Immunohistochemical Analysis of Prostein in Needle Core Biopsies of Acinar and Intraductal Prostatic Adenocarcinoma in Western Kenya Population
Article Information
Tyrus Omondi Swaya1,2,4*, Dedan Opondo4, David O. Atandi5, Benard Guyah1, Ng’wena Gideon Magak3
1Department of Biomedical Science and Technology, School of Public Health and Community Development, Maseno University, Kenya
2Department of Medical laboratory Science, School of Public Health and Biomedical Sciences, Masinde Muliro University of Science and Technology, Kenya
3Department of Medical physiology, School of Medicine, Maseno University, Kenya
4Division of Urology, Synergy Clinics, Kisumu, Kenya
5ScanLab Laboratory, Nakuru, Kenya
*Corresponding Author: Tyrus Omondi Swaya, Department of Biomedical Science and Technology, School of Public Health and Community Development, Maseno University, Kenya
Received: 25 June 2022; Accepted: 12 July 2022; Published: 21 July 2022
Citation: Tyrus Omondi Swaya, Dedan Opondo, David O. Atandi, Benard Guyah, Ng’wena Gideon Magak. Immunohistochemical Analysis of Prostein in Needle Core Biopsies of Acinar and Intraductal Prostatic Adenocarcinoma in Western Kenya Population. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 269-275
Share at FacebookAbstract
Background: Prostein is a newly reported prostate cancer biomarker. Nonetheless, no reports on African population are available. The current study aimed to determine the prostein expression in archived prostatic core biopsies in Western Kenya.
Methods: This was a retrospective study conducted on malignant and benign prostatic tissue core biopsies of 106 patients from Jaramogi Oginga Odinga Teaching and Referral Hospital and division of urology at Synergy Clinics, Kisumu between January 2018 to May 2021. Manual Immunohistochemical technique was performed on each of the 106 samples and on the following non-prostatic male control biopsies; Testis, Penis, Liver and Esophagus. Cellular location of prostein staining was evaluated microscopically and classified as cytoplasmic or nucleocytoplasmic. Intensity of prostein expression was assessed and graded according the immunohistochemistry composite score.
Results: The mean (SE) age was 72.00 ± 0.93 years. 97.2% of malignant and all the benign prostate tissue stained positive for prostein whereas the four non-prostatic male tissues were negative. Staining intensities were weak (24.5%), Moderate (17.0%), strong (55.7%) and non-stained (2.8%). The staining was highly immunolocalized within the cytoplasm (95.1% cases) as compared to nucleocytoplasmic (2.0% cases). The mean immunoreactivity composite score was 1.91 ± 0.96 (0.0-3.14). Strongly stained sections of both acinar and intraductal adenocarcinoma had a staining pattern clustered within the cytoplasm in a perinuclear location whereas the weakly stained sections had less coarse brown cytoplasmic granular appearing.
Conclusion: Prostein is expressed in both acinar and intraductal adenocarcinoma and can be routinely used in differential diagnosis of prosta
Keywords
Adenocarcinoma; Prostein; Biomarker; Prostate; Immunohistochemistry
Adenocarcinoma articles; Prostein articles; Biomarker articles; Prostate articles; Immunohistochemistry articles
Adenocarcinoma articles Adenocarcinoma Research articles Adenocarcinoma review articles Adenocarcinoma PubMed articles Adenocarcinoma PubMed Central articles Adenocarcinoma 2023 articles Adenocarcinoma 2024 articles Adenocarcinoma Scopus articles Adenocarcinoma impact factor journals Adenocarcinoma Scopus journals Adenocarcinoma PubMed journals Adenocarcinoma medical journals Adenocarcinoma free journals Adenocarcinoma best journals Adenocarcinoma top journals Adenocarcinoma free medical journals Adenocarcinoma famous journals Adenocarcinoma Google Scholar indexed journals Prostein articles Prostein Research articles Prostein review articles Prostein PubMed articles Prostein PubMed Central articles Prostein 2023 articles Prostein 2024 articles Prostein Scopus articles Prostein impact factor journals Prostein Scopus journals Prostein PubMed journals Prostein medical journals Prostein free journals Prostein best journals Prostein top journals Prostein free medical journals Prostein famous journals Prostein Google Scholar indexed journals Biomarker articles Biomarker Research articles Biomarker review articles Biomarker PubMed articles Biomarker PubMed Central articles Biomarker 2023 articles Biomarker 2024 articles Biomarker Scopus articles Biomarker impact factor journals Biomarker Scopus journals Biomarker PubMed journals Biomarker medical journals Biomarker free journals Biomarker best journals Biomarker top journals Biomarker free medical journals Biomarker famous journals Biomarker Google Scholar indexed journals Prostate articles Prostate Research articles Prostate review articles Prostate PubMed articles Prostate PubMed Central articles Prostate 2023 articles Prostate 2024 articles Prostate Scopus articles Prostate impact factor journals Prostate Scopus journals Prostate PubMed journals Prostate medical journals Prostate free journals Prostate best journals Prostate top journals Prostate free medical journals Prostate famous journals Prostate Google Scholar indexed journals Immunohistochemistry articles Immunohistochemistry Research articles Immunohistochemistry review articles Immunohistochemistry PubMed articles Immunohistochemistry PubMed Central articles Immunohistochemistry 2023 articles Immunohistochemistry 2024 articles Immunohistochemistry Scopus articles Immunohistochemistry impact factor journals Immunohistochemistry Scopus journals Immunohistochemistry PubMed journals Immunohistochemistry medical journals Immunohistochemistry free journals Immunohistochemistry best journals Immunohistochemistry top journals Immunohistochemistry free medical journals Immunohistochemistry famous journals Immunohistochemistry Google Scholar indexed journals Prostate cancer articles Prostate cancer Research articles Prostate cancer review articles Prostate cancer PubMed articles Prostate cancer PubMed Central articles Prostate cancer 2023 articles Prostate cancer 2024 articles Prostate cancer Scopus articles Prostate cancer impact factor journals Prostate cancer Scopus journals Prostate cancer PubMed journals Prostate cancer medical journals Prostate cancer free journals Prostate cancer best journals Prostate cancer top journals Prostate cancer free medical journals Prostate cancer famous journals Prostate cancer Google Scholar indexed journals malignant tumor articles malignant tumor Research articles malignant tumor review articles malignant tumor PubMed articles malignant tumor PubMed Central articles malignant tumor 2023 articles malignant tumor 2024 articles malignant tumor Scopus articles malignant tumor impact factor journals malignant tumor Scopus journals malignant tumor PubMed journals malignant tumor medical journals malignant tumor free journals malignant tumor best journals malignant tumor top journals malignant tumor free medical journals malignant tumor famous journals malignant tumor Google Scholar indexed journals biopsies articles biopsies Research articles biopsies review articles biopsies PubMed articles biopsies PubMed Central articles biopsies 2023 articles biopsies 2024 articles biopsies Scopus articles biopsies impact factor journals biopsies Scopus journals biopsies PubMed journals biopsies medical journals biopsies free journals biopsies best journals biopsies top journals biopsies free medical journals biopsies famous journals biopsies Google Scholar indexed journals immunohistochemistry articles immunohistochemistry Research articles immunohistochemistry review articles immunohistochemistry PubMed articles immunohistochemistry PubMed Central articles immunohistochemistry 2023 articles immunohistochemistry 2024 articles immunohistochemistry Scopus articles immunohistochemistry impact factor journals immunohistochemistry Scopus journals immunohistochemistry PubMed journals immunohistochemistry medical journals immunohistochemistry free journals immunohistochemistry best journals immunohistochemistry top journals immunohistochemistry free medical journals immunohistochemistry famous journals immunohistochemistry Google Scholar indexed journals nucleocytoplasmic articles nucleocytoplasmic Research articles nucleocytoplasmic review articles nucleocytoplasmic PubMed articles nucleocytoplasmic PubMed Central articles nucleocytoplasmic 2023 articles nucleocytoplasmic 2024 articles nucleocytoplasmic Scopus articles nucleocytoplasmic impact factor journals nucleocytoplasmic Scopus journals nucleocytoplasmic PubMed journals nucleocytoplasmic medical journals nucleocytoplasmic free journals nucleocytoplasmic best journals nucleocytoplasmic top journals nucleocytoplasmic free medical journals nucleocytoplasmic famous journals nucleocytoplasmic Google Scholar indexed journals
Article Details
1. Introduction
Prostate cancer (PCa) is the most frequent malignant tumor in the male population worldwide, and has remained one of the leading causes cancer-associated mortality in men [1-4]. In East Africa, prostate cancer ranks third in both incidence and mortality, and leads to an estimated 9,000 (9% of all male cancers) cases and 7,300 (8.5% of all male cancer) deaths annually [5]. It is significant to note that PCa incidences increased by 64.5% between 1990 and 2010 [6]. In 2019, the prevalence of Pca in Western Kenya was at 7.0% [7-9]. However, the incidences and the mortalities of the prostate cancer are extremely variable worldwide with higher incidence rate occurring most among the Africa-American Men as compared to the white [10]. The clinical, natural history and pathological behavior of the disease has also been reported to be variable [11].
Presently the diagnosis of prostatic cancer relies on histopathological features (Stage and Gleason score) supported by combination of Prostate Specific Antigen (PSA) levels and imaging techniques [12]. However, information such as PSA level, cancer stage, and Gleason score, are limited in their ability to determine the disease severity thus complicating most of the clinical decisions [13]. Both PSA and Gleason score are not able to clearly distinguish between indolent and aggressive cancers since tumors with similar histological patterns may have different clinical outcome [14-16]. This has led to overtreatment and unnecessary biopsies in some cases. As a biomarker PSA lacks specificity and sensitivity [17,18]. Due to increased number of specimens with limited number of suspicious glands and minimal atypia [19], histomorphological findings in the biopsied tissues sometimes are difficult to report. Distinguishing between aggressive and indolent tumors is a major challenge [20]. Moreover, differentiating between high-grade urothelial carcinoma (UC) and high-grade Prostate adenocarcinoma (PAC) is frequently a diagnostic and prognostic challenge. According to European guidelines, PCa validated biomarkers are urgently needed for guiding the pre-treatment decision processes [21]. These scenarios can be improved by using Prostate specific and sensitive immunohistochemical biomarkers, which would adjunct PSA levels and help pathologist to make much more accurate differential diagnosis.
Prostein (also known as prostate cancer-associated protein 6 / P501S / SLC45A3) is a protein present in the Golgi apparatus of benign and malignant prostatic glandular epithelium. It is encoded by the Solute carrier family 45, member 3 (SLC45A3) gene, an androgen-regulated gene and a prostate specific marker expressed in prostatic glandular cells [22]. It shows perinuclear cytoplasmic localization in immunohistochemical experiments [22,25]. Because it is highly specific for prostate glandular cells, this target is useful for differentiating prostatic metastases from other carcinomas such as urothelial carcinomas or colorectal carcinomas [22-25]. Prostein is sometimes expressed in PSA-negative prostate carcinomas, and thus used in combination can lead to increased sensitivity in the identification of prostate cancer metastases [25,26]. Most cohorts for evaluation of the current diagnostic biomarkers are restricted to the Caucasian Population with little or no representation of other geographic, ethnic population and then directly applied in general way to other population irrespective of their genetic variability [13].
Since prostate cancer is highly heterogeneous and its incidence and mortality vary strikingly among ethnic, racial, and national groups and this is an area worth exploring. There have been no reported studies on manual immunohistochemical diagnostic utility of Prostein in detection of prostate cancer among the African populations. The current study aims to evaluate immunohistochemical expression levels of Prostein, P501s, in archived formalin fixed paraffin embedded (FFPE) prostatic core biopsy from prostate cancer patients in Western Kenya.
2. Materials and Methods
This study involved 106 archived formalin fixed paraffin embedded prostatic core biopsy specimens consecutively collected between January 2018 and May 2021 from prostate cancer patients at Jaramogi Oginga Odinga Teaching and Referral Hospital, Pathology Department and Division of urology at Synergy Clinics, Kisumu. All the clinical prostate tissue samples used in this study were accompanied by demographic information and pathological reports such as Tribe, Age, and type of malignancy. Non-prostatic tissues from the neighboring organs such as Testis, Penile; distant organs such as Liver and Oesophagus were included in the study as control specimen.
Following approval by Jaramogi Oginga Odinga Teaching and Referral Hospital Ethics and review committee (IERC/JOORTH/353/2021); the eligible patients or their families were contacted and consent obtained via recorded telephonic interview and their demographics obtained as well as their histopathology reports retrieved from the hospital laboratory management information system. The tissue blocks, selected using non-probability approach, were retrieved from the laboratory archive, deidentified, sectioned and manually stained using prostein immunohistochemical stains.
2.1 Immunohistochemistry
Immunohistochemistry (IHC) was performed manually following manufacturer’s instructions. The FFPE tissue specimens were cut into sections of 4 μm using Micros Razor rotary microtome. After preparation of the tissue, the sections were mounted on charged poly-l-lysine FLEX IHC Microscope Slides (Code K8020) flat and wrinkle-free. The tissue sections mounted on the slides were dewax at 600C for 45Minutes in a hot oven then subjected to 3- in -1 pretreatment procedure with HIER using 1:50 diluted EnVision FLEX Target Retrieval Solution, High pH (50x) (Code K8004) in Dako PT Link. Deparaffinization, rehydration and Heat induced epitope retrieval were performed in Dako PT Link at automated Pre-heat temperature: 65°C; epitope retrieval temperature and time: 97°C for 20 minutes; cool down to 65°C. The slide rack was then removed from PT tank and immediately dipped into jar/tank (e.g., PT Link Rinse Station (Code PT109)) containing diluted room temperature EnVision FLEX Wash Buffer (20x) (Code K8007). The slides were left in Wash Buffer for 5 minutes.
The immunolabeling procedures and incubation times were carried out manually but in accordance to the manufacturers' instruction using Dako Envision Labelled monomer-HRP anti-mouse (Dako, Glostrup, Denmark). The protocol involved each slide being rinsed with wash Buffer for further cooling to room temperature and pressure. Thereafter, 100µL FLEX Ready to Use Primary Antibody was added to the slides and incubated for 5minutes. This was again rinsed twice with Wash Buffer and then followed by addition of 100µL EnVision™ FLEX /HRP (RTU) and subjected to incubation for 20minutes. The slides were rinsed in Wash buffer and incubated for 5minutes then flooded with 200µL Envision TM FLEX Substrate working solution prepared by mixing 1ml of Substrate with one drop of 3,3′-Diaminobenzidine (DAB) and incubated further for another 20minutes. The slides were further rinsed using wash buffer and finally counterstained using 100µL EnVision™ FLEX Hematoxylin (RTU) incubated for 20minutes and rinsed in two changes of Deionized water and two changes of Distilled water. The incubations were done at room temperature.
After staining the slides were dehydrated in an increasing concentration of alcohol (70%, 90%, 95%, 100% 100%), cleared in two changes of xylene and mounted using aqueous Dako Glycergel™ Mounting Medium, Code C0563 then examined and confirmed by a surgical pathologist and immunohistochemical features recorded. The results were compared to the positive control provided by the manufacturer.
A negative control, section treated with a tris-buffer solution instead of primary antibody, were run simultaneously using the same protocol as the patient specimens and control biopsies. Caution was taken not to allow tissue sections to dry out during treatment or immunohistochemical staining procedure.
2.2 Interpretation of the immunohistochemical stains
Photomicrograph of prostein immunohistochemical staining pattern in PCa Benign, HGPIN and non-prostatic specimens was taken at x4, x10 and x40 objective lens using Euromex Oxion Microscope. The expression proportion and intensity of immunopositivity were scored, using a method similar to Yin et al.; Zhao et al. and Hao et al. (27-29). The percentage of cells with immunopositivity was graded as follows: 0 (< 5%), 1 (5-25%), 2 (26-50%), 3 (51-75%), 4 (> 75%), and the staining intensity was graded as negative (0, no staining), weak (1+), moderate (2+), or intense (3+, as strong). A weighted immunoreactivity score was obtained by multiplying the scores for distribution and intensity positivity in the respective lesions yielding a score ranging from 0 to 12. For example, if 60% of tumor cells are scored weak (1=Weak), 30% scored 2 (2= Moderate), and 5% Strong (3=Strong); the immunoreactivity score of this case is [3 × 1] + [2× 2] + [0 × 3] = 7. Scores of 1-4 were defined as greatly reduced staining, 5-8 as reduced, 9-12 were defined as strong staining, and 0 as negative. A board-certified surgical pathologists confirmed the evaluation of the specimens from this study. The data was presented as mean ± standard errors (SE).
2.3 Data analysis
The frequency and descriptive statistic for age, tribe, tumor type, staining immunolocalization and intensity were analyzed using IBM SPSS version 23.0. For each dataset within groups, the distribution, mean and standard deviation within the 95% confidence limits are shown.
3. Results
In the present study, the median age of the subjects was 72.00 ± 0.93 years (49.0 - 107.0) and Modal age group being 70.0 -79.0years. Majority of patients were Nilotic (81.1%). Other ethnic groups were Bantu (17.9%), and Cushites (0.9%) (Figure 1A-B). The Population Mean Age 72.00 ± 9.62 years with a range of 58 (49-107) Years. (Figure 1A). The modal 70-79 years (35.8%) (Figure 1B). Majority were Nilotes (81.1%), Bantu 17.9% (Table 1). Prostein expression had a cytoplasmic immunolocalization in most of the prostatic core biopsies cases (95.1%) with varying degree of homogeneous staining pattern (Figure 2). Prostein expression had a cytoplasmic immunolocalization in most of the prostatic core biopsies cases (95.1%) with varying degree of homogeneous staining pattern. Prostein was strongly expressed (55.7%), weakly expressed in 24.5%, moderately expressed in 17.0% and non-stained in 2.8% of the prostatic core biopsies. 95.3% of staining pattern was majorly cytoplasmic with 1.9% cases of Nucleocytoplasmic staining characteristics and 2.8% non-stained (Figure 2).
Most prevalent tumor was acinar adenocarcinoma (81.7%) followed by 5.7% intraductal Carcinoma, 1.9% High grade Prostatic Intraepithelial Neoplasms. 4.7% of the cases were Benign prostatic hyperplasia. The acinar adenocarcinoma was stained as follows; 51% strong, 23% weak, 16% moderate and 3% unstained. Intraductal carcinoma were stained as follows 2% strong, 4% moderate and 3%weak. The most common type of prostate malignancy among the prostatic core biopsies was acinar adenocarcinoma (91.2%) followed by intraductal carcinoma (5.9%). 2.9% of the tissues had High grade Prostatic Intraepithelial Neoplasms (HGPIN) (Figure 3).
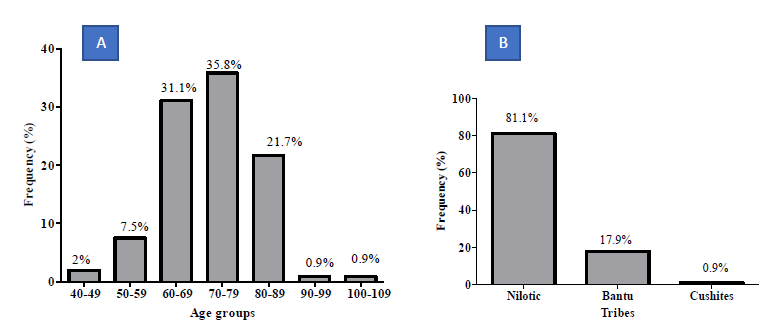
Figure 1: Demographic Characteristics of the population as percentages of the cases.
Table 4.1a: Demographic Characteristics of Prostate Cancer patients.
|
Patients Characteristics |
Frequencies % (n) |
|
Age (Mean ± SE) |
72.21 ± 0.96 years |
|
Specimen source |
|
|
Synergy urology clinic |
68.7% (70) |
|
JOOTRH |
31.4% (32) |
|
Tribe |
|
|
Nilotic |
83.3% (85) |
|
Bantus |
16.7% (17) |
|
Marital status |
|
|
Married/Partnered |
97.1% (99) |
|
Widowed |
2.9% (3) |
|
Education Level |
|
|
Primary |
41.2% (42) |
|
Secondary |
23.5% (24) |
|
Tertiary |
35.3% (36) |
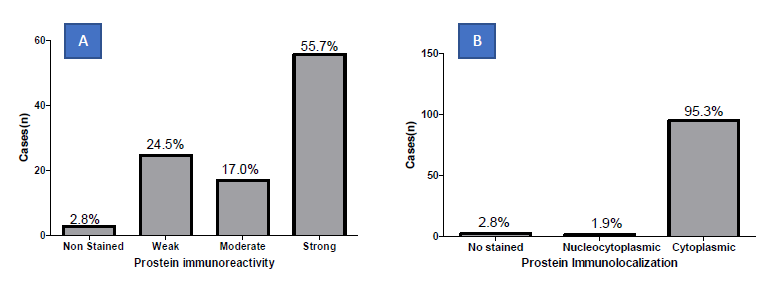
Figure 2: Frequency distribution of prostein immunohistochemical staining intensity (A) and location (B) in malignant biopsies.
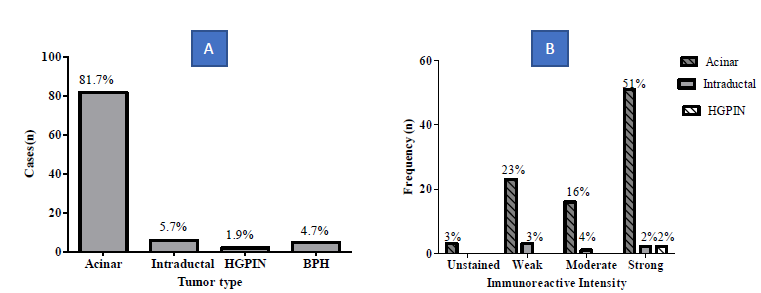
Figure 3: Tumor type (A) and Immunoreactivity (B).
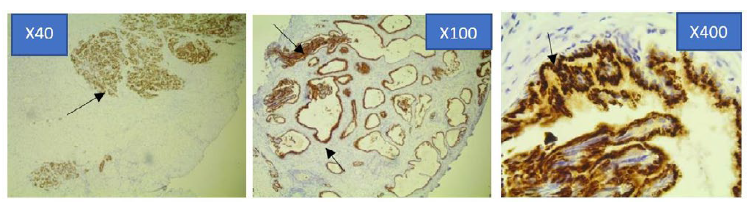
Figure 4a: Photomicrograph of Strong Prostein immunohistochemical expression pattern in PCa specimen at x40, x100 and x400 Magnification using Euromex Oxion Microscope.
The sections were stained with Mouse monoclonal antibody 10E3-G4-D3 against Prostein. The intensity of the immunohistochemical analysis shows (Arrows) a strong punctate plasma membrane staining pattern with mouse monoclonal anti-prostein antibody, Clone 10E3. The stained areas are clustered within the cytoplasm in a perinuclear location. The staining characteristics has a completely random polarity from cell to cell but remained polarized within each cell (x400).
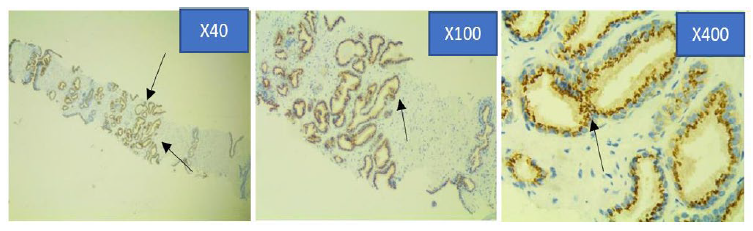
Figure 4b: Photomicrograph of Moderate Prostein immunohistochemical staining pattern in PCa specimen at x40, x100 and x400 objective lens using Euromex Oxion Microscope.
The intensity of the immunohistochemical analysis shows (Arrows) a moderate punctate plasma membrane staining pattern with mouse monoclonal anti-prostein antibody, Clone 10E3. Prostein is expressed in the cytoplasm showing circumscribed, cytosolic brown dense granular staining pattern.
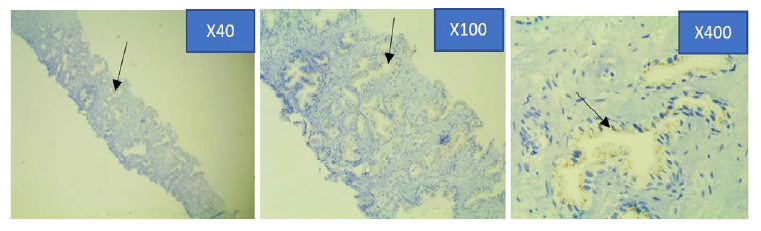
Figure 4c: Photomicrograph of weak Prostein immunohistochemical expression in PCa specimen at x4, x10 and x40 objective lens using Euromex Oxion Microscope.
The intensity of the immunohistochemical analysis shows (Arrows) a faintly stained punctate plasma membrane staining pattern with mouse monoclonal anti-prostein antibody, Clone 10E3. The granules are relatively faint and punctate showing focal loss of expression. However, the granules were still visible in the apical region of the cells using higher magnifications.
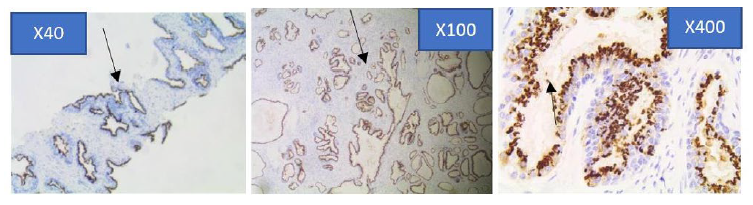
Figure 4d: Strong immunohistochemical expression pattern of prostein in Benign Prostatic hyperplasia core biopsy sections at x40, x100 and x400 objective lens using Euromex Oxion Microscope.
The intensity of the immunohistochemical analysis shows (Arrows) a moderately stained punctate plasma membrane staining pattern with mouse monoclonal anti-prostein antibody, Clone 10E3. The polarity of the staining characteristics appears remained polarized within each cell.
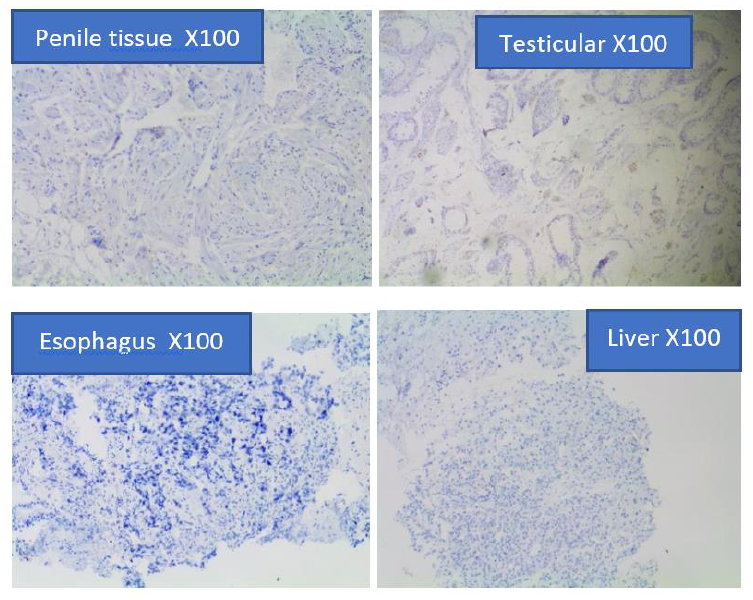
Figure 4e: Prostein Immunohistochemical staining on metastatic carcinomas of Penile, benign testicular tissue, benign tissue, esophageal squamous carcinoma, and hepatocellular carcinoma.
The immunohistochemical findings indicated that none of the four non-prostatic tissues (Liver Penile, Testis, and Esophagus) that were stained with mouse monoclonal anti-prostein antibody were positive for Prostein. Prostein was exclusively expressed only in the Prostatic epithelium tissues. The expression is specific for prostate and is not detected in other tumor tissues, including liver, penile, testis, and esophageal tissues (Figure 4e).
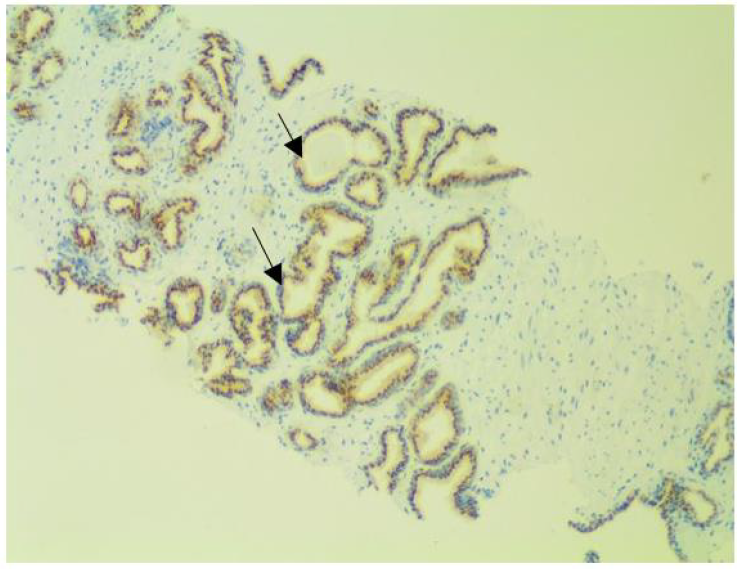
Figure 4f: Intraductal carcinoma at x100 magnification.
The sections comprise of many large atypical glands with loose and dense cribriform architecture. The prostein immunostaining pattern is similar to that of acinar carcinoma as characterized by marked expansile growth of atypical cells that forms large dense cribriform.
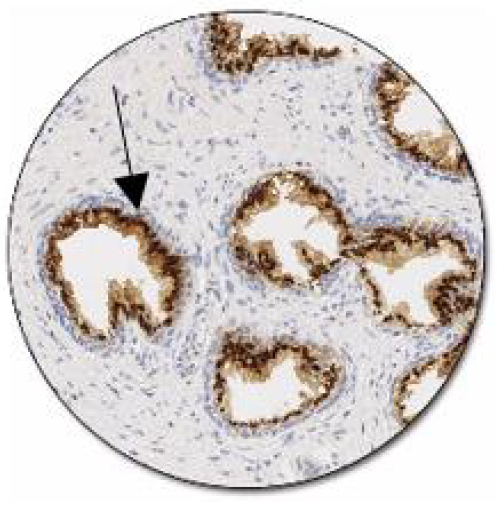
Figure 5: Positive control of prostein expression, Clone 10E in normal prostate: Luminal epithelial cells show a moderate to strong granular cytoplasmic staining reaction (www. Dako/Agilent/Prostein).
4. Discussion
The findings of the present study have demonstrated that prostein, P501s, is expressed in both benign and malignant prostate tumor tissues with varying degrees of brown punctate cytoplasmic staining pattern (Figure 4a-d; Table 1). The immunohistochemical staining showed stained areas clustered within the cytoplasm in a perinuclear location. This confirmed that Prostein has a cytoplasmic immunolocalization. The staining pattern corresponds to the location of Golgi complex as was similarly demonstrated by previous studies [12, 30]. The granular perinuclear cytoplasmic expression of prostein is pivotal feature in establishing the prostatic origin of the tumors [31]. The different variation in staining patterns could be attributed to the different tumor progression as the more the tumor the less the staining intensities. These findings agree with previous studies that also reported various degrees of staining intensities ranging from weak, moderate to strong [22, 27]. These findings indicate that prostein is expressed in a similar way among the African cohort as the Caucasian. Therefore, despite prostate tumour heterogeneity [12, 32], prostein expression is unaffected by geographical settings and race and thus is a potential biomarker for prostate cancer diagnosis. The staining intensity appeared reduced in metastatic cases, a phenomenon which was similarly observed previously in studies conducted with PSA and NKX3.1 IHC stains [33-36]. The polarity of the staining in moderately and strongly stained tissue sections were completely random within each cell (Figure 4a-b, d). However, in weakly stained cases, the granules were relatively faint and punctate, but were still visible in the apical region of the cells using higher magnifications (Figure 4c). The staining characteristics is consistent with the manufacturer’s positive controlled pictorial atlas (Figure 5). The luminal epithelial cells show a moderate to strong granular cytoplasmic staining reaction. The staining characteristics from cell to cell was completely random but remained polarized within each cell.
None of the non-Prostatic specimens in our study including liver, penile, testicular and esophagus tissues showed prostein immunopositivity (Figure 4e). This is because prostein is a prostate specific biomarker and are only expressed by epithelial tissues of the prostate glands. These findings indicate that prostein is a potential biomarker for prostate cancer diagnosis. Previous studies demonstrated that prostein appears to be expressed immunohistochemically in an exclusively prostate-specific pattern and could not be detected even at mRNA expression levels in any of the non-prostatic tissues [22, 25, 37]. It is useful as a target for differentiating extra-prostatic metastases from other carcinomas such as urothelial carcinomas or colorectal carcinomas due to its excellent specificity [12, 22-25]. It is also still expressed in poorly differentiated and PSA-negative adenocarcinoma [38]. Moreover, these two targets used in combination can lead to increased sensitivity in the identification of prostate cancer metastases [25, 26, 39]. Prostein immunostaining pattern is similar to that of acinar carcinoma and is characterized by marked expansile growth of atypical cells that forms large dense cribriform (Figure 4e). These results therefore confirm prostein as a prostate-specific marker with potential utility in the diagnosis of prostatic origin of metastatic adenocarcinomas among the African population.
The photomicrographs taken at different objective lenses showed excellent comparison of the cytoplasmic immunolocalization of the marker. However, preanalytical factors such as Ischemic time, amount and duration of fixation as well as tumor heterogeneity seems to affect the prostein staining characteristics resulting into non-staining features of some areas or sections (2.8%). Prescott and colleagues attributed 42.1% of the diagnostic discrepancies in immunohistochemistry to poor antibody selection [40]. Nevertheless, according to the findings from this study, prostein immunohistochemistry can be used even in resource limited setting as adjunct prostate cancer specific and sensitive biomarker. This will enhance both the accuracy and reduce the false positive and negative diagnostic rates of poorly differentiated prostate cancer. While Prostein immunostaining may be of value for the differential diagnosis of clinically significant Prostate cancer in H&E diagnostically challenging cases, a correlation with the Gleason grades should be considered.
5. Conclusion
Our findings indicate that prostein can be detected using manual IHC techniques in both acinar and intraductal adenocarcinoma of the prostate glands without expression in non-prostatic glands. It is exclusively expressed with an excellent brown punctate cytoplasmic granular staining pattern in prostate tissues of African cohort. We propose the utility of Prostein as an additional marker in the diagnosis of poorly differentiated prostatic carcinoma of unknown origin. Further studies can be conducted on the prostein expression and staging pattern of prostate adenocarcinoma and as a target for development of prostein-specific antibody therapeutic regimen for prostate cancer among the African population.
Declaration
Financial support and Sponsorship
This research received no external funding.
Conflict of Interest
The authors declare that there was no conflict or competing of interest.
Acknowledgement
We are grateful to acknowledge ScanLab laboratory Nakuru for help with PT link and conducting IHC procedures. Equally, we acknowledge PathExpert Laboratory where tissue samples from patients seeking treatment at Synergy Urology Clinic were kept and obtained during the study period.
Ethical Statement
All the methods were performed in accordance with the relevant guidelines and regularities as stipulated by Jaramogi Oginga Odinga Teaching and Referral Hospital Ethics and review committee (IERC/JOORTH/353/2021) approval. All the eligible participants or their families were contacted and consent obtained via recorded telephonic interview. Their demographics were obtained for the retrieval of their tissue blocks as well as their histopathology reports retrieved from the hospital histology laboratory and management information system.
Data Availability
The datasets generated and analyzed during the current study are available as additional supporting files, in spreadsheet format alongside other supporting documents. Part of the data contain information that would compromise research participant privacy thus are available on the manuscript as pictorials.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (2018): 394-424.
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer 144 (2019): 1941-1953.
- Siegel R, Naishadham D, Jemal A. Cancer statistics CA: a cancer journal for clinicians 63 (2013): 11-30.
- Cimadamore A, Mazzucchelli R, Lopez-Beltran A, et al. Prostate Cancer in 2021: Novelties in Prognostic and Therapeutic Biomarker Evaluation. Cancers 13 (2021): 3471.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127 (2011): 2893-2917.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London England) 380 (2012): 2095-2128.
- Macharia L, Mureithi M, Anzala O. Cancer in Kenya: types and infection-attributable. Data from the adult population of two National referral hospitals (2008-2012). AAS Open Res 1 (2019): 25.
- Wambalaba FW, Son B, Wambalaba AE, et al. Prevalence and Capacity of Cancer Diagnostics and Treatment: A Demand and Supply Survey of Health-Care Facilities in Kenya. Cancer control: journal of the Moffitt Cancer Center 26 (2019).
- Luke Awichi. Cancer prevalence rate per county-The Star https://www.the-star.co.ke/news/2019-07-30-cancer-prevalence-rate-per-county (2019).
- Panigrahi K, Praharaj P, Kittaka H, et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer medicine 8 (2019): 1110-1123.
- Ouattara A, Hodonou R, Avakoudjo J, et al. Epidemiology of urologic cancers at the university teaching hospital of Cotonou Benin. Review of about 158 cases of urologic cancers. Prog urol 22 (2012): 261-265.
- Garudadri G, Rao BV, Sundaram C, et al. Diagnostic utility of immunohistochemical marker prostein for evaluation of primary and metastatic prostatic carcinomas. Indian journal of pathology & microbiology 63 (2020): S18-S24.
- Narayan M, Konety BR, Warlick C. Novel biomarkers for prostate cancer: An evidence-based review for use in clinical practice. International journal of urology: official journal of the Japanese Urological Association 24 (2017): 352-360.
- Shen M, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes & development 24 (2010): 1967-2000.
- Squire A, Park C, Yoshimoto M, et al. Prostate cancer as a model system for genetic diversity in tumors. Advances in cancer research 112 (2011): 183-216.
- Schoenborn R, Nelson P, Fang M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clinical cancer research: an official journal of the American Association for Cancer Research 19 (2013): 4058-4066.
- Epstein JI, Egevad L, Humphrey PA, et al. Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. The American journal of surgical pathology 38 (2014): e6-e19.
- Romero J, Garcia B, Campos F, et al. Prostate cancer biomarkers: an update. Urologic oncology 32 (2014): 252-260.
- Magi-Galluzzi C. Prostate cancer: diagnostic criteria and role of immunohistochemistry. Modern pathology: an official journal of the United States and Canadian Academy of Pathology Inc 31 (2018): S12-S21.
- Sequeiros T, García M, Montes M, et al. Molecular markers for prostate cancer in formalin-fixed paraffin-embedded tissues. BioMed research international (2013): 283-635.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening Diagnosis and Local Treatment with Curative Intent. European urology 71 (2017): 618-629.
- Xu J, Kalos M, Stolk JA, et al. Identification and characterization of prostein a novel prostate-specific protein. Cancer research 61 (2001): 1563-1568.
- Lane Z, Hansel DE, Epstein JI. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. The American journal of surgical pathology 32 (2008): 1322-1326.
- Chuang AY, DeMarzo AM, Veltri RW, et al. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. The American journal of surgical pathology 31 (2007): 1246-1255.
- Sheridan T, Herawi M, Epstein JI, et al. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. The American journal of surgical pathology 31 (2007): 1351-1355.
- Perner S, Rupp NJ, Braun M, et al. Loss of SLC45A3 protein (prostein) expression in prostate cancer is associated with SLC45A3-ERG gene rearrangement and an unfavorable clinical course. International journal of cancer 132 (2013): 807-812.
- Yin M, Dhir R, Parwani AV. Diagnostic utility of p501s (prostein) in comparison to prostate specific antigen (PSA) for the detection of metastatic prostatic adenocarcinoma. Diagnostic pathology 2 (2007): 41.
- Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDK4 correlated to prognosis in colorectal carcinoma. World journal of gastroenterology 9 (2003): 2202-2206.
- Hao XP, Willis JE, Pretlow TG, et al. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer research 60 (2000): 18-21.
- Kalos M, Askaa J, Hylander BL, et al. Prostein expression is highly restricted to normal and malignant prostate tissues. The Prostate 60 (2004): 246-256.
- Srinivasan M, Parwani AV. Diagnostic utility of p63/P501S double sequential immunohistochemical staining in differentiating urothelial carcinoma from prostate carcinoma. Diagnostic pathology 6 (2011): 67.
- Armenia J, Wankowicz S, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nature genetics 50 (2018): 645-651.
- Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82 (1998): 2256-2261.
- Renshaw AA, Granter SR. Metastatic sarcomatoid and PSA- and PAP-negative prostatic carcinoma: diagnosis by fine-needle aspiration. Diagnostic cytopathology 23 (2000): 199-201.
- Bowen C, Bubendorf L, Voeller HJ, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer research 60 (2000): 6111-6115.
- Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Human pathology 34 (2003): 646-653.
- Wolfgang Curt D, Magnus Essand James J. Vincent Byungkook Lee Ira Pastan TARP: A nuclear protein expressed in prostate and cancer cells derived from an alternate reading frame of the T cell receptor γ chain locus. Proceedings of the National Academy of Sciences 97 (2000): 9437-9442.
- Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. American journal of clinical pathology 117 (2002): 471-477.
- Amin MB, Epstein JI, Ulbright TM, et al. Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the International Society of Urological Pathology consensus conference. The American journal of surgical pathology 38 (2014): 1017-1022.
- Prescott J, Wells S, Bisset L, et al. Audit of tumour histopathology reviewed by a regional oncology centre. Journal of clinical pathology 48 (1995): 245-249.
