Immune Response in Patients Diagnosed with Non-Muscle Invasive Bladder Cancer Treated with CIMAvax-EGF Concomitant with Intravesical Bacillus Calmette-Guerin
Article Information
Jenysbel de la Caridad Hernández1#, Laura Martínez2#, Amnely González1, Patricia Lorenzo-Luaces1, Victor Manuel Medina3, Eduardo Ibañez4, Kirenia Camacho5, Eduardo Santiesteban5, Rosa María Amador6, Yanet Caveda6, Meylan Cepeda1, Leticia Cabrera1, Annia Gorte1, Yuliannis Santiesteban1, Pedro Camilo Rodríguez1, Zaima Mazorra1, Tania Crombet1, Maurenis Hernandez1, Danay Saavedra1
1Clinical Immunology Direction, Center of Molecular Immunology, Havana, Cuba
2Faculty of Biology, University of Havana, Havana, Cuba
3National Institute of Oncology and Radiobiology, Havana, Cuba
4Celestino Hernández Hospital, Villa Clara, Cuba
5José Ramón López Tabranes Hospital, Matanzas, Cuba
6III Congreso Oncologic Hospital, Pinar del Rio, Cuba
*Corresponding Author: Dr. Danay Saavedra, Clinical Immunology Lab, Center of Molecular Immunology, 216 St, corner 15, PO box 16040, Atabey, Havana, Cuba
Received: 03 August 2020; Accepted: 08 September 2020; Published: 07 December 2020
Citation: Jenysbel de la Caridad Hernández, Laura Martínez, Amnely González, Patricia Lorenzo-Luaces, Victor Manuel Medina, Eduardo Ibañez, Kirenia Camacho, Eduardo Santiesteban, Rosa María Amador, Yanet Caveda, Meylan Cepeda, Leticia Cabrera, Annia Gorte, Yuliannis Santiesteban, Pedro Camilo Rodríguez, Zaima Mazorra, Tania Crombet, Maurenis Hernandez, Danay Saavedra. Immune Response in Patients Diagnosed with Non-Muscle Invasive Bladder Cancer Treated with CIMAvax-EGF Concomitant with Intravesical Bacillus Calmette-Guerin. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 574-587
Share at FacebookAbstract
Bladder cancer is the ninth most common malignancy worldwide and the seventh in male patients. Most of new patients are diagnosed with non-muscle invasive bladder cancer. It usually courses with a favorable prognosis, although there is a high risk of disease recurrence and progression to muscle-invasive disease. The standard treatment for intermediate and high-risk patients is a transurethral resection of bladder tumor, complemented by intravesical therapy with Bacillus Calmette-Guerin. EGFR immunoreactivity is present in about 50% of bladder cancers and correlates with recurrence, time to recurrence, and survival. Here we show the immunological results of an open, randomized, controlled, exploratory clinical trial in patients diagnosed with non-muscle invasive bladder cancer treated with CIMAvax-EGF therapeutic vaccine concomitant with intravesical BCG (n=20) or treated with intravesical BCG alone (n=24). The combination was safe and well tolerated. In vaccinated patients anti-EGF antibody titers increased during the first months of immunization and 80% of patients developed a good anti-EGF antibody response. Anti-EGF response was predominantly against the central region of the EGF molecule (loop B). IgG1 and IgG4 subclasses were the most relevant among the IgG anti-EGF antibodies generated after vaccination. The EGF serum levels were undetectable after six months of treatment. Vaccination with CIMAvax-EGF in combination with intravesical BCG develops a significant inverse correlation between anti-EGF antibody titers and baseline EGF serum concentration suggesting that in this context, the combination allowed the development of CIMAvax-EGF mechanism of action.
Keywords
Non-muscle invasive bladder cancer; Bacillus Calmette-Guerin; CIMAvax-EGF; Cancer immunotherapy
Non-muscle invasive bladder cancer articles; Bacillus Calmette-Guerin articles; CIMAvax-EGF articles; Cancer immunotherapy articles
Non-muscle invasive bladder cancer articles Non-muscle invasive bladder cancer Research articles Non-muscle invasive bladder cancer review articles Non-muscle invasive bladder cancer PubMed articles Non-muscle invasive bladder cancer PubMed Central articles Non-muscle invasive bladder cancer 2023 articles Non-muscle invasive bladder cancer 2024 articles Non-muscle invasive bladder cancer Scopus articles Non-muscle invasive bladder cancer impact factor journals Non-muscle invasive bladder cancer Scopus journals Non-muscle invasive bladder cancer PubMed journals Non-muscle invasive bladder cancer medical journals Non-muscle invasive bladder cancer free journals Non-muscle invasive bladder cancer best journals Non-muscle invasive bladder cancer top journals Non-muscle invasive bladder cancer free medical journals Non-muscle invasive bladder cancer famous journals Non-muscle invasive bladder cancer Google Scholar indexed journals Bacillus Calmette-Guerin articles Bacillus Calmette-Guerin Research articles Bacillus Calmette-Guerin review articles Bacillus Calmette-Guerin PubMed articles Bacillus Calmette-Guerin PubMed Central articles Bacillus Calmette-Guerin 2023 articles Bacillus Calmette-Guerin 2024 articles Bacillus Calmette-Guerin Scopus articles Bacillus Calmette-Guerin impact factor journals Bacillus Calmette-Guerin Scopus journals Bacillus Calmette-Guerin PubMed journals Bacillus Calmette-Guerin medical journals Bacillus Calmette-Guerin free journals Bacillus Calmette-Guerin best journals Bacillus Calmette-Guerin top journals Bacillus Calmette-Guerin free medical journals Bacillus Calmette-Guerin famous journals Bacillus Calmette-Guerin Google Scholar indexed journals CIMAvax-EGF articles CIMAvax-EGF Research articles CIMAvax-EGF review articles CIMAvax-EGF PubMed articles CIMAvax-EGF PubMed Central articles CIMAvax-EGF 2023 articles CIMAvax-EGF 2024 articles CIMAvax-EGF Scopus articles CIMAvax-EGF impact factor journals CIMAvax-EGF Scopus journals CIMAvax-EGF PubMed journals CIMAvax-EGF medical journals CIMAvax-EGF free journals CIMAvax-EGF best journals CIMAvax-EGF top journals CIMAvax-EGF free medical journals CIMAvax-EGF famous journals CIMAvax-EGF Google Scholar indexed journals Cancer immunotherapy articles Cancer immunotherapy Research articles Cancer immunotherapy review articles Cancer immunotherapy PubMed articles Cancer immunotherapy PubMed Central articles Cancer immunotherapy 2023 articles Cancer immunotherapy 2024 articles Cancer immunotherapy Scopus articles Cancer immunotherapy impact factor journals Cancer immunotherapy Scopus journals Cancer immunotherapy PubMed journals Cancer immunotherapy medical journals Cancer immunotherapy free journals Cancer immunotherapy best journals Cancer immunotherapy top journals Cancer immunotherapy free medical journals Cancer immunotherapy famous journals Cancer immunotherapy Google Scholar indexed journals malignancy articles malignancy Research articles malignancy review articles malignancy PubMed articles malignancy PubMed Central articles malignancy 2023 articles malignancy 2024 articles malignancy Scopus articles malignancy impact factor journals malignancy Scopus journals malignancy PubMed journals malignancy medical journals malignancy free journals malignancy best journals malignancy top journals malignancy free medical journals malignancy famous journals malignancy Google Scholar indexed journals transurethral resection articles transurethral resection Research articles transurethral resection review articles transurethral resection PubMed articles transurethral resection PubMed Central articles transurethral resection 2023 articles transurethral resection 2024 articles transurethral resection Scopus articles transurethral resection impact factor journals transurethral resection Scopus journals transurethral resection PubMed journals transurethral resection medical journals transurethral resection free journals transurethral resection best journals transurethral resection top journals transurethral resection free medical journals transurethral resection famous journals transurethral resection Google Scholar indexed journals immunoreactivity articles immunoreactivity Research articles immunoreactivity review articles immunoreactivity PubMed articles immunoreactivity PubMed Central articles immunoreactivity 2023 articles immunoreactivity 2024 articles immunoreactivity Scopus articles immunoreactivity impact factor journals immunoreactivity Scopus journals immunoreactivity PubMed journals immunoreactivity medical journals immunoreactivity free journals immunoreactivity best journals immunoreactivity top journals immunoreactivity free medical journals immunoreactivity famous journals immunoreactivity Google Scholar indexed journals intravesical BCG articles intravesical BCG Research articles intravesical BCG review articles intravesical BCG PubMed articles intravesical BCG PubMed Central articles intravesical BCG 2023 articles intravesical BCG 2024 articles intravesical BCG Scopus articles intravesical BCG impact factor journals intravesical BCG Scopus journals intravesical BCG PubMed journals intravesical BCG medical journals intravesical BCG free journals intravesical BCG best journals intravesical BCG top journals intravesical BCG free medical journals intravesical BCG famous journals intravesical BCG Google Scholar indexed journals therapeutic vaccine articles therapeutic vaccine Research articles therapeutic vaccine review articles therapeutic vaccine PubMed articles therapeutic vaccine PubMed Central articles therapeutic vaccine 2023 articles therapeutic vaccine 2024 articles therapeutic vaccine Scopus articles therapeutic vaccine impact factor journals therapeutic vaccine Scopus journals therapeutic vaccine PubMed journals therapeutic vaccine medical journals therapeutic vaccine free journals therapeutic vaccine best journals therapeutic vaccine top journals therapeutic vaccine free medical journals therapeutic vaccine famous journals therapeutic vaccine Google Scholar indexed journals tumor articles tumor Research articles tumor review articles tumor PubMed articles tumor PubMed Central articles tumor 2023 articles tumor 2024 articles tumor Scopus articles tumor impact factor journals tumor Scopus journals tumor PubMed journals tumor medical journals tumor free journals tumor best journals tumor top journals tumor free medical journals tumor famous journals tumor Google Scholar indexed journals
Article Details
1. Introduction
Bladder cancer (BCa) is the 9th most common malignancy worldwide and the seventh in male patients [1]. In Cuba it is the fifth cause of diseases by tumor malignancies, with a higher incidence and mortality in men than in women [2]. Ninety percent of patients diagnosed with bladder cancer are over 55 years and the average age at diagnosis is 73 years [1]. Aging and male sex are considered non-modifiable risk factors to develop this type of tumor [3], but, cigarette smoking is the most common risk with an estimated causal association for half of BCa in both genders [3, 4]. Approximately 75-80% of new diagnosed BCa patients are diagnosed with non-muscle invasive bladder cancer (NMIBC), while the other 25-30% of have muscle-invasive (MIBC) or metastatic disease at presentation [5, 6].
Although NMIBC usually courses with a favorable prognosis, there is a high risk of disease recurrence and risk of progression to muscle-invasive disease. The standard treatment for intermediate and high-risk patients is a transurethral resection of bladder tumor (TURBT), with both diagnostic and therapeutic purposes, complemented by intravesical therapy with Bacillus Calmette-Guerin (BCG). It is a non-specific immunotherapy and has been the standard of care treatment for some decades [7]. However, even after complete endoscopic resection and BCG treatment, there is a high recurrence rate of around 50-70% and 10 to 30% of patients will progress to MIBC [3, 7].
Many causes have been postulated for the BCG failure, including insufficient treatment, occult invasive or metastatic disease, inadequate immune response and gradual impairment of immune response [8]. Therefore, new therapies to enhance the antitumor immune response of BCG and the development of novel immunotherapeutic agents are needed. The combination of BCG therapy with PD-1 inhibitors such as pembrolizumab either intravesical or intravenously are currently being evaluated [6, 9].
The epidermal growth factor receptor (EGFR) family has been implicated in tumor cell growth and differentiation [10]. Expression and overexpression of the EGFR have been demonstrated in human solid tumors, such as in non-small cell lung cancer (NSCLC), prostate, colorectal, gastric and cancers of the bladder [11] . The intracellular domain is associated with protein tyrosine kinase activity and its overexpression by tumor cells alters the regulation of the cell cycle, blocks apoptosis, promotes angiogenesis, and increases the invasiveness of the tumor cells [12]. Protein overexpression of EGFR is found in 23% of bladder tumors. EGFR overexpression is associated with poorer prognosis and more aggressive tumor behavior than other low grade tumor. EGFR immunoreactivity is present in about 50% of BCa and correlates with bladder cancer recurrence, time to recurrence, and survival [13]. The epidermal growth factor (EGF) is one of the main ligands of the EGFR. Its binding induces receptor dimerization resulting in autophosphorylation and the transduction of mitogenic signals [14].
The strategy of “sequestering” EGF has been extensively studied by our group in the clinical setting, mainly in patients diagnosed with NSCLC. CIMAvax-EGF is a therapeutic cancer vaccine composed by human recombinant EGF coupled to a carrier protein (recombinant P64k of Neisseria meningitides) and exerts its anti-cancer activity by targeting the immune system, inducing anti-EGF antibodies that result in the decrease of the circulating EGF in sera [12, 15] Several clinical trials have demonstrated that the vaccine is immunogenic and safe [15].
Here we show the immunological results of an open, randomized, controlled, exploratory clinical trial in patients diagnosed with NMIBC who were treated with CIMAvax-EGF concomitant to intravesical BCG or intravesical BCG alone.
2. Patients and Methods
2.1 Patients
Eligible patients were 18 years or older diagnosed with NMIBC Ta, in situ carcinoma (CIS) or T1 tumor, previously treated by transurethral resection of bladder tumor (TURBT) or partial cystectomy. Other eligibility criterion was an Eastern Cooperative Oncology Group (ECOG) performance status index ranging from 0 to 2. Secondary malignancies, patients not suitable for treatment with intravesical BCG or history of hypersensitivity to foreign proteins rendered patients ineligible.
Forty-four NMIBC patients were recruited in four sites (Celestino Hernández Hospital, José Ramón López Tabranes Hospital, III Congreso Oncologic Hospital and María Curie Oncologic Hospital) from May 2013 to September 2016. Patients have been followed for 24 months and the clinical follow up will continue for 5 years. All patients provided a written informed consent.
2.2 Study design
A multicenter, controlled, randomized, open exploratory clinical trial was conducted with two study groups: patients diagnosed with NMIBC receiving conventional treatment with intravesical BCG concomitant with CIMAvax-EGF therapeutic vaccine (vaccine group) and patients diagnosed with NMIBC receiving only intravesical BCG (control group). The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practices guidelines. The ethics boards from all participating institutions approved the study protocol and the informed consent.
2.3 Treatments
Patients in the vaccine group received the intravesical BCG treatment that comprises an induction course (6 weekly treatments) and a maintenance course with instillations at 3rd and 6th months in the first year of treatment and every six months during the second year. CIMAvax-EGF was administered bi-weekly for the first four doses (induction phase), followed by monthly immunizations (maintenance). Patients in the control group received intravesical BCG treatment alone.
CIMAvax-EGF consists of human recombinant EGF manufactured in yeast (hu-recEGF) chemically conjugated to the P64K Neisseria meningitides recombinant protein (reP64K), manufactured in Escherichia coli. Both hu-recEGF and P64K were supplied by the Center for Genetic Engineering and Biotechnology, Havana, Cuba. The rhEGF-rrP64K conjugate is stored at 4ºC. As adjuvant was employed Montanide ISA 51 VG (NC0962946, Seppic) and emulsified in it, in a proportion 1:1 (v/v) immediately before injection. The vaccine formulation (rhEGF- rP64k/ Montanide) was administered at 2.4 mg of total EGF dose per vaccination (divided into four equal 0.6-mg intramuscular injections at four sites; two in the deltoids and two in the gluteus) (Figure 1)
The BCG is produced by the pharmaceutical laboratory “Carlos J. Finlay”. Each vial contains BCG for immunotherapy 50 mg/ml containing 5.03461 ± 3.62329 x 108 colony-forming units (CFU). The treatment schedule consisted in weekly repeated instillation during 6 weeks, followed by 3 consecutive weekly instillations at 3 months, at 6 months and thereafter every 6 months up to 24 months (Figure 1).
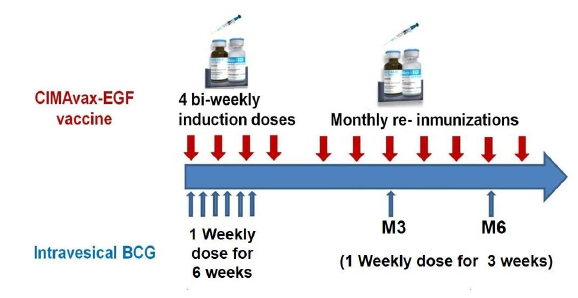
Figure 1: Treatment schedules in patients diagnosed with non-muscle invasive bladder cancer.
2.4 Safety and tolerability
All patients included in the study were evaluated for safety. The frequency, nature, causality, and severity of the adverse events were evaluated at each dose level. Severity was graded according to the NIH Common Terminology Criteria for Adverse Events, version 3.0. Special attention was given to administration related symptoms and allergic reactions.
2.5 Immune response measurements
2.5.1 Measurement of antibody titers by ELISA: Antibody titers against human EGF were measured by ELISA, as previously described [16]. Patients were classified in good antibody responders (GAR) if they developed anti-EGF antibody titers equal or higher than 1:4000 and super-good antibody responders if they reached anti-EGF antibody titers equal or higher than 1:64000. Those vaccinated patients that did not have titers above 1:4000 were classified as poor antibody responders.
2.5.2 Determination of EGF’s immunodominant epitope by ELISA: To identify the epitopes recognized by the serum of vaccinated patients, three peptides that represent different zones of the EGF molecule were synthesized. As previously described, both pre- and post-immune sera (1:100 dilution) from vaccinated patients were tested for antibody responses specific to different peptides from EGF molecule. EGF-derived peptide immunodominance was defined as an optical density signal (405 nm) of at least two times the one obtained with the rest of the peptides used in the assay [14, 16].
2.5.3 Determination of anti-EGF subclasses by ELISA: To characterize the anti-EGF IgG subclass, antihuman IgG1 (B6775, Sigma), IgG2 (B3398, Sigma) IgG3 (B3523, Sigma) and IgG4 (B3648, Sigma) subclass-specific secondary antibodies and alkaline phosphatase-conjugated streptavidin (189732, Sigma) were used in the ELISA assay previously described. All serum samples were diluted 1:100 and performed by triplicates. The optical density (OD) was read at 405 nm [14].
2.5.4 Measurement of EGF sera concentration: EGF concentration in serum was measured using the Human EGF Quantikine ELISA Kit (Cat# SEG00) from R&D Systems (Minneapolis, USA).
2.6 Statistical analyses
Kolmogorov–Smirnov test was used to explore normality of data. For the analyses of the immunological data, non-parametric Wilcoxon signed-rank test was used for matched-pairs taking into account that the samples were not normally distributed. Dunn’s multiple comparison test was employed, as post hoc analysis, after applying ANOVA. Spearman rank correlation was applied to estimate the correlation between anti-EGF Ab titers and EGF concentration. The statistical analyses were performed with GraphPadPrism 5 (San Diego, CA, USA) and SPSS program for Windows, v.19.0. The statistical data were considered significant if p<0.05.
3. Results
3.1 Patients characteristics
Forty-four patients diagnosed with NMIBC were randomly assigned 1:1 to CIMAvax-EGF vaccine concomitant with BCG or BCG alone from May 16, 2013 to September 23, 2016. Only three patients (12.5%) from the control arm and one patient from the vaccine group (5%) did not complete the BCG induction phase, while one patient who started vaccination did not complete CIMAvax-EGF induction (four doses). The main causes of early dropout were withdrawal of consent and disease progression. Demographic and baseline characteristics were comparable between both groups (Table 1).
|
Patient characteristics |
CIMAvax-EGF (%) |
Control (%) |
|
|
Age |
Mean |
62.84 |
63.91 |
|
years |
CI |
56-69 |
58-69 |
|
Ethnic group |
White |
16 (80) |
22 (91.8) |
|
Black |
1 (5) |
1 (4.1) |
|
|
Other |
3 (15) |
1 (4.1) |
|
|
ECOG |
0 |
14 (70) |
18 (75) |
|
1 |
2 (10) |
4 (16.6) |
|
|
2 |
4 (20) |
2 (8.4) |
|
|
Sex |
Female |
4 (20) |
8 (33.4) |
|
Male |
16 (80) |
16 (66.6) |
|
|
Smoking history |
Current |
11 (55) |
10 (41.7) |
|
Pass |
1 (5) |
0 |
|
|
Never |
8 (40) |
14 (58.3) |
|
ECOG: eastern cooperative oncology group; CI: confidence interval
Table 1: Demographic characteristics of patients diagnosed with non-muscle invasive bladder cancer according to the treatment group.
3.2 Safety of treatments
As previously described [17, 18], CIMAvax-EGF was safe. A total of 170 adverse events were reported: 146 in the group receiving CIMAvax-EGF and 24 in the group receiving BCG alone. Adverse events (AE) were mainly classified as grade 1 (mild) or 2 (moderate) (Table 2). Most frequent AE reported were fever (16.4%) and injection-site reactions (15.8%) and were related with CIMAvax-EGF administration. Grade 3-4 adverse events were seen in two of the vaccinated patients and one control patient, and consisted of urinary tract infection (UTI), painful urination and low back pain respectively. No serious AE were observed.
|
Adverse event |
CIMAvax-EGF |
Control |
||
|
No. |
% |
No. |
% |
|
|
Fever |
24 |
16.4 |
1 |
4.2 |
|
Injection-site reaction |
23 |
15.8 |
0 |
0 |
|
Dysuria |
9 |
6.2 |
4 |
16.7 |
|
Hematuria |
6 |
4.1 |
0 |
0 |
|
Myalgia |
6 |
4.1 |
0 |
0 |
|
Asthenia |
5 |
3.4 |
1 |
4.2 |
|
Dizziness |
4 |
2.7 |
0 |
0 |
|
UTI |
2 |
1.2 |
1 |
4.2 |
No.: number of events; UTI: urinary tract infection
Table 2: Adverse events according to treatment group.
3.3 Immune response induced by CIMAvax-EGF vaccination
3.3.1 Antibody response: Vaccination with CIMAvax-EGF in patients diagnosed with NMIBC was immunogenic. Anti-EGF antibodies were evaluated in 25 patients (15 vaccinated and 10 controls). Gradual increases in the geometric mean of anti-EGF titers were observed in the vaccine group after repeated immunizations. Out of 15 vaccinated patients, 12 (80%) were classified as good responders; among them, one patient (6.66%) was categorized as super-good responder. In the control group, the titers of anti-EGF antibodies remained similar to the baseline during all the evaluation period (Figure 2a).
3.3.2 Immunodominance of anti-EGF antibodies against EGF peptides: To evaluate the immunodominance of the antibody response induced by vaccination, serum from eight patients classified as GAR were tested against three peptides corresponding to N-terminal, central (Loop B) and C-terminal (Loop C) regions of the EGF molecule. Before immunization started, there was a similar distribution of anti-EGF IgG Ab response to all peptides. At 6th month of vaccination, the EGF-specific Ab response was significantly directed against the loop B (p=0.0350, Wilcoxon test); for 56% (n=5) of the analyzed patients, the anti-EGF antibodies were focused on the central peptide corresponding to loop B. In these patients, the Absorbance of the antibody ELISA against the central peptide was twice the mean Absorbance of the response against the other peptides. In the remaining patients, the antibody response was distributed against all regions of the EGF molecule without a clear dominance (Figure 2b).
3.3.3 Elicited anti-EGF IgG subclasses: The distribution of IgG subclasses of anti-EGF Ab was evaluated in eight patients classified as GAR, during the first year of vaccination. After 3 months of vaccination, the humoral response against EGF was predominantly IgG3 (p=0.0167, Mann Whitney test). At this moment also IgG1 was a relevant IgG subclass (p=0.0728, Mann Whitney test). As previously reported, after 6 months of treatment with CIMAvax-EGF, IgG4 was the predominant subclass (M6: p=0.0025, M12: p=0.0139, Mann Whitney test). However, IgG1 subclass was also predominant (M6: p=0.0175, M12: p=0.0017, Mann Whitney test). This pattern of IgG1/IgG4 anti-EGF IgG subclasses was maintained for the rest of the evaluated period. For the first time, IgG1 subclass is found among the anti-EGF IgG relevant subclasses after CIMAvax-EGF immunization. These results suggest that the concomitance between CIMAvax-EGF and intravesical BCG induced this new pattern of IgG subclasses distribution (Figure 2c).
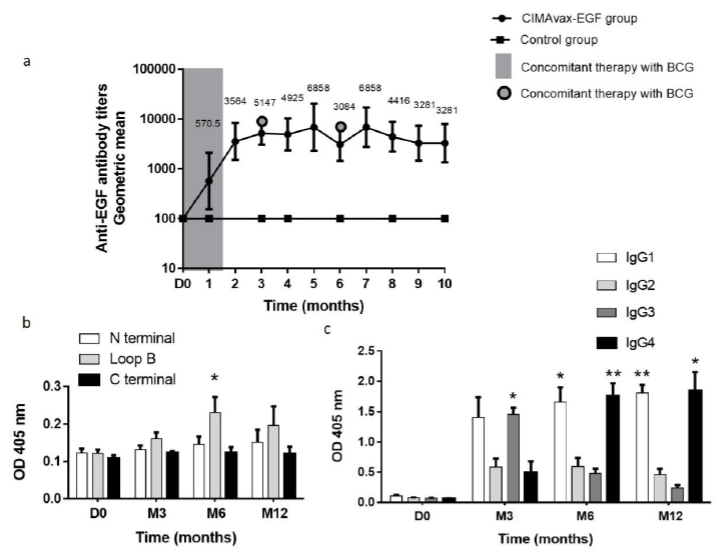
Figure 2: Induction of EGF-specific humoral immune response in patients diagnosed with NMIBC. a Kinetics of anti-EGF Ab titers in serum from CIMAvax-EGF vaccinated patients (n=15) and controls (n=10) during the first ten months of immunization. Serum EGF IgG antibody titers were determined by ELISA at indicated time points and presented as the inverse of serum dilution. b IgG response to EGF-derived peptides from vaccinated patients classified as GAR (n=8). Antibody levels against different regions of EGF molecule were determined by ELISA at indicated time points and presented as values of Absorbance at 405 nm. Asterisks (*) represent significant differences according to Dunn test: (*) p<0.05. c Levels of EGF-specific IgG subclasses from vaccinated patients classified as GAR (n=8). Serum levels of EGF-specific IgG1, IgG2, IgG3 and IgG4 levels were determined by ELISA using subclass-specific antibodies and presented as values of Absorbance at 405 nm. Asterisks (*) represent significant differences according to Dunn test: (*) p<0.05, (**) p<0.01. EGF: epidermal growth factor; OD: optical density.
3.4 Serum EGF concentrations
The reduction of EGF serum levels during treatment with CIMAvax-EGF in patients diagnosed with NSCLC has been interpreted as an in vivo effect of the anti-EGF Ab generated after immunization [12, 18, 19]. The serum from 23 patients (14 vaccinated and 9 controls) were tested to detect EGF concentrations before vaccination. Low to medium levels of circulating EGF were detected (mean 280 pg/mL, range: 0-1187 pg/mL). Fourteen patients (61%) had detectable EGF concentration at baseline evaluation. More than 70% of the vaccinated patients decreased their EGF serum levels to undetectable values after four administrations of CIMAvax-EGF. In all the vaccinated patients, serum EGF concentration decreased to undetectable levels at month 6 of treatment (Figure 3a). On the contrary, no variation in the EGF levels was observed in serum from control patients (Figure 3b)
As it has been described in previous works [16, 18], there was a statistically significant inverse correlation between the EGF serum concentration and the anti-EGF antibody response over time in the vaccine group according to the Pearson correlation coefficient (r=-0.3988, p=0.0082), as shown in Figure 3c.
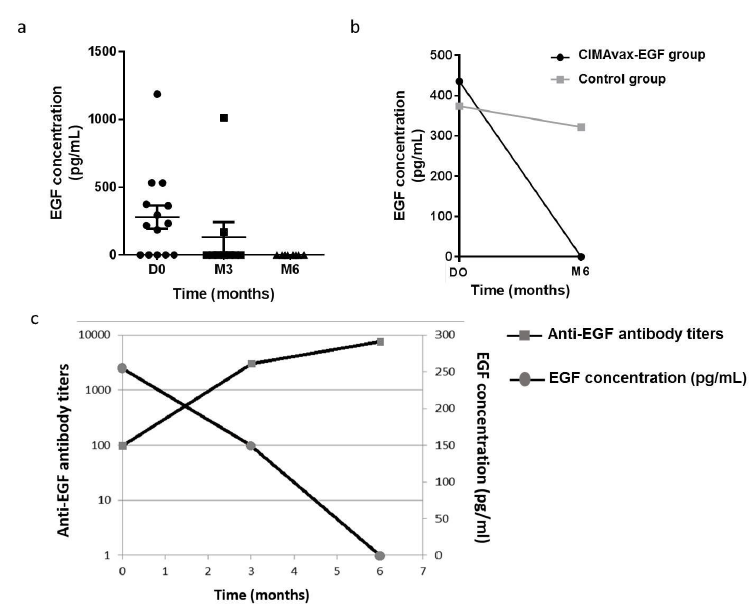
Figure 3: Serum levels of EGF in patients diagnosed with NMIBC. a Circulating EGF levels in vaccinated patients (n=14) patients during 6 months. b Circulating EGF concentrations in vaccinated patients (n=14) patients and control patients (n=9) before treatment and at 6th month of vaccination. c Inverse correlation between anti-EGF Ab titers and EGF serum levels in patients vaccinated with CIMAvax-EGF. Spearman correlation p<0.05.
4. Discussion
Bladder cancer is the ninth most commonly encountered malignancy worldwide and sixth cause of death by cancer in men, with an increase of new cases every year [20]. Nearly 75% of diagnosed bladder cancer cases are non-muscle invasive and are treated by TURBT followed by intravesical treatment with BCG [13, 21]. However, recurrence or progression of these lesions is frequent. Probability of recurrence at 5 years ranges from 31 to 78% [22-24]. Novel therapeutic agents such as immune checkpoint inhibitors (CPIs), cancer vaccines, and immunogene therapy, have emerged as potential immunotherapeutic options [6, 9].
Due to the relevance of EGF-EGFR couple in tumor cell growth and differentiation of some human cancers, among them, bladder cancer, anti-EGF strategies emerged as attractive therapies [12, 25]. This work describes, by the first time, the immune response against EGF generated in patients diagnosed with NMIBC treated with CIMAvax-EGF vaccine in combination with intravesical BCG. The rational of this combination was to induce systemic Abs against EGF capable to neutralize this growth factor and ultimately inhibit its binding to EGFR and the phosphorylation of the receptor. It has been described that NSCLC patients who exhibit a high Ab response against EGF able to inhibit its binding to EGFR, achieve an increased survival [16].
Based on previous clinical results showed the safely profile and the immunogenicity of CIMAvax-EGF when used in patients diagnosed with NSCLC [17, 18], an exploratory clinical trial was performed in patients suffering NMIBC. The vaccine was well tolerated. The most frequent adverse events were fever and injection-site reactions, similar with previous reports [17, 18].
The existence of a natural response specific to EGF was detected in all evaluated patients. The existence of natural anti-EGF Abs has been described in healthy subjects [26] and has been also found in patients with NSCLC before and after chemotherapy [18]. The levels of anti-EGF Abs gradually increased from the beginning of vaccination. At third month of immunization, the 80% of vaccinated patients were classified as GAR. These results are consistent with previous study, although generated in a different subset of patients [18]. Therefore, these results suggest that the vaccination with CIMAvax-EGF concomitant with BCG did not interfere with the immunogenicity of the vaccine.
During the evaluation of the antibodies, we checked the specific response against different regions of the EGF molecule. It was observed an increase in the response against the central region of EGF (Loop B) at sixth month of vaccination, while the humoral response against the rest of peptides representing the EGF molecule did not change during treatment. These results are similar to those reported in a cohort of patients diagnosed with advanced NSCLC using CIMAvax-EGF as switch maintenance after platinum-based chemotherapy [14, 19].
An anti-EGF IgG3 Ab response was generated after 3 months of vaccination. This response was promptly switched for IgG4 after 6 months of treatment with the vaccine. Previous studies from our lab have described the same behavior of anti-EGF subclasses [14, 19]. The development of IgG4 responses is often the outcome of repeated or prolonged antigen exposure [27]. The IgG4 subclass has also been associated with strong neutralization activity [28], which is very appropriate in the case of CIMAvax-EGF because of its mechanism of action.
Interesting, by the first time, it was detected the presence of IgG1 among the subclasses generated after CIMAvax-EGF treatment. It appeared very early from the 3rd month of vaccination and continued during all the evaluated period. IgG1 is generally formed against protein antigens. Functionally, is a strong inducer of Fc-mediated effector mechanisms, such as antibody-dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity, and antibody-dependent cellular phagocytosis (ADCP) [27]. Intravesical BCG elicits a potent local immune response and recruits effector T cells into the NMIBC tumor microenvironment (TME) [29]. Some works associated the success of intravesical BCG with the induction of a T helper (Th) 1 response, characterized by production of interleukin (IL) -2, interferon gamma (IFNγ) and IL-12. CD4+ and CD8+ T cells, NK cells and granulocytes are the main cell types in mediating the immunotherapeutic effect of BCG [30-32]. However, it has been proposed that humoral factors and antibodies also contribute to the immune activation observed in the TME. It has been also suggested that the IFNγ produced by Th1 cells induces IgG2a and IgG2b type IgG subclass in mice and IgG1 and IgG3 type in human [33]. In line with this evidences, we hypothesized that intravesical BCG in combination with CIMAvax-EGF generated an IgG1 anti-EGF Ab response from the stimulation of a Th1 response induced by local treatment with BCG.
Lau and colleagues demonstrated the presence of cytoplasmic EGF in urothelial and renal carcinomas. Due to the evidences of internalization and fast degradation of receptor-bound EGF, these authors suggested its active local synthesis and proposed that EGF synthesized by urothelial and renal carcinomas may be involved in an autocrine mechanism of malignant proliferation [34]. From this, anti-EGF IgG1 Abs could bind EGF not only in circulation but also in tumor cells. Therefore, we hypothesize that the possible mechanism of action of the combination of CIMAvax-EGF with BCG in NMIBC patients could be the blockade of the interaction EGF-EGFR by antibodies, together with the anti-tumoral and cytotoxic effect supported by the ADCP and ADCC capacity of IgG1 anti-EGF specific Abs.
It has been well demonstrated that CIMAvax-EGF is not only immunogenic but also able to reduce EGF serum concentration [14, 18]. In this work, EGF serum levels reduced to undetectable values after four administrations of CIMAvax-EGF in the majority of vaccinated patients and in all of them at 6th month of vaccination. Surprisingly, baseline serum EGF concentration was lower compared to the values found in previous studies in patients diagnosed with NSCLC [16, 18]. EGF has been also detected in urine of patients with urothelial tumors [35, 36], and associated its decrease with invasion of tumors [36]. We could not explore the presence of this growth factor in the urine of our patients. However, we are very interested performing this study, looking for possible subrogates biomarker of the efficacy of CIMAvax-EGF vaccination in patients with bladder cancer.
Our group has previously described an inverse correlation between serum EGF levels and the anti-EGF antibody titers also demonstrated in the present study, confirming that the reduction of EGF serum concentration represents an indicator of the effect of the antibodies generated by immunization [18].
The aberrant expression of EGFR [25] and the local production of the EGF molecule in urothelial carcinomas [36] point to the relevance of the EGF-EGFR system among the therapeutic armamentarium against NMIBC. The effect of the combination in the clinical outcome of treated patients remains to be analyzed (manuscript in preparation).
5. Conclusion
Our results showed that the administration of CIMAvax-EGF vaccine concomitantly with intravesical BCG in NMIBC patients is safe and induces the increase of anti-EGF Abs titers and the reduction of circulating serum EGF to undetectable levels, suggesting that in this context it is possible to trigger the proposed mechanism of action of CIMAvax-EGF.
Acknowledgments
The authors are especially grateful to the patients and their families, as well as the staff of all institutions involved in this study.
Author’s Contributions
Conception and design: PC. Rodríguez, M. Hernández, D. Saavedra
Clinical investigators (recruited and treated patients): V.M. Medina, E. Ibañez, E. Santiesteban, Y. Caveda, K. Camacho, RM. Amador
Immunological assessments: L. Martínez, A. González, D. Saavedra
Trial conduction and supervision: M. Hernández, M. Cepeda, L. Cabrera, A. Gorte
Data collection: M. Hernández, Y. Santiesteban, M. Cepeda, L. Cabrera, A. Gorte, J.C. Hernández, D. Saavedra
Analysis and interpretation of data: L. Martínez, J.C. Hernández, P. Lorenzo-Luaces, D. Saavedra, Z. Mazorra, T. Crombet
Writing and or/revision of the manuscript: J.C. Hernández, Z. Mazorra, D. Saavedra
Financial Support
This study did not receive any financial support.
Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68 (2018): 394-424.
- Dirección de registros médicos y estadísticas de salud. Ministerio de Salud Pública de Cuba. Anuario Estadístico de Salud 2019. accesible in:https://filessldcu/bvscuba/files/2020/05/Anuario-Electr%C3%B3nico-Espa%C3%B1ol-2019-ed-2020pdf, (2020).
- Oliveira MC, Caires HR, Oliveira MJ, et al. Urinary Biomarkers in Bladder Cancer: Where Do We Stand and Potential Role of Extracellular Vesicles. Cancers (Basel) 12 (2020).
- Sanli O, Dobruch J, Knowles MA, et al. Bladder cancer. Nat Rev Dis Primers 3 (2017): 17022.
- Sanguedolce F, Russo D, Mancini V, et al. Human Epidermal Growth Factor Receptor 2 in Non-Muscle Invasive Bladder Cancer: Issues in Assessment Methods and Its Role as Prognostic/Predictive Marker and Putative Therapeutic Target: A Comprehensive Review. Urol Int 102 (2019): 249-261.
- Boegemann M, Aydin AM, Bagrodia A, et al. Prospects and progress of immunotherapy for bladder cancer. Expert Opin Biol Ther 17 (2017): 1417-1431.
- Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 36 (2018): 440-447.
- Butt SU, Malik L. Role of immunotherapy in bladder cancer: past, present and future. Cancer Chemother Pharmacol 81 (2018): 629-645.
- Gupta M, Kates M, Bivalacqua TJ. Immunotherapy in nonmuscle invasive bladder cancer: current and emerging treatments. Curr Opin Oncol 31 (2019): 183-187.
- Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 21 (2009): 177-184.
- Li W, Wang Y, Tan S, et al. Overexpression of Epidermal Growth Factor Receptor (EGFR) and HER-2 in Bladder Carcinoma and Its Association with Patients' Clinical Features. Med Sci Monit 24 (2018): 7178-7185.
- Saavedra D, Crombet T. CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Front Immunol 8 (2017): 269.
- Railkar R, Krane LS, Li QQ, et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol Cancer Ther 16 (2017): 2201-2214.
- Popa X, García B, Fuentes KP, et al. Anti-EGF antibodies as surrogate biomarkers of clinical efficacy in stage IIIB/IV non-small-cell lung cancer patients treated with an optimized CIMAvax-EGF vaccination schedule. OncoImmunology 9 (2020): 1762465.
- Crombet Ramos T, Rodriguez PC, Neninger Vinageras E, Garcia Verdecia B, Lage Davila A. CIMAvax EGF (EGF-P64K) vaccine for the treatment of non-small-cell lung cancer. Expert review of vaccines 14 (2015): 1303-1311.
- Garcia B, Neninger E, de la Torre A, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clinical cancer research : an official journal of the American Association for Cancer Research 14 (2008): 840-846.
- Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol 26 (2008): 1452-1458.
- Rodriguez PC, Popa X, Martinez O, et al. A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clinical cancer research: an official journal of the American Association for Cancer Research 22 (2016): 3782-3790.
- Saavedra D, Neninger E, Rodriguez C, et al. CIMAvax-EGF: Toward long-term survival of advanced NSCLC. Semin Oncol 45 (2018): 34-40.
- Golla V, Lenis AT, Faiena I, Chamie K. Intravesical Therapy for Non-muscle Invasive Bladder Cancer-Current and Future Options in the Age of Bacillus Calmette-Guerin Shortage. Rev Urol 21 (2019): 145-153.
- O'Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol 24 (2006): 481-487.
- Ucpinar B, Erbin A, Ayranci A, et al. Prediction of recurrence in non-muscle invasive bladder cancer patients. Do patient characteristics matter? J BUON 24 (2019): 1659-1665.
- Kamat AM, Colombel M, Sundi D, et al. BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol 14 (2017): 244-255.
- Nykopp TK, Batista da Costa J, Mannas M, Black PC. Current Clinical Trials in Non-muscle Invasive Bladder Cancer. Curr Urol Rep 19 (2018): 101.
- Yang X, Flaig TW. Novel targeted agents for the treatment of bladder cancer: translating laboratory advances into clinical application. Int Braz J Urol 36 (2010): 273-282.
- Gonzalez G, Montero E, Leon K, Cohen IR, Lage A. Autoimmunization to epidermal growth factor, a component of the immunological homunculus. Autoimmun Rev 1 (2002): 89-95.
- de Taeye SW, Rispens T, Vidarsson G. The Ligands for Human IgG and Their Effector Functions. Antibodies (Basel) 8 (2019).
- Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 39 (2009): 469-477.
- Kates M, Nirschl T, Sopko NA, et al. Intravesical BCG Induces CD4(+) T-Cell Expansion in an Immune Competent Model of Bladder Cancer. Cancer Immunol Res 5 (2017): 594-603.
- Ayari C, LaRue H, Hovington H, et al. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guerin immunotherapy. Eur Urol 55 (2009): 1386-1395.
- Saint F, Kurth N, Maille P, et al. Urinary IL-2 assay for monitoring intravesical bacillus Calmette-Guerin response of superficial bladder cancer during induction course and maintenance therapy. Int J Cancer 107 (2003): 434-440.
- Pryor K, Goddard J, Goldstein D, et al. Bacillus Calmette-Guerin (BCG) enhances monocyte- and lymphocyte-mediated bladder tumour cell killing. Br J Cancer 71 (1995): 801-807.
- Macedo LT, Ribeiro J, Curigliano G, et al. Multidisciplinary approach in the treatment of patients with small cell bladder carcinoma. Eur J Surg Oncol 37 (2011): 558-562.
- Lau JL, Fowler JE, Jr., Ghosh L. Epidermal growth factor in the normal and neoplastic kidney and bladder. J Urol 139 (1988): 170-175.
- Saika T, Tsushima T, Nasu Y, et al. Epidermal growth factor in urine from patients with bladder cancer. Urol Res 28 (2000): 230-234.
- Fuse H, Mizuno I, Sakamoto M, et al. Epidermal growth factor in urine from the patients with urothelial tumors. Urol Int 48 (1992): 261-264.
