (n = 6) |
100% (6) |
0% (0) |
0% (0) |
4.7% (6/129) |
|
POD2 (n = 9) |
55.6% (5) |
44.4% (4) |
0% (0) |
7.1% (9/127) |
|
POD3 (n = 3) |
66.7% (2) |
33.3% (1) |
0% (0) |
5.2% (3/58) |
|
POD4 (n = 1) |
100% (1) |
0% (0) |
0% (0) |
2.0% (1/51) |
|
POD5 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/35) |
|
POD6 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/21) |
|
With Preexisting Normal Renal Function Test |
||||
|
Postop day |
AKI stage 1 |
AKI stage 2 |
AKI stage 3 |
AKI stages 1-3 |
|
POD1 (n = 44) |
84.1% (37) |
15.9% (7) |
0% (0) |
5.8% (44/757) |
|
POD2 (n = 38) |
92.1% (35) |
7.9% (3) |
0% (0) |
5.2% (38/725) |
|
POD3 (n = 3) |
100% (3) |
0% (0) |
0% (0) |
1.1% (3/271) |
|
POD4 (n = 2) |
100% (2) |
0% (0) |
0% (0) |
1.0% (2/202) |
|
POD5 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/93) |
|
POD6 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/63) |
Table 4: Postoperative AKI in different stages (in % and (n)) in patients receiving celecoxib perioperatively and with preexisting renal impairment and with preexisting normal renal function test.
Patients not taking celecoxib (189 patients) were further divided into groups with and without preexisting renal impairment and the incidence of postoperative AKI was compared. The results for postoperative AKI are summarized in Table 5.
|
Postop day (POD) |
Percentage of postop AKI |
P value (preexisting renal impairment vs. normal RFT) |
||
|
Overall |
Preexisting renal impairment |
Preexisting Normal RFT |
||
|
POD1 (n = 187) |
4.8% (9/187) |
2.4% (2/85) |
6.9% (7/102) |
0.186 |
|
POD2 (n = 183) |
5.5% (10/183) |
8.3% (7/84) |
3.0% (3/99) |
0.19 |
|
POD3 (n = 101) |
5.9% (6/101) |
7.1% (4/56) |
4.4% (2/45) |
0.69 |
|
POD4 (n = 82) |
2.4% (2/82) |
4.9% (2/41) |
0% (0/41) |
0.494 |
|
POD5 (n = 47) |
2.1% (1/47) |
3.8% (1/26) |
0% (0/21) |
1 |
|
POD6 (n = 38) |
0% (0/38) |
0% (0/19) |
0% (0/19) |
1 |
|
Overall (n = 189) |
9.5% (18/189) |
11.6% (10/86) |
7.8% (8/103) |
0.368 |
|
Value (in %) of AKI (number/total) |
||||
Table 5: Incidence of postoperative AKI in patients not taking celecoxib perioperatively.
For those not taking perioperative celecoxib , the overall incidence of postoperative AKI for those with and without preexisting renal impairment was 11.6% and 7.8%, respectively (Table 5). This was not statistically significant. For those with preexisting renal impairment, the duration of AKI was 5 days and peaked at postoperative day 2. For those with preexisting normal renal functinon, the duration of AKI was 3 days and peaked at postoperative day 1. All AKI cases involved were Stage 1 for both groups (Table 6).
|
With Preexisting Renal Impairment |
||||
|
Postop day |
AKI stage 1 |
AKI stage 2 |
AKI stage 3 |
AKI stages 1-3 |
|
POD1 (n = 2) |
100% (2) |
0% (0) |
0% (0) |
2.4% (2/85) |
|
POD2 (n = 7) |
100% (7) |
0% (0) |
0% (0) |
8.3% (7/84) |
|
POD3 (n = 4) |
100% (4) |
0% (0) |
0% (0) |
7.1% (4/56) |
|
POD4 (n = 2) |
100% (2) |
0% (0) |
0% (0) |
4.9% (2/41) |
|
POD5 (n = 1) |
100% (1) |
0% (0) |
0% (0) |
3.8% (1/26) |
|
POD6 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/19) |
|
With Preexisting Normal Renal Function Test |
||||
|
Postop day |
AKI stage 1 |
AKI stage 2 |
AKI stage 3 |
AKI stages 1-3 |
|
POD1 (n = 7) |
100% (7) |
0% (0) |
0% (0) |
6.9% (7/102) |
|
POD2 (n = 3) |
100% (3) |
0% (0) |
0% (0) |
3.0% (3/99) |
|
POD3 (n = 2) |
100% (2) |
0% (0) |
0% (0) |
4.4% (2/45) |
|
POD4 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/41) |
|
POD5 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/21) |
|
POD6 (n = 0) |
0% (0) |
0% (0) |
0% (0) |
0% (0/19) |
Table 6: Postoperative AKI in different stages (in % and (n)) in patients not receiving celecoxib perioperatively and with preexisting renal impairment and with normal renal function.
4. Discussion
This is the first retrospective study in which researchers comprehensively evaluated the effect of perioperative celecoxib as one of the standard multimodal analgesic protocols on the risk of postoperative AKI in terms of incidence, severity, duration and possible reversibility in patients with and without preexisting renal impairment (nomal renal function). AKI is a common postoperative complication with an average rate ranging from 1.6% -1.69% according to the Scottish Arthroplasty Project Annual Report 2011 [23]. Non-Steroid Anti-Inflammatory Drugs (NSAIDs) have been regarded as potentially nephrotoxic and may contribute to postoperative AKI, especially in elderly patients with preexisting renal impairment.
Patient characteristics were quite homogenous in terms of ASA status and percentage of patients having preoperative ACEIs or ARBs among patients receiving perioperative celecoxib with and without preexisting renal impairment. Patient characteristics were also quite homogenous in terms of ASA status and percentage of patients having preoperative ACEIs or ARBs among patients not receiving perioperative celecoxib, with and without preexisting renal impairment.
We have shown that the overall incidence of AKI during hospitalization for those taking perioperative celecoxib was 9.2%, while it was 9.5% for those not taking perioperative celecoxib. There was no significant difference (Table 2). This implies that the use of perioperative celecoxib may not significantly contribute to postoperative AKI. The odds ratio for having perioperative celecoxib was 0.97 (<1), suggesting that patients with perioperative celecoxib were 0.97 times (95% CI: 0.57–1.65) more likely to develop postoperative AKI than those without perioperative celecoxib. Therefore, no additional AKI risk was imposed on patients taking perioperative celecoxib.
To further evaluate the impact of perioperative celecoxib, it is essential to further investigate the incidence of AKI for those with preexisting renal impairment and those with normal renal function. We have shown that the overall incidence of postoperative AKI for those with preexisting renal impairment and with normal renal function was 9.3% and 9.2%, respectively. This was not statistically significant (Table 3). Our findings suggest that prescribing perioperative celecoxib in patients with preexisting renal impairment did not impose additional AKI risk. There was a similar pattern of AKI associated with preexisting renal impairment, and those with normal renal function – duration of AKI in both groups were in the first 4 postoperative days with; severity of AKI, which was Stage 1 for both groups. This demonstrated similar reversibility and severity of AKI associated with perioperative celecoxib for both having preexisting renal impairment and normal renal function. Therefore, preexisting renal impairment was not a sole determining factor for not prescribing perioperative celecoxib. This is especially true if perioperative celecoxib is prescribed in short duration and in carefully selected patients with close monitoring of renal function test, urine output and fluid status.
After balancing the risks of celecoxib and the potential benefits of better pain control and shorter length of hospitalization, NSAIDs play an important role in multimodal analgesic protocols. Nevertheless, care should be taken for patients with risk factors for AKI, as shown in clinical studies [10,13-22]. These included advanced age, higher body mass index, hypertension, diabetes mellitus and preoperative use of nephrotoxic drugs, e.g., ACEIs and ARBs. Patients with increased risk should be closely monitored in terms of urine output and fluid intake with serial monitoring of renal function since the development of AKI is a known risk factor for future renal diseases [24].
5. Limitations
We acknowledge the relatively small sample size of this study, single center and retrospective nature as the major limitations. It is very hard for this study to identify whether celecoxib represents a risk factor for the development of AKI as there is no comparator group. Other risk factors of AKI should also been included, such as underlying diseases, perioperative complications, preoperative use of nephrotoxic agents (e.g., ACEIs and ARBs), obesity, blood loss, urine output, intraoperative fluids and blood pressure. This study only described the local patient population without drawing any inference that implies causation. The long-term effect of celecoxib beyond 6 days after the operation was not evaluated. It is also assumed that reversibility of renal function was achieved once the serum creatinine level returned to the patient’s baseline value. Moreover, comparison of pain control for those having and not having perioperative celecoxib should be evaluated to further justify the use of perioperative use of NSAIDs, balancing potential side effects and efficacy of NSAIDs.
6. Conclusion
As part of the multimodal analgesic protocol in an enhanced recovery program, 82.5% of our patients received perioperative celecoxib. In this retrospective clinical study of elective hip and knee arthroplasty, the overall postoperative AKI rate was 9.2% in patients taking perioperative celecoxib and 9.5% in those not taking perioperative celecoxib. There was no association between perioperative celecoxib and postoperative AKI. Patients taking perioperative celecoxib probably had no additional AKI risk. For those with perioperative celecoxib, the incidence of AKI was similar for those with preexisting renal impairment and for those with normal renal function. Most patients who developed AKI were cured by postoperative day 5. Appropriate risk stratification is still necessary in prescribing celecoxib for patients with preexisting renal impairment. It is generally safe to administer perioperative NSAIDs even with preexisting renal impairment provided that it is for a short duration with good perioperative fluid management and postoperative monitoring of renal function.
7. Ethics Approval and Consent to Participate
This retrospective review was approved by the Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (HKU/ HA HKW IRB). IRB reference number is UW22-230. The requirement for informed consent was waived because it was for service audit purposes and this review did not affect clinical decision making and patient management. It was registered at ClinicalTrials.gov (NCT05595694) on 27 October 2022.
All methods were carried out in accordance with relevant guidelines and regulations in the Declaration of Helsinki.
Conflicts of interest
The authors declare that they have no competing interests.
Authors’ contributions
MTHC: Design of the work, analysis of data, draft the manuscript.
TCWC: Design of the work, analysis of data, supervised the process of study and approved the final version of the manuscript.
HCYM: Interpretation of data.
WSHC: Interpretation of data.
SSCW: Draft the manuscript.
VKCW: Interpretation of data, draft the manuscript.
LPKC: Design of the work, draft the manuscript.
CWC: Design of the work, approved the final version of the manuscript.
All authors have read and approved the final manuscript and agreed with its submission to BMC Anesthesiology. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by the Department of Anaesthesiology, The University of Hong Kong. No benefits in any for have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 325 (2021): 568-78.
- Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop 82 (2011): 577-581.
- Christelis N, Wallace S, Sage CE, et al. An enhanced recovery after surgery program for hip and knee arthroplasty. Med J Aust 202 (2015): 363-368.
- Frassanito L, Vergari A, Nestorini R, et al. Enhanced Recovery After Surgery (ERAS) in hip and knee replacement surgery: description of a multidisciplinary program to improve management of the patients undergoing major orthopedic surgery. Musculoskelet Surg 104 (2020): 87-92.
- Gaffney CJ, Pelt CE, Gililland JM, et al. Perioperative Pain Management in Hip and Knee Arthroplasty. Orthop Clin North Am 48 (2017): 407-419.
- Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 10 (2017): 2287-2298.
- Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. Acta Orthop 91 (2020): 3-19.
- Fischer HB, Simanski CJ, Sharp C, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia 63 (2008): 1105-1123.
- Bell S, Prowle J. Postoperative AKI-Prevention Is Better than Cure? J Am Soc Nephrol 30 (2019): 4-6.
- Warth LC, Noiseux NO, Hogue MH, et al. Risk of Acute Kidney Injury After Primary and Revision Total Hip Arthroplasty and Total Knee Arthroplasty Using a Multimodal Approach to Perioperative Pain Control Including Ketorolac and Celecoxib. J Arthroplasty 31 (2016): 253-255.
- Singh JA, Cleveland JD. Acute kidney injury is associated with increased healthcare utilization, complications, and mortality after primary total knee arthroplasty. Ther Adv Musculoskelet Dis 12 (2020): 1759720X20908723.
- Yayac M, Aman ZS, Rondon AJ, et al. Risk Factors and Effect of Acute Kidney Injury on Outcomes Following Total Hip and Knee Arthroplasty. J Arthroplasty 36 (2021): 331-338.
- Filippone EJ, Yadav A. Acute kidney injury after hip or knee replacement: Can we lower the risk? Cleve Clin J Med 86 (2019): 263-276.
- Jafari SM, Huang R, Joshi A, et al. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 25 (2010): 49-53.
- Jamsa P, Jamsen E, Lyytikainen LP, et al. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 88 (2017): 370-376.
- Sehgal V, Bajwa SJ, Sehgal R, et al. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 12 (2014): e16713.
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 59 (2012): 1111-1118.
- Kimmel LA, Wilson S, Janardan JD, et al. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 7 (2014): 546-551.
- Perregaard H, Damholt MB, Solgaard S, et al. Renal function after elective total hip replacement. Acta Orthop 87 (2016): 235-238.
- Hassan BK, Sahlstrom A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 10 (2015): 158.
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 34 (2016): 520-523.
- Jiang EX, Gogineni HC, Mayerson JL, et al. Acute Kidney Disease after Total Hip and Knee Arthroplasty: Incidence and Associated Factors. J Arthroplasty 32 (2017): 2381-2385.
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Engl 99 (2017): 307-312.
- Ponte B, Felipe C, Muriel A, et al. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant 23 (2008): 3859-66.
