Heterogeneity of Low Voltage Areas Spatial Distribution in Sinus Rhythm during Electroanatomic Mapping of Persistent Atrial Fibrillation
Article Information
Antoine Lepillier1*, Xavier Copie1, William Escande1, Marjorie Niro1, Olivier Paziaud1, Olivier Piot1
1Centre Cardiologique du Nord - 32, rue des Moulins Gémeaux - 93200 Saint-Denis, France
*Corresponding author: Antoine Lepillier, Centre Cardiologique du Nord - 32, rue des Moulins Gémeaux - 93200 Saint-Denis, France.
Received: 14 Febraury 2023; Accepted: 20 Febraury 2023; Published: 28 Febraury 2023
Citation: Antoine Lepillier, Xavier Copie, William Escande, Marjorie Niro, Olivier Paziaud, Olivier Piot. Heterogeneity of Low Voltage Areas Spatial Distribution in Sinus Rhythm during Electroanatomic Mapping of Persistent Atrial Fibrillation. Cardiology and Cardiovascular Medicine 7 (2023): 106-111.
Share at FacebookAbstract
Background: Current ablation strategies for persistent AF have shown a limited success rate with frequent arrhythmia recurrences. Several studies suggest PVI and complementary ablation of low voltage area (LVA) may represent an efficient strategy.
Aim: Distribution of (LVA) in sinus rhythm in patients undergoing persistent AF ablation may represent a marker of AF recurrence during follow-up.
Methods: We prospectively included patients with persistent AF (age: 63 +/- 8.7 years, men 69.3%). The ablation strategy consisted in circumferential PVI followed by a electro-anatomic mapping in sinus rhythm. Complementary radiofrequency (RF) ablation was guided by LVA < 0.5 mV. Success was defined as freedom from AF/ atrial flutter or atrial arrhythmia (AT) for a period ranging from 3 months to 18 months or more.
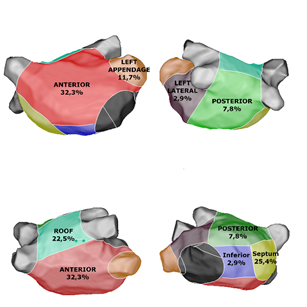
Results: 101 patients with persistent AF were included. LVA were identified in 48 patients (47%). Two or more different sites of LVA were found in 40 patients. The distribution of the 108 different LVA was: 33 anterior wall (32.3%), 26 septum (25.4%), 8 posterior (7.8%), 3 lateral (2.9%), 12 left appendage (11.7%), 23 roof (22.5%), 3 inferior (2.9%). RF ablation was performed in all low voltage areas. At the end of procedure, 76 patients (75.2%) were non inducible. At the end of FU of 18 months, and after a single procedure, 72.3% of patients were free of symptomatic AF (n=73 patients) and 65.3% of patients (n=66) were free of AF/AT recurrence. LVA distribution was not associated with AF/AT recurrence at 18 months.
Conclusion: Patients with persistent AF have an heterogeneity of LVA distribution in SR, a tailored ablation in SR can be an efficient strategy in addition to PVI to maintain SR.
Keywords
Atrial Fibrillation; Low Voltage Areas; Radiofrequency Ablation
Atrial Fibrillation articles; Low Voltage Areas articles; Radiofrequency Ablation articles
Article Details
1. Introduction
Pulmonary veins represent the major triggers for paroxysmal atrial fibrillation (AF), circumferential ablation of PV to isolate them from the rest of the atrium is the cornerstone of ablation [1]. In case of persistent AF, current ablation strategies have shown a limited success rate with frequent arrhythmia recurrences [2, 3]. To improve the maintenance of sinus rhythm, substrate ablation strategies has been considered for use in conjunction to pulmonary vein isolation (PVI). The most common approaches for substrate modification were the creation of linear lesions in the left atrium [4] and focal ablation to eliminate complex atrial signals [5]. STAR-AF 2 trial assessed that PVI only is as effective as other ablation strategies [6]. In another arm, recent studies suggest that the atrial arrhythmogenic sites are related to regions with heterogeneities and increased fibrosis which is detected by reduced bipolar voltage areas [7, 8]. Targeting low voltage areas in addition to PVI may represent an efficient strategy for the ablation of persistent AF. We hypothesized that additional lesion beyond PVI, whether at low voltage potentials, should enhance the single-procedure efficacy of ablation in patients with persistent AF.
2. Methods
This was a single-center study in which participating subjects were symptomatic patients for persistent AF and referred for ablation procedures at our center from June 2016 to June 2018. All subjects with drug-refractory AF undergoing their first ablation procedure that fulfilled European Society of Cardiology (ESC) and Heart Rhythm Society [1] defined criteria for persistent or long lasting persistent AF were eligible to participate in the study. Persistent AF was defined according to ESC guidelines as an episode of “AF lasting longer than 7 days, including episodes that are terminated by cardioversion, either with drugs or by direct current cardioversion, after 7 days or more”. The exclusion criteria included aged 18 years, inability to provide informed consent, prior AF ablation, paroxysmal AF and any contraindication to undergoing AF ablation (mechanical prosthetic mitral valve, contraindications for anticoagulation, pregnancy, left atrial thrombus). Primary study end point was freedom from AF and/or organized atrial tachyarrhythmias at 1 year after a single-ablation procedure. The first 3 months after the ablation (blanking period) were censored. Beyond this, any symptomatic or asymptomatic AF or AT episode that lasted for 30 seconds was categorized with a 24-hour ambulatory Holter monitor. The secondary study end points were as follows: (1) total procedure time; (2) total fluoroscopy time; and (3) occurrence of serious adverse events that included death, pericardial effusion causing tamponade or requiring pericardiocentesis, cerebrovascular events, significant PV stenosis (symptomatic or asymptomatic 70% reduction in PV diameter in 1 veins), left atrial-esophageal fistula, diaphragmatic paralysis, and any vascular complication requiring transfusion or intervention.
2.1 Ablation Procedure
Anticoagulation therapy was continued throughout the ablation period. A CT scan before the ablation procedure was performed in order to assess pulmonary vein anatomy. Transoephageal and transthoracic echocardiography were realised to exclude left atrial (LA) thrombus, and mesured LA diameter, LA surface, left ventricle ejection fraction. The ablation procedures were performed with the patient under general anesthesia. Right femoral venous access was obtained, a decapolar catheter was placed in the coronary sinus (EZ-CS decapolar, Biosense Webster), a single transeptal puncture was performed using an SL0 sheath (St. Jude Medical), and a BRK needle. A bolus of unfractionned heparin (50–100 IU/kg) was administered immediately after the trans-septal puncture and additional boluses of heparin were administered throughout the procedure in order to maintain the activated clotting time (ACT) between 300 and 350 seconds. PVI guided by a three-dimensional non fluoroscopic mapping system (CARTO3; Biosense Webster) using an irrigated-tip contact force–sensing radiofrequency ablation catheter (Thermocool SmartTouch or SmartTouch Surround Flow; Biosense Webster). Radiofrequency (RF) energy was delivered with a power of 30 Watts at the posterior wall and 35 Watts at the anterior wall. The contact force targeted before lesion delivery was 10 grams with minimum individual lesion duration of 350-400 gram-seconds force-time integral. Circumferential ablation lesions were delivered around each of the PV ostia until each vein was isolated electrically from the left atrium (ie, bidirectional conduction block). An electrical cardioversion was realized for patients in AF to convert to sinus rhythm. PVI was assessed by entry block and exit block in sinus rhythm. A voltage map was realized with a multi-electrode Penta-Ray (Biosense Webster) in sinus rhythm to identify low voltage areas (Figure 1), the contact with atrial walls was assessed by Tissue Proximity Index (TPI®, Biosense Webster). Low voltage potentials were defined by all amplitude electrograms < 0.5 mV. Complementary RF was guided by low voltage areas, window of interest of the map was adjusted between 0.2 and 0.4 mV to achieve homogenization and complete elimination of all low amplitude and/or fractionated electrograms (Figure 2). RF energy was delivered targeting a power of 30 Watts at the posterior wall and 35 Watts at the anterior wall, during 20 seconds. Inducibility at end of procedure was tested for all patients.
2.2 Follow-up
Success was defined as freedom from symptomatic AF/atrial flutter or atrial arrhythmia for a period ranging from 3 months to 18 months. After catheter ablation, patients received oral anticoagulation for at least 3 months. Patients were followed for 12 months after the ablation procedure with clinical visits, a 12-lead ECG, and a 24-hour ambulatory Holter monitor at 3, 6, 12 and 18 months. The primary end point was time to first recurrence of symptomatic or asymptomatic atrial tachyarrhythmia (AF, atrial flutter or atrial tachycardia) documented by any form of monitoring, between 3 to 18 months after ablation, or a repeat ablation procedure. An atrial tachyarrhythmia qualified as an arrhythmia if it lasted for 30 seconds or longer. A standard 3 months blanking period for early AF recurrence was used.
2.3 Statistical Analysis
Continuous variables are expressed as mean ± 1 SD or median and interquartile range (IQR) (25th, 75th percentile). A chi-square test or Fisher exact probability test was used for comparison of categorical variables. Logistic regression analysis was used to estimate the effects on the presence of LVA. All significant variables in the univariate analysis were included in a multivariate analysis. A 2-tailed p value of < 0.05 indicated statistical significance.
3. Results
3.1 Baseline Characteristics
A total of 101 consecutive patients referred for ablation of persistent symptomatic AF at our center, were included in the study from June 2016 to June 2018. Baseline characteristics are resumed in Table 1. The majority of patients were male (69.3%), aged 63 +/- 8.7 years. Six patients presented with long standing AF (5.8%). Mean functional EHRA class was 3 +/- 0.6. Fifty-seven patients were treated for hypertension (56.4 %), diabetes in 18 patients (17.6 %), obesity (IMC > 30) was found in 19 patients (18.6 %). Mean CHA2DS2-VASC Score: 2.6 +/- 0.5. Most of the patients (87.2 %) were treated with direct oral anticoagulants. 59 patients (57.8%) were taken antiarrhythmic therapy (Amiodarone or AAR class I), and 79 patients (77.4%) were taking beta-blockers. Mean left ventricle ejection fraction (LVEF) was 51.9 +/- 12.2%. A total of 27 patients (26.4%) had reduced ejection fraction (LVEF < 40%) and 3.9% had coronary artery disease. Mean LA diameter was 48.3 +/- 6.6 mm and mean LA surface was 27 +/- 7.4 cm².
|
N = 101 patients |
Total |
AF/AT free |
AF recurrence |
p |
|
Age (Years) |
63±8.7 |
63.1±8.9 |
62.9±8.5 |
0,902 |
|
Sexe F (n, %) |
31 (30.3) |
19 (29.2) |
12 (32.4) |
0,735 |
|
Long standing AF (n, %) |
6 (5.8) |
4 (6.1) |
2 (5.4) |
0,877 |
|
EHRA class |
Mean 3 +/- 0.6 |
|||
|
EHRA 2 |
14 (13.7) |
12 (18.4) |
2 (5.4) |
0,065 |
|
EHRA 3 |
65 (63.7) |
41 (63) |
24 (64.8) |
0,856 |
|
EHRA 4 |
24 (23.5) |
12 (18.4) |
12 (32.4) |
0,109 |
|
High Blood Pressure (n, %) |
57 (56.4) |
34 (52.3) |
23 (63.8) |
0,26 |
|
Diabetes (n, %) |
18 (17.6) |
11 (16.9) |
7 (18.9) |
0,799 |
|
Obesity (IMC> 30) (n, %) |
19 (18.6) |
11 (16.9) |
8 (21.6) |
0,557 |
|
LVEF (%) |
51.9±12.2 |
53.1±12.1 |
49.8±12.3 |
0,194 |
|
DCM (LVEF<40%) (n,%) |
27 (26.4) |
15 (23) |
12 (32.4) |
0,303 |
|
CAD (n, %) |
4 (3.9) |
4 (6.1) |
0 (0) |
0,123 |
|
Medications (n, %) |
||||
|
Amiodarone |
54 (52.9) |
39 (60) |
15 (40.5) |
0,058 |
|
AAR I |
5 (4.9) |
1 (1.5) |
4 (10.8) |
0,037 |
|
Betablockers |
79 (77.4) |
48 (73.8) |
31 (83.7) |
0,248 |
Table 1: Characteristics of the Patients.
LVEF- Left Ventricle Ejection Fraction; DCM- Dilated Cardiomyopathy; CAD- Coronary Artery Disease.
3.2 Procedural Data
Procedural datas are resumed in table 2. Fifteen patients (14.8%) were in sinus rhythm at the beginning of procedure. Patients with AF (84.2%) had a mean cycle-length (coronary sinus) 170.9±28.2 msec. Sinus rhythm was obtained after electrical cardioversion for all patients. Complete PVI was obtained for 98% of patients with entry and exit block. Mean LA volume 182.6±44.8 mL. Low voltage areas (<0.5 mV) were found in 48 patients (47%). Two or more different sites of LVA were found in 40 patients (83.3%). The distribution of the 108 different areas of low voltage electrograms < 0.5 mV was (Figure 2): 33 anterior wall (32.3%), 26 septum (25.4%), 8 posterior (7.8%), 3 lateral (2.9%), 12 left appendage (11.7%), 23 roof (22.5%), 3 inferior (2.9%). At the end of the procedure, 76 patients (75.2%) were non inducible, 9 patients (8%) were still inducible for AF, and 17 patients (16.8%) for organized atrial tachycardia. Acute complication rate was 2%: two patients presented cardiac tamponade.
|
N = 101 patients |
Total |
AF/AT free |
AF recurrence |
p |
|
Left Atrium dimension |
||||
|
LA diameter (cm) |
48.3±6.6 |
47.6±7 |
49±6.2 |
0,519 |
|
LA Surface (cm²) |
27.2±7.4 |
26.2±6.1 |
29±9 |
0,066 |
|
LA Volume (mL) |
182.6±44.8 |
177±44.3 |
192.7±44.6 |
0,097 |
|
Cycle CS (ms) |
170.9±28.2 |
175.2±28.4 |
163.7±26.8 |
0,068 |
|
Low Voltage Areas (< 0.5mV) (n, %) |
48 (47) |
26 (40) |
22 (59.4) |
0,058 |
|
Distribution of LVA (n, %) |
||||
|
Anterior |
33 (32.3) |
19 (29.2) |
14 (37.8) |
0,371 |
|
Septum |
26 (25.4) |
16 (24.6) |
10 (27) |
0,788 |
|
Posterior |
8 (7.8) |
7 (10.7) |
1 (2.7) |
0,145 |
|
Lateral |
3 (2.9) |
3 (4.6) |
0 (0) |
0,184 |
|
Left Appendage |
12 (11.7) |
5 (7.6) |
7 (18.9) |
0,09 |
|
Roof |
23 (22.5) |
11 (16.9) |
12 (32.4) |
0,071 |
|
Inferior |
3 (2.9) |
3 (4.6) |
0 (0) |
0,184 |
|
Procedural datas |
||||
|
Procedure Duration (min) |
144.6±33.7 |
142.6±34.7 |
147.9±32.1 |
0,457 |
|
Radiation Exposure (sec) |
154.8±95.8 |
146±101.5 |
170.2±84 |
0,229 |
|
Radiation Exposure (mGym²) |
0.5±0.3 |
0.5±0.3 |
0.6±0.3 |
0,136 |
Table 2: Procedural Datas.
CS- Coronary Sinus.
3.3 Follow-up
At the end of FU of 18 months, and after a single procedure, 72.3% of patients were free of symptomatic AF (n=73 patients) and 65.3% of patients (n=66) were free of AF/AT recurrence. In the 28 patients with recurrence of AF, different types were found: 5 paroxysmal AF, 15 persistent AF and 8 permanent AF. Redo procedure was successfully achieved in 13 patients and consisted in PVI only strategy in the 4 paroxysmal AF, and PVI and low voltage guided strategy for the 9 persistent AF. 8 patients were considered for rate control-only strategy. Atrial tachycardia occurred in 9 patients. The mechanisms of AT were typical cavo-tricuspid flutter in 3 patients, peri-mitral flutter in 3 patients, and atrial focal tachycardia in 3 patients : one from left appendage, one focal tachycardia close to RSPV and one from mid-septal LA. All these tachycardia were ablated with success, without any recurrence during FU. No predictive factor of AF/AT recurrence was found.
4. Discussion
Catheter ablation of atrial fibrillation (AF) has become a successful therapeutic option in patients whose quality of life is severely disturbed by AF and in whom anti-arrythmic drug therapy is inefficient or non-tolerated [1-2]. Pulmonary veins are a major determinant in the initiation of paroxysmal AF [9], and PVI is associated with a significant reduction of AF recurrence. However in persistent AF, current ablation strategies have shown a limited success rate [10]. For instance, the STAR-AF 2 trial [6], showed that PVI-only was as effective as PVI with defragmentation and PVI with lines. Recently, left atrial bipolar endocardial voltage mapping has emerged as an invasive tool used during radiofrequency ablation procedures for defining the AF substrate. MRI analyses [11] demonstrated the atrial fibrosis impact on AF ablation outcome: circumferential PV antral scarring predicted ablation success in mild atrial fibrosis. Thus, the detection of atrial fibrosis may be useful in selecting the appropriate strategy in catheter ablation of AF. Atrial scar quantified automatically in MRI, spatially correlates with endocardial voltage. Our study suggests these low voltage areas (LVA) seems to have a major implication in the perpetuation of AF. Jadidi et al. [12], reported that ablation of low-voltage areas in addition to PVI was more effective than a PVI-only approach in persistent AF ablation. LVA were defined as a voltage below 0.5mV during AF and associated with fractionated or rotational activity or discrete rapid local activity during AF. Our strategy was to define low voltage with a threshold inferior to 0.5 mV in sinus rhythm. It confirms previous studies, reported [13] that the mean bipolar electrogram voltage during AF was significantly lower in dense delayed enhancement regions detected by cardiac MRI. For Spragg et al, a late gadolinium enhancement detect areas of low voltage (<0.5 mV) with a sensitivity of 84% but with specificity of 68% [14]. In another study, 21 patients with paroxysmal AF [15], increased MRI signal intensity correlated with progressively lower bipolar voltage. AF triggers and low voltage areas depend critically on the voltage thresholds chosen to define low voltage. Histological correlation between low voltage and ventricular scar has been demonstrated in a porcine ventricular infarct model [16]. Lin et al [17] compared voltages of patients undergoing left-sided accessory pathway ablation with AF patients in sinus rhythm. In patients without AF, 95% of all electrograms were above 0.38 mV. It confirms our hypothesis that the detection of low voltage areas in sinus rhythm may represent a major determinant in the perpetuation of AF. The distribution of low voltage areas is not homogenous between different atrial regions: we found a low incidence of LVA in the inferior and posterior areas. It confirms previous studies: highest voltage [18] was found in the left atrial floor and the lowest in the left atrium–pulmonary vein junctions. The posterior wall tends to have higher voltages than other segments of the left atrium when mapped in AF. The left atrial appendage and mid anterior wall have the highest voltages. If the posterior wall is a healthy tissue, it is probably unnecessary needed to apply complementary RF ablation on this area. As known, this region is close to esophagus with a risk of injury. Our result suggests this approach can preserve healthy atrial tissue and limit the risk of complications. We observed a low incidence of atrial tachycardia of 9% (AT) during follow-up with this strategy. Previous studies [19] reported a higher prevalence of AT following ablation of persistent AF ablation, when large defragmentation or linear ablation strategies were performed (29-39 %) in apparently healthy tissue. Scherr a al reported the importance of repeat procedures [10] to maintain sinus rhythm at long term. Our study has however several significant limitations because of its observational and mono-centric nature. Indeed, there is no comparative group with PVI-only or PVI with defragmentation. Yagishita et al. [20] compared low voltage area-guided ablation, with additional complex electrogram ablation, or standard stepwise ablation including linear ablation but did not show a significant improvement in outcomes. Further comparative studies with longer follow-up are needed in order to confirm long term efficacy of such strategy on the recurrence of AF and the low incidence of AT.
5. Conclusion
Low voltage ablation associated to PVI may be a safe and efficient strategy for the ablation of persistent AF. We noted heterogeneity of the spatial distribution of LVA, this approach may preserve healthy atrial tissue, resulting in a low incidence of atrial tachycardia. Further interventional studies are needed to compare such strategy to a PVI-only approach in the ablation of persistent AF.
Acknowledgment
We would like to thank the ADREC team (Association Dionysienne de Recherche et d'Enseignement Cardiologique) for its support in the preparation of the manuscript.
Conflict of Interest
None to declare.
References
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 14 (2017): e275-e444.
- Parkash R, Verma A, Tang AS. Persistent atrial fibrillation: current approach and controversies. Curr Opin Cardiol 25 (2010): 1-7.
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 7 (2010): 835-846.
- Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 110 (2004): 2996-3002.
- Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 43 (2004): 2044-2053.
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372 (2015): 1812-1822.
- Haissaguerre M, Shah AJ, Cochet H, et al. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiologic mechanism of human persistent atrial fibrillation. J Physiol (2016).
- Jadidi AS, Cochet H, Shah AJ, et al. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: Combined magnetic resonance imaging and high-density mapping. JACC 62 (2013): 802-812.
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339 (1998): 659-666.
- Scherr D, O´Neil M, Knecht S, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circulation Arrhythm Electrophysiol 8 (2015): 18-24.
- Akoum N, Daccarett M, Mcgann C, et al. Atrial Fibrosis Helps Select the Appropriate Patient and Strategy in Catheter Ablation of Atrial Fibrillation: A DE-MRI Guided Approach. J Cardiovasc Electrophysiol 22 (2011): 16-22
- Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 9 (2016):
- Miyamoto K, Tsuchiya T, Narita S, et al. Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation. Europace 11 (2009): 1597-1605.
- Spragg DD, Khurram I, Zimmerman SL, et al. Initial experience with magnetic resonance imaging of atrial scar and coregistration with electroanatomic voltage mapping during atrial fibrillation: Success and limitations. Heart Rhythm 9 (2012): 2003-2009.
- Malcolme-Lawes LC, Juli C, et al. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: A 2-center study. Heart Rhythm 10 (2013): 1184-1189.
- Callans DJ, Ren JF, Michele J, et al. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction: Correlation with intracardiac echocardiography and pathological analysis. Circulation 100 (1999): 1744-1750.
- Lin Y, Yang B, Garcia FC, et al. Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. J Interv Card Electrophysiol 39 (2014): 57-67.
- Kapa S, Desjardins B, Callans DJ, et al. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 25 (2014): 1044-1052.
- Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2 (2005): 464-471.
- Yagishita A, Gimbel JR, De Oliveira S, et al. Long-term outcome of left atrial voltage-guided substrate ablation during atrial fibrillation: A novel adjunctive ablation strategy. J Cardiovasc Electrophysiol 28 (2017): 147-155.