Immunotherapy-Induced Acute Gastritis: First Case Report of Nivolumab Re-Challenge after Severe Immune-Mediated Inflammatory Response
Article Information
Ari Raphael1*, Yoram Menachem2, Eli Barazovski3, Oren Shibolet2, Ravit Geva1
1Department of Oncology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel
2Department Gastroenterology and Hepatology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel
3Department Pathology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel
*Corresponding Author: Ari Raphael, Departments of Oncology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel.
Received: 02 February 2022; Accepted: 14 February 2022; Published: 09 March 2022
Citation: Ari Raphael, Yoram Menachem, Eli Barazovski, Oren Shibolet, Ravit Geva. Immunotherapy-Induced Acute Gastritis: First Case Report of Nivolumab Re-Challenge after Severe Immune-Mediated Inflammatory Response. Archives of Clinical and Medical Case Reports 6 (2022): 182-188.
Share at FacebookAbstract
Background: The use of Checkpoint Inhibitors (CPIs) exposes patients to CPI immune-related adverse events (irAEs) that affect a variety of body organs and systems. IrAEs of the upper gastrointestinal tract and gastritis are rare manifestations of CPI-related IrAEs, with only a few cases reported in the literature. Currently, there are no guidelines for the evaluation, treatment and re-challenge after CPI-mediated gastritis.
Case Presentation: A fifty-eight-year-old male patient with hepatocellular carcinoma developed a rare irAE of acute gastritis after treatment with nivolumab. The immunotherapy was withdrawn and corticosteroids were administered, leading to complete symptom resolution. Nivolumab was then reinstated with good anti-tumor response and without recurrence of gastritis.
Conclusion: In our experience, nivolumab re-challenge after immune related gastritis was safe and resulted in a continued good clinical response.
Keywords
Gastritis; Immune-related adverse events; Checkpoint inhibitors; Nivolumab; Immunotherapy re-challenge; hepatocellular carcinoma
Gastritis articles; Immune-related adverse events articles; Checkpoint inhibitors articles; Nivolumab articles; Immunotherapy re-challenge articles; hepatocellular carcinoma articles
Article Details
Abbreviations:
CPIs: Check point inhibitors; GI: Gastrointestinal; HCC: Hepatocellular carcinoma; HP: Helicobacter pylori; irAEs: immune related adverse events.
1. Background
Anti- programmed death-ligand 1 with its receptor, programmed cell death protein 1 (PD-1/PD-L1), are widely used Checkpoint Inhibitors (CPIs) with a unique toxicity profile that manifests as a late-onset autoimmune or inflammatory reaction in a variety of organs [1-5]. CPI-Immune-Related Adverse Events (IrAEs) may be explained by activation of T cell populations which cross-react against healthy tissue. The exact pathophysiology of CPI-irAEs, however, is still not well-understood [6-11].
Upper Gastrointestinal (GI) irAEs are much rarer than the relatively common lower GI irAEs (e.g., colitis), with CPI-mediated gastritis having been reported in less than 1% of CPI-treated patients [12]. Acute gastritis usually presents with epigastric discomfort and pain, nausea, vomiting, loss of appetite and bloating. Etiologies of acute gastritis can be infectious (Helicobacter Pylori [HP], syphilis, cytomegalovirus, etc.), pharmacological (non-steroidal anti-inflammatory drugs, iron salts, alcohol, alendronate, cocaine), idiopathic (eosinophilic, collagenous, lymphocytic) and miscellaneous (Crohn's disease, sarcoidosis), all of which have a similar clinical presentation but distinct histological patterns. The diagnosis of an upper GI irAE is made by eliciting a specific history of indigestion and the findings of endoscopy and biopsy. In the rare case of CPI-induced gastritis, the histology will show lymphocytic infiltration, mostly by CD8+ T lymphocytes [13-16]. Corticosteroids with or without CPI withdrawal are the mainstay of therapy [17-18]. Importantly, discontinuation of CPI puts the patient at risk of malignant disease progression. There are currently no published recommendations regarding re-challenge with CPI following an IrAE. Nivolumab (Opdivo®) is an anti PD-1 mononuclear antibody with established activity against a variety of cancers including Hepatocellular Carcinoma (HCC). We describe the course and management of a rare case of CPI-induced gastritis.
2. Case Presentation
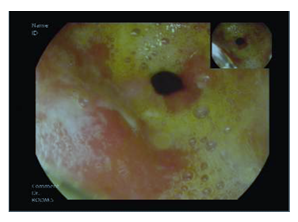
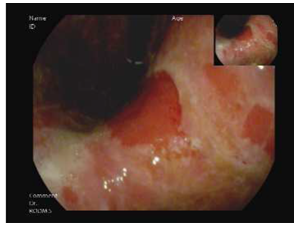
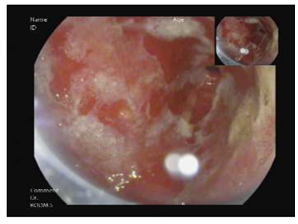
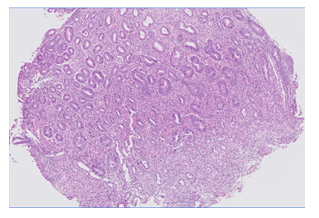
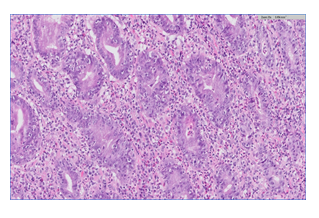
A fifty-eight-year-old male with hepatitis C virus-related cirrhosis secondary to an infected blood transfusion was diagnosed in 2011 with a non-resectable Hepatocellular Carcinoma (HCC). He was initially treated loco-ablative therapies, including radio-frequency ablation, trans-arterial chemo-embolization and selective internal radiation therapy until 2016. He was then treated for extra-hepatic spread with sorafenib from 9/2016–7/2017 until progression. At the time of progression, the patient was categorized as Child-Pugh A, Barcelona clinic liver cancer C and Eastern Cooperative Oncology Group performance status 0 and without varices. He was enrolled into a phase IIA second-line trial of oral milciclib maleate (an orally bioavailable inhibitor of cyclin-dependent kinases and tropomyosin receptor kinase A) for patients who have progressed on sorafenib. Three months after enrollment, the study medication was discontinued because of a retinal tear. From 5/2018 to 11/2018, he received third-line therapy with regorafenib until lung metastasis progression. In 12/2018, he started fourth-line therapy with nivolumab 240 mg fixed dose every 2 weeks followed by 480 mg every 4 weeks and achieved complete radiological response of the lung metastasis and partial response of the liver disease according to immune response criteria in solid tumors [19]. In 6/2020, approximately 18 months after starting nivolumab therapy, the patient developed epigastric pain, difficulty in drinking and eating, early satiety and heartburn which led him to cease eating and drinking altogether. He had lost 10 kg and was hospitalized. The patient’s physical exam was unremarkable, and his blood test results were all within normal range. An abdominal computerized tomographic scan showed his already known liver findings and no evidence of bowel obstruction. Gastroscopy showed a normal esophagus and duodenum. The gastric mucosa was erythematic and friable with easy bleeding and covered with diffuse exudate with multiple erosions involving the entire stomach (Figures A1-3). Biopsies were taken and revealed oxyntic mucosa showing severe chronic active lymphocytic gastritis with erosions. HP testing was negative. No tumor infiltration was seen (Figures B1-2). He was diagnosed as having CPI-induced acute gastritis and was treated with intravenous hydrocortisone and high-dose proton pump inhibitor. The nivolumab was stopped due to the severity of symptoms. The patient reported a very rapid relief of symptoms, and started to eat within a single day of having received the steroids. He was discharged on a regimen of oral prednisone 40 mg per day and esomeprazole 40 mg twice daily. All symptoms were resolved after two weeks, and the corticosteroids were tapered down to 10 mg. Following deliberations in a multi-disciplinary gastroenterological oncology teams, nivolumab was re-challenged on 8/2020. Since then, the patient has remained asymptomatic and continues on the monthly regimen of nivolumab, with stable HCC findings on imaging.
Figure A: Endoscopic findings revealing a diffuse erythematous exudate throughout the stomach of a patient diagnosed as having gastritis (1: pylorus and antrum, 2: body, 3: fundus).
Figure B: Gastric biopsy showing diffuse and severe gastritis. The lamina propria is infiltrated by mixed inflammatory infiltrate containing lymphocytes, plasma cells, neutrophils and eosinophils. High amount of epithelial apoptosis and many intraepithelial lymphocytes are present. Laboratory findings of Helicobacter Pylori (HP), Cytomegalovirus (CMV) and syphilis were negative. There is no evidence of tumor infiltration (B1: zoom x5, B2: zoom x20; hematoxylin-eosin stain).
3. Discussion
CPI-induced gastritis is a rare pathology. To date, only few cases of CPI-induced gastritis that included the use of nivolumab have been described, and none involving patients with HCC. The average onset of the gastritis after CPI initiation was 5.5 months [20]. We present a rare case of nivolumab-induced acute gastritis in a patient with metastatic HCC. None of the patients reported in the literature had been re-challenged with CPI. Based upon his severely altered eating pattern and need for hospitalization, our patient’s gastritis was grade 3 according to the common terminology criteria for adverse events 4 [21]. Endoscopy showed diffuse erythematous exudate throughout the stomach, and histology showed severe chronic active lymphocytic gastritis with erosions negative for HP. Taken together with the late onset of symptoms (18 months after introduction of nivolumab) and rapid symptom resolution under prednisone, these findings led to the diagnosis of CPI-induced gastritis. Suspending immunotherapy until symptom resolution was considered mandatory due to a risk of gastric perforation. Based upon his excellent and sustained response, we re-challenged nivolumab and today, almost two and one-half years since reinstating treatment with nivolumab, our patient continues treatment with evidence confirming disease control and no irAEs, including no recurrence of gastritis.
To the best of our knowledge, this is the first report of the reintroduction of immunotherapy after CPI-mediated gastritis. Re-challenge in this patient was safe and the response appears to be durable. Our experience leads us to call for more case presentations and case series of reintroducing CPI after gastritis, in the hope that they will lead to specific guidelines of treatment and re-challenge.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
All authors declared no conflict of interest.
References
- Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 57 (2017): 36-49.
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22 (2016): 886-894.
- Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering Cancer Center. J Clin Oncol 33 (2015): 3193-3198.
- Lu J, Firpi-Morell RJ, Dang LH, et al. An unusual case of gastritis in one patient receiving PD-1 blocking therapy: coexisting immune-related gastritis and cytomegaloviral infection. Gastroenterology Res 11 (2018): 383-387.
- Nishimura Y, Yasuda M, Ocho K, et al. Severe gastritis after administration of nivolumab and ipilimumab. Case Rep Oncol 11 (2018): 549-556.
- Zhang ML, Neyaz A, Patil D, et al. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology 76 (2020): 233-243.
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26 (2008): 677-704.
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 45 (2016): 7-18.
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 4 (2015): 560-575.
- Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44 (2016): 51-60.
- Boike J, Dejulio T. Severe esophagitis and gastritis from nivolumab therapy. ACG Case Rep 12 (2017): 57.
- Gonzalez RS, Salaria SN, Bohannon CD, et al. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis. Histopathology 70 (2017): 558-567.
- Onuki T, Morita E, Sakamoto N, et al. Severe upper gastrointestinal disorders in pembrolizumab-treated nonsmall cell lung cancer patient. Respirol. Case Rep 6 (2018): 1-3.
- Wang DY, Johnson DB, Davis EJ. Toxicities associated with PD-1/PD-L1 blockade. Cancer J 24 (2018): 36-40.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54 (2016): 139-148.
- Gaffuri P, Espeli V, Fulciniti F, et al. Immune-related acute and lymphocytic gastritis in a patient with metastatic melanoma treated with pembrolizumab immunotherapy. Pathologica 111 (2019): 92-97.
- Teufel A, Zhan T, Hartel N, et al. Management of immune related adverse events induced by immune check-point inhibition. Cancer Lett 456 (2019): 80-87.
- Johncilla M, Grover S, Zhang X, et al. Morphological spectrum of immune checkpoint inhibitor therapy associated gastritis. Histopathology 76 (2020): 531-539.
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutic. The Lancet Oncology 18 (2017): 143-152.
- Noriko H, Hiroaki I, Yuichirou H, et al. Severe gastritis due to pembrolizumab treatment in a lung cancer patient. Respirology Case Reports 8 (2020): 00636.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US Department of Health and Human Services National Institutes of Health National Cancer Institute 4 (2009).