High Expression Levels of Foxp3 and VISTA in Advanced Human Gliomas and Impact on Patient’s Prognosis
Article Information
Amina Ghouzlani1, Sarah Kandoussi1, Soumaya Rafii1, Abdelhakim Lakhdar2,3, Abdallah Badou1,*
1Cellular and Molecular Pathology Laboratory, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
2 Department of Neurosurgery, UHC Ibn Rochd, Casablanca, Morocco
3Laboratory of research on neurologic, neurosensorial diseases and handicap, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
*Corresponding author: Abdallah Badou, Cellular and Molecular Pathology Laboratory, Faculty of medicine and pharmacy, University Hassan II, Casablanca, Morocco
Received: 28 October 2020; Accepted: 05 November 2020; Published: 17 November 2020
Citation: Amina Ghouzlani, Sarah Kandoussi, Soumaya Rafii, Abdelhakim Lakhdar, Abdallah Badou. High Expression Levels of Foxp3 and VISTA in Advanced Human Gliomas and Impact on Patient’s Prognosis. Archives of Clinical and Biomedical Research 4 (2020): 691-703.
Share at FacebookAbstract
Gliomas are considered the most malignant cancers of the body. Despite the advances in cancer therapy, this type of tumor continues to progress and be more aggressive. In human gliomas, anti-tumor T cell responses are inhibited through induction of local and systemic immunosuppression. Cancer immunotherapy using immune checkpoint blockade has made a great stride in mending patient’s clinical outcome for multiple types of cancers. However, many studies reported that treatment of glioblastoma patients with anti-CTLA4 and anti-PD-1 has no survival benefit compared to standard chemotherapy. The aim of this study was to investigate the role of regulatory T cells as well as VISTA in the suppression of immune cell functions within glioma microenvironment, using molecular biology and bioinformatics approaches. Foxp3 and VISTA mRNA expression were assessed in human glioma patients at different grades using 2 independent cohorts, a set of 20 Moroccan patients, and a series of 667 patients from TCGA. The expression of Foxp3, a transcription factor specific for Treg cells, and VISTA, a newly identified immune checkpoint molecule, significantly correlated with gliomas grading and with the most aggressive histological type. Indeed, this expression was associated with bad overall survival of patients. Thus, finding new strategies for blocking VISTA on Treg cells in the tumor microenvironment could be beneficial in stopping the tumor progression. Our study highlighted a correlation between high levels of VISTA expression and Foxp3 with a bad prognosis in glioma patients. VISTA expression in Treg cells might be involved in glioma progression and could be considered as a possible new therapeutic target especially in glioblastoma.
Keywords
Foxp3; VISTA; Treg; Glioblastoma; Immunotherapy; Immune checkpoint
Foxp3 articles; VISTA articles; Treg articles; Glioblastoma articles; Immunotherapy articles; Immune checkpoint articles
Article Details
1. Introduction
Gliomas are the most frequent and aggressive primary brain tumors in adults. They constitute a deep and unresolved human clinical problem. Although, considerable progress has been made in the treatment of other types of cancers, many questions remain unanswered around gliomas [1]. Despite current treatments, including surgery, chemotherapy, and radiation therapy, these tumors continue to grow and recur with a more aggressive and resistant phenotype [2].
An interesting way to kill malignant cells would be to induce an immune response against the tumor. It should be able to distinguish tumor cells from normal cells [3]. However, it is interesting to exploit this potential to fight gliomas. Ongoing and recently completed clinical trials include the use of cancer vaccines, adoptive transfer of effector cells, and the use of checkpoint inhibitors to reverse the immunosuppression prevalent in the tumor microenvironment [4].
In the last decades, immunotherapy has brought new hope as a potential novel therapeutic approach for glioma patients [5]. However, the majority of glioma patients did not respond to the blockade of usual immune checkpoints pathways [5-8]. This has increased our interest in finding novel therapeutic strategies through the activation of effector cells of the immune system so that it could be beneficial for glioma patients.
Forkhead box protein P3 (Foxp3) was shown to be a key regulatory gene for development of regulatory T cells (T reg). It was reported in the 1980s that CD4+ CD25+ T cells could inhibit anti-tumor immunity [9]. However, in 2003 it was demonstrated that Foxp3 plays an important role in the differentiation, development, and function of regulatory T cells [9]. In addition, in 2005, Foxp3+ CD25+ CD4+ T cells naturally expressing Foxp3 were defined as Treg cells [10].
Glioma microenvironment is known to be profoundly immunosuppressed, and understanding whether these tumors have Treg-mediated immune resistance would be interesting to develop and initiate specific immunotherapeutic approaches [11].
VISTA belongs to the CD28 receptor family and is expressed primarily on myeloid and granulocyte cells, NK cells, macrophages and DCs, but not on B cells. Its expression is highest in naive cells and FoxP3+ T reg cells [12]. Through the interaction with the receptor on T cells, VISTA negatively inhibits T cell responses [13]. In vitro, it induces the development of Treg cells with the help of TGF-β [14]. The present study aimed to investigate the potential relationship between Foxp3 and VISTA expression, according to human glioma progression.
2. Materials and Methods
2.1 Patients and samples
mRNA expression was assessed in a total of 20 glioma tissues at different grades: 8 glioblastomas of grade IV, 2 Astrocytomas of grade III, 1 Astrocytomas of grade II, 5 Astrocytomas of grade I, 2 Oligodendrogliomas of grade II, and 2 Ependymoma of grade II. Patients were at the Ibn Rochd University Hospital, neurosurgery department (Casablanca, Morocco), and had been previously diagnosed with glioma. Patients had not undergone any therapy before tumor resection. All glioma tissues were graded according to the World Health Organization (WHO). Clinical information, including gender, age and smoking status were obtained from the medical records of the patients.
2.2 TCGA data analysis
Transcriptome data of patients diagnosed with glioma (WHO II-IV) were collected from The Cancer Genome Atlas (TCGA) dataset (n = 667) (http://cancergenome.nih.gov/). Data analysis as well as statistical tests were carried out by two different people in the lab. During the analysis with TCGA RNAseq data, expression values were log converted.
2.3 Total RNA isolation and Reverse transcription (RT)
Total RNA was extracted from frozen glioma samples using TRisur reagent (Bioline, France) as previously described [15]. RNA concentration and quality were measured using the NanoVueTM plus Spectrophotometer (GE Healthcare, UK). cDNA was synthesized using Tetro Reverse Transcriptase Enzyme (Bioline, France) from 0.5μg of total RNA in a 20μl reaction mixture according to the manufacturer’s instructions, with 1μl Random Hexamer Primer 25µg (Bioline, France) and 4μl of RNase-Free Water added and incubated at 70°C for 5min to break the secondary structure of RNA. Next, the mixture was maintained on ice. 4μl Tetro Reverse Transcriptase buffer, 4 μl of dNTP (10mM), 0.5μl of RNase Inhibitor (Invitrogene, France), 0.5μl Tetro Reverse Transcriptase Enzyme (Bioline, France) and 1μl of RNase-Free Water were added and incubated at 25°C for 10min then at 45°C for 30min then at 85 °C for 5min.
2.4 Real time RT-PCR assays
Relative quantification of gene expression was analyzed by real-time PCR in the presence of the fluorescent dye SYBR ™ Green PCR Master Mix (Thermofischer). β-actin was used as an internal control to evaluate relative expression of VISTA and Foxp3. Experiments were performed in a 20μL reaction volume with specific primer pairs used at 10 µM for all genes.
PCR was programmed as follows: 10min at 95°C for polymerase activation and sample denaturation, then 40cycles of 15s at 95°C and 60s at 60°C for annealing and extension. Fluorescence readings at the end of the extension phase of each cycle were used to estimate the values for the threshold cycles (Ct).
Primer pairs were as follows:
β-actin Forward: 5’-TGGAATCCTGTGGCATCCATGAAAC-3’
Reverse: 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’
VISTA Forward: 5-TGTAGACCAGGAGCAGGATG-3’
Reverse: 5-ATGCACCATCCAACTGTGTG-3’
Foxp3 Forward: 5’TCTTCCTTGAACCCCATGCC-3’
Reverse: 5’GCATGAAATGTGGCCTGTCC-3’
The Ct values for each gene were converted into relative quantification (2-ΔCt).
2.5 Statistical analysis
In this research work, statistical analysis was executed using GraphPad Prisme 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance between mean values was determined by using Student’s t-test and one-way ANOVA. Survival curve was created by Kaplan-Meier method based on log-rank test.
3. Results
3.1 Foxp3 and VISTA gene expression were upregulated in advanced glioma grades
In total, 20 glioma tissues (8 men and 12 women) were recruited in the current study. The characteristics of glioma patients were described in Table 1. Glioma patients were classified according to the WHO as follows: 8 glioblastomas of grade IV, 2 Astrocytomas of grade III, 1 Astrocytomas of grade II, 5 Astrocytomas of grade I, 2 Oligodendrogliomas of grade II, and 2 Ependymoma of grade II (Table 1).
|
Variable |
Cases (%) |
|
(n= 20) |
|
|
Sex |
|
|
• Male |
8 (40) |
|
• Female |
12 (60) |
|
Age |
|
|
• Children (≤ 18 years) |
5 (25) |
|
• Adults (>18 years) |
15 (75) |
|
WHO Grade |
|
|
• Low grade (I-II) |
10 (50) |
|
• High grade (III-IV) |
10 (50) |
|
Histological type |
|
|
• Astrocytomas |
16 (80) |
|
• Oligodendrogliomas |
2 (10) |
|
• Ependymomas |
2 (10) |
|
Smoking status |
|
|
• Yes |
14 (70) |
|
• No |
6 (30) |
Table 1: Clinic-pathological characteristics of glioma patients.
|
Variable |
Cases n (%) |
|
|
|
|
Sex |
|
|
• Male |
327 (49,1) |
|
• Female |
339 (50,9) |
|
Age |
|
|
• Children (≤ 18 years) |
3 (0,45) |
|
• Adults (>18 years) |
664 (99,55) |
|
WHO Grade |
|
|
• Low grade (II-III) |
518 (77.66) |
|
• High grade (IV) |
149 (22,33) |
|
Histological type |
|
|
• Astrocytoma |
245 (36.73) |
|
• Oligoastrocytoma |
129 (19,34) |
|
• Oligodendroglioma |
293 (43.92) |
|
Glioblastoma Subtype |
|
|
• Mesenchymal |
49 (34,3) |
|
• Classical |
39 (27,3) |
|
• Neural |
26 (18,2) |
|
• Proneural |
29 (20,2) |
|
Karnofsky score |
|
|
•>80 |
204 (69,6) |
|
• 80-60 |
84 (28,7) |
|
•<60 |
5 (1,7) |
|
IDH mutation status |
|
|
• Yes |
135 (23,6) |
|
• No |
437 (76,4) |
Table 2: Clinic-pathological characteristics of glioma patients in TCGA dataset.
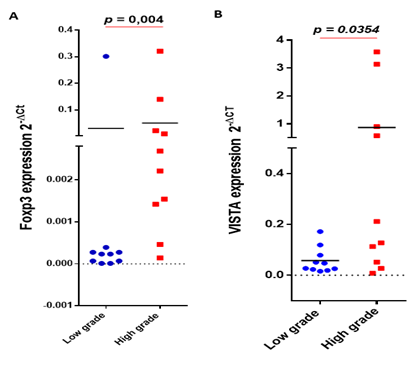
To assess the association between Foxp3 expression and glioma pathogenesis, 20 glioma cases were analyzed. mRNA expression levels of Foxp3 were evaluated by real time RT-PCR. Foxp3 gene showed a significantly higher mRNA expression in advanced grades of gliomas (p = 0.004) (Figure 1A). On the other hand, we evaluated the expression of VISTA, an immune checkpoint molecule that is known to be highly expressed on myeloid cells and Foxp3+ CD4+regulatory cells [14]on the same set of glioma patients. VISTA transcripts were also significantly elevated in high grade glioma patients compared to low grades (p = 0.0354) (Figure 1B).
Figure 1: Foxp3 and VISTA gene expression were upregulated in advanced glioma grades. Foxp3 and VISTA transcript expression was evaluated using real time RT-PCR analysis. (A) Foxp3 gene was highly expressed in grades III-IV compared to grades I-II of glioma patients; (B) VISTA was strongly expressed in advanced glioma grades.
3.2 Foxp3 transcripts positively correlated with VISTA and other critical regulatory T cell-secreted cytokines
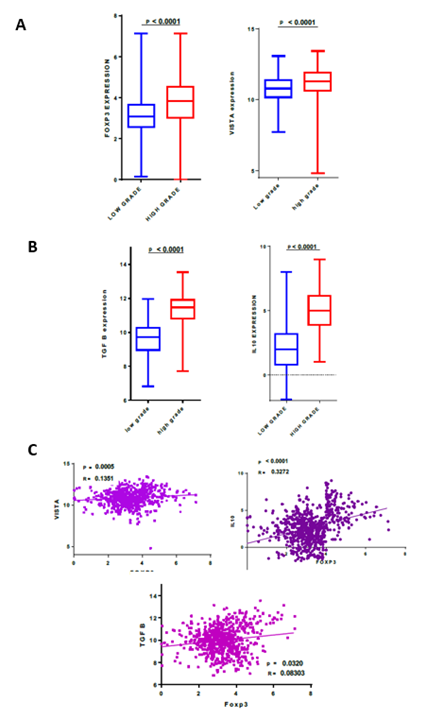
In order to study the expression of Foxp3 and VISTA in a distinct cohort, we analyzed the RNA-sequencing data of gliomas from the TCGA dataset. 667 samples were evaluated and were organized according to the WHO grading system. Compared to low grades, high grade gliomas present a significantly higher Foxp3 and VISTA expression (p<0.0001) (Figure 2A).
Using the TCGA dataset, the expression pattern of Foxp3 was then compared to the expression of VISTA and two critical cytokines (TGFβ, IL-10), known to be expressed by regulatory T cells [16]. Indeed, high glioma grades (glioblastoma) showed significantly higher TGFβ and IL-10 expressions compared to low grades, exhibiting a similar expression profile to VISTA and Foxp3 (p<0.0001) (Figure 2B). Subsequently, a correlation study was done between Foxp3 expression and the same immune molecules (VISTA, TGFβ and IL-10). Foxp3 was positively correlated with VISTA (p = 0.0005, r= 0.1351), TGFβ (p = 0.0320, r= 0.08303) and IL-10 (p< 0.0001, r= 0.3272) gene expression (Figure 2C), suggesting that tumor cells likely use regulatory T cells that express VISTA gene to escape the immune system.
Figure 1: Foxp3 and VISTA gene expression were upregulated in advanced glioma grades. Foxp3 and VISTA transcript expression was evaluated using real time RT-PCR analysis. (A) Foxp3 gene was highly expressed in grades III-IV compared to grades I-II of glioma patients; (B) VISTA was strongly expressed in advanced glioma grades.
Figure 2: Foxp3 transcripts positively correlated with VISTA and other critical regulatory T cell-secreted cytokines. RNAseq of 667 glioma patients were analyzed using TCGA dataset. (A) Foxp3 and VISTA mRNA exhibited high expression levels in advanced grades of gliomas; (B) TGFβ and IL-10 showed elevated expression in high grade glioma (glioblastoma) compared to low grade; (C) Foxp3 expression was positively correlated with VISTA, TGFβ and IL-10.
3.3 Elevated expression of Foxp3 and VISTA in glioma patient’s microenvironment associated to a poor overall survival
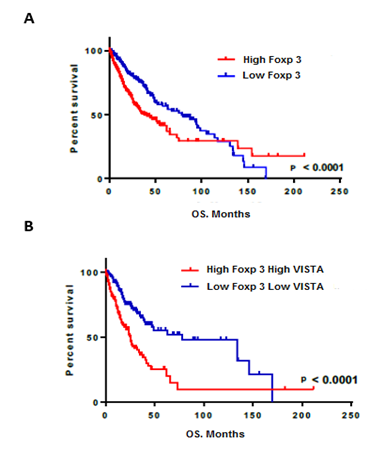
In order to investigate the impact on patient survival, we evaluated the prognostic value of Foxp3 and VISTA in the TCGA dataset. Survival data were available for 666 human glioma patients. As showed using Kaplan–Meier curves, patients with lower Foxp3 expression had better survival in comparison with patient with higher expression of Foxp3 (p<0.0001) (Figure 3A). Remarkably, glioma patients having higher expression levels of both Foxp3 and VISTA showed a profound bad survival compared with those presenting low expression of both genes (p<0.0001) (Figure 3B). These results indicated that regulatory T cells that express VISTA could be considered as a bad prognostic factor for patients with glioma.
4. Discussion
Gliomas are known to be the most frequent and fatal primary brain tumors in adults [17]. Despite treatment of glioblastoma patients with conventional therapies, the prognosis is still poor [6]. Immune checkpoint inhibitors blockade is one of the newest and promising approaches to boost the anti-tumor immune response and it has made great stride in improving patient's clinical outcome for multiple cancer types [18, 19]. Unluckily, the majority of glioma patients did not respond to the blockade of usual immune checkpoint pathways [5-8], which catalyzed our interest in exploring additional targets to enhance the immune system against glioma progression.
Thus, the main objective of this study was to evaluate the expression and role of Foxp3 and VISTA in human gliomas. At the best of our knowledge, this is the first exploration of the role of Foxp3 and VISTA in clinically resected glioma tumors using two independent cohorts (TCGA and a Moroccan cohort).
In this study, we attempted to investigate the role of regulatory T cells (Tregs) in glioma. A wide variety of markers are expressed in Treg cells, including Foxp3 which is the most specific and the main regulator of these cells [18]. We tried to evaluate the Foxp3 and VISTA (Immune checkpoint molecule that is highly expressed on myeloid cells and Foxp3+ CD4+regulatory cells [14]) expression according to glioma grade. We found that Foxp3 expression was significantly correlated with the histological grade of glioma. Patients with high grade glioma (grade III and IV) presented a significant overexpression of Foxp3 gene compared to those with a low grade (grade I and II). This result correlates with the study reported by Wang et al., that showed that strong expression of Foxp3 is linked to a poor prognosis for patients (Wang et al., 2014).
Regulatory T cells have been widely described in several cancer types, but their value as predictors of disease outcome is debatable in glioblastoma. Independent researchers described that there is an increased infiltration of Foxp3+Treg in advanced grades of various brain tumor types, including glioblastoma [19, 20].
V-domain Immunoglobulin suppressor of T cell activation (VISTA) is a recently discovered new Immunoglobulin (Ig) superfamily ligand [13]. VISTA expression is observed in the majority of immune cells, including CD4+ and CD8+ T cells, NK cells, macrophages, DCs and neutrophils, but not B cells [21]. In this current study, VISTA mRNA was highly expressed in advanced glioma grades. The elevated expression of both genes (Foxp3 and VISTA) suggests that regulatory T cells strongly infiltrate VISTA-expressing advanced grades of human gliomas, which may lead to glioma progression.
One main way of immune cell suppression involves the secretion of inhibitory cytokines such us IL-10 and TGF-β [22]. Using The Cancer Genome Atlas "TCGA", we attempted to explore their expression in glioma patients according to different parameters and in correlation with Foxp3 and VISTA. The expression of these 2 potent inhibitory molecules was significantly increased in patients with high grade gliomas. Their presence in the glioma microenvironment could be explained by their immune suppression ability. TGFβ can inhibit the expression of MHC classe I and II molecules in glioma cells, which allows tumor cells to invade surrounding tissues without being detected by immune cells [23]. IL-10 negatively regulates MHC class II molecule expression on monocytes and positively regulates the PD-L1 checkpoint molecule on glioma-associated macrophages [24]. Also, it inhibits the production of IFN-γ and TNF-α by immune cells [25], which causes energy and apoptosis of T cells [26, 27]. Overall, these data would explain the invasive potential of glioma cells.
Regarding immune checkpoints, the positive and significant correlation between the expression of Foxp3 and VISTA has been described by DiDomenico et al. [28]. In the case of sarcoma, overexpression of VISTA inhibits antitumoral immunity, and the blockade of VISTA effectively inhibited tumor growth by decreasing Treg cell and increasing CD8+ and CD4+ effector T cell infiltration in tumors [29]. We also detect a significant improvement in survival for patients with low expression of VISTA and foxp3 compared to those with high expression of VISTA and Foxp3. Wang et al. demonstrated that in the presence of TGF-ß, VISTA Ig promoted partially the differentiation of iTreg, and that this effect could be observed in both murine and human CD4+ T cells [14, 30]. Interestingly, blocking VISTA in tumor cells improved the survival of mice that were inoculated with VISTA-overexpressing ovarian cancer cells, although combined therapy using anti-PD-1 and anti-VISTA did not further enhance mice survival compared to anti-VISTA treatment alone [31].
In summary, our data revealed a correlation between VISTA and Foxp3 expression with glioma progression in patients. This study also indicated that VISTA and regulatory T cells could represent a bad prognostic factor in gliomas, and pinpoints VISTA and Foxp3 as a possible new therapeutic target, especially in advanced stages of gliomas.
Nomenclature
WHO: World Health Organization
GBM: Glioblastoma
PD-1: Programmed cell death1
VISTA: V-domain Immunoglobulin suppressor of T cell activation
Foxp3: Forkhead box protein P3
PCR: Polymerase chain reaction
IL-2: Interleukin-2
IL-10: Interleukin-10
TGFβ: Transforming growth factor β
IFNγ: Interferon gamma
TCGA: The Cancer Genome Atlas
mRNA: Messenger RNA
Conflict of Interest
The Authors declare that they have no conflicts of interest.
Funding
This work was supported by the Moroccan Ministry of Higher Education and Research and The National Center for Scientific and Technical Research (CNRST) through a “PPR1” project coordinated by A.B. A.G. was supported by a “CNRST” fellowship.
Author Contributions
A.G. collected, analyzed, and interpreted data; wrote the manuscript; K.S. analyzed, and interpreted data; S.R. collected, analyzed, data; A.L. collected and analyzed data; A.B. designed research, analyzed and interpreted data, wrote the manuscript, and supervised the study.
Acknowledgment
We would like to thankall members of the Neurosurgery department, including, Pr. A. El azhari, Pr. S. Hilmani, Pr. K. Ibahioin, Pr. A. Bertal, Pr. A. Naja, Pr. A. Challaoui, Dr. A. Bocco, Dr .T. Mesbahi, Dr. Z. Ennhaili, Dr. M. Fatihi, Dr. O. Benhayoun, Dr. M. Haous, Dr. D. Kaba, Dr. I. Mahazou, Dr. A. Jehri, and nurses (S. Khayam, S. Watti, H. Bchira, Fz. Remiany).
References
- Koo Y-E L, et al. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev 58 (2006): 1556-1577.
- Bondy ML, et al. Brain Tumor Epidemiology: Consensus from the Brain Tumor Epidemiology Consortium (BTEC). Cancer 113 (2008): 1953-1968.
- Brain Tumor Immunotherapy. Humana Press (2001).
- Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nature Reviews Cancer 20 (2020): 12-25.
- Han SJ, Zygourakis C, Lim M, Parsa AT. Immunotherapy for glioma: promises and challenges. Clin. N. Am 23 (2012): 357-370.
- Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Med 8 (2002): 793-800.
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Med 5 (1999): 1365-1369.
- Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4 (2012):
- T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med 151 (1980): 69-80.
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6 (2005): 345-352.
- Heimberger AB, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg 56 (2009): 98-106.
- Nowak EC, et al. Immunoregulatory functions of VISTA. Rev 276 (2017): 66-79.
- Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. Exp. Med 208 (2011): 577-592.
- Mercier IL, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res 74 (2014): 1933-1944.
- Moutia M, et al. Capparis Spinosa L. promotes anti-inflammatory response in vitro through the control of cytokine gene expression in human peripheral blood mononuclear cells. BMC Immunol 17 (2016):
- Yue Q, et al. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J Neurooncol 116 (2014): 251-259.
- Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic Cancer. Cancer Res 23 (2017): 3129-3138.
- Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol 38 (2008): 925-927.
- El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol 83 (2207): 145-152.
- Jacobs JFM, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. Journal of Neuroimmunology 225 (2010): 195-199.
- Wang L, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Natl. Acad. Sci. U.S.A. 111 (2014): 14846-14851.
- Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology 8 (2008): 523-532.
- Nana AW, Yang PM, Lin HY. Overview of transforming growth factor β superfamily involvement in glioblastoma initiation and progression. Asian Pacific journal of cancer prevention?: APJCP 16 (2015): 6813-6823.
- Bloch O, et al. Gliomas Promote Immunosuppression through Induction of B7-H1 Expression in Tumor-Associated Macrophages. Clin Cancer Res 19 (2013): 3165-3175.
- Kennedy RH, Silver R. Neuroimmune Signaling: Cytokines and the Central Nervous System. in Neuroscience in the 21st Century: From Basic to Clinical (eds. Pfaff, D. W. and Volkow, N. D.) (2016): 601-641.
- Wintterle S, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res 63 (2003): 7462-7467.
- De Waele J, et al. Poly(I:C) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade. Oncoimmunology 7 (2017).
- DiDomenico J et al. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology 7 (2018):
- Kondo Y, et al. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncology 57 (2016): 54-60.
- Lines JL, et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res 74 (2014): 1924-1932.
- Mulati K, et al. VISTA expressed in tumour cells regulates T cell function. British Journal of Cancer 120 (2019):