Fluoroscopy Guided Minimally Invasive Swine Model of Myocardial Infarction by Left Coronary Artery Occlusion for Regenerative Cardiology
Article Information
Finosh G Thankam*, Mohamed Radwan, Angelo Keklikian, Manreet Atwal, Taj Rai and Devendra K Agrawal*
Department of Translational Research, Western University of Health Sciences, Pomona, CA 91766, USA
*Corresponding authors: Devendra K Agrawal, Department of Translational Research, Western University of Health Sciences, Pomona, CA 91766, USA.
Finosh G Thankam, Department of Translational Research, Western University of Health Sciences, Pomona, CA 91766, USA.
Received: 04 August 2022; Accepted: 12 August 2022; Published: 24 August 2022
Citation:
Finosh G Thankam, Mohamed Radwan, Angelo Keklikian, Manreet Atwal, Taj Rai and Devendra K Agrawal. Fluoroscopy Guided Minimally Invasive Swine Model of Myocardial Infarction by Left Circumflex Artery Occlusion for Regenerative Cardiology. Cardiology and Cardiovascular Medicine 6 (2022): 466-472.
Share at FacebookAbstract
Background: Despite the recent advancements in the cardiac regenerative technologies, the lack of an ideal translationally relevant experimental model simulating the clinical setting of acute myocardial infarction (MI) hurdles the success of cardiac regenerative strategies.
Methods: We developed a modified minimally invasive acute MI model in Yucatan miniswine by catheter-driven controlled occlusion of LCX branches for regenerative cardiology. Using a balloon catheter in three pigs, the angiography guided occlusion of LCX for 10-15 minutes resulted in MI induction which was confirmed by the pathological ECG changes compared to the baseline control.
Results: Ejection fraction was considerably decreased post-procedure compared to the baseline. Importantly, the highly sensitive MI biomarker Troponin I was significantly increased in post-MI and follow-up groups along with LDH and CCK than the baseline control. The postmortem infarct zone tissue displayed the classical features of MI including ECM disorganization, hypertrophy, inflammation, and angiogenesis confirming the MI at the tissue level.
Conclusions: The present model possesses the advantage of minimal mortality, simulating the pathological features of clinical MI and the suitability for injectable regenerative therapies suggesting the translational significance in regenerative cardiology.
Keywords
Ischemic Injury; Infarct Zone; LCX occlusion; Myocardial Infarction; Regenerative Cardiology
Article Details
1. Introduction
The lifetime menace of myocardial infarction (MI) and heart failure (HF) continue to rise drastically, affecting more than 6 million people of >20 years of age in the United States [1]. Additionally, the mortality rate in cardiovascular subjects is increasing, despite the developments in lifesaving conventional management strategies [2]. Interestingly, the unmet demands for definitive management strategies give birth to next generation approaches focusing to address the permanent loss of cardiomyocytes (CM) following the ischemic injury and associated contractile dysfunction [3]. Importantly, the emerging approaches in regenerative cardiology and cardiac tissue engineering aiming to rebuild the infarcted myocardium following MI promise novel translational opportunities in the management of HF [4]. Despite the recent advancements in the cardiac regenerative technologies, the lack of an ideal translationally relevant experimental model simulating the clinical setting of acute MI hurdles the success of cardiac regenerative strategies.
The current knowledge about the molecular pathology of acute MI largely owes to small animal models which were created mostly by coronary artery ligation, interventional embolization, and/or drug induction. The increased mortality rate, uncontrolled infarct zone, and minimal structural, genetic and physiological resemblance with native human myocardium pose translational challenges to small animal models of acute MI [5,6]. Thus, there is a great interest in developing large animal models to unveil the molecular pathology and regenerative signaling underlying acute MI. Such animal models require anatomical and physiological similarities with human system, adequate size, resemblances in biological responses with that of humans, comparative anatomical distribution of coronary arteries, and accommodation of the devices/surgical instruments intended for use in human [7]. Based on these requirements, sheep and dogs have been used as cardiovascular diseases (CVD) models for surgical/interventional procedures owing to their similarities with the human CV system [7-10]. However, these animals encountered immediate and intractable ventricular fibrillation following coronary occlusion or even after slight cardiac manipulations, unable to sustain ischemia, developed wound infections with overall high mortality rate [7]. Among the large animal models, the swine model has been considered to be superior for coronary interventions, and hence for MI induction, owing to the anatomical, vascular and physiological/pathological similarities with human CV system [11]. In addition, the swine model exhibits the advantage of the utility of ECG assessment and the applicability of human imaging and surgical tools/approaches; however, presents a limited cardiac acoustic window which hurdles the functional Ultrasound (US) assessment of the left ventricular walls [12]. Also, the swine model simulates the oxygen consumption, metabolic pattern and CV physiology [13,14]. Interestingly, the swine MI model has been attempted by the occlusion of the left anterior descending (LAD) branch of the coronary artery as it supplies more than half of left ventricle and the occlusion results in the ischemia and infarction of anteroapical, lateral and septal walls of left ventricle simulating clinical MI. However, LAD occlusion demonstrated increased procedural mortality affecting the reproducibility of infarct size and pathology challenging the regenerative approaches [15]. Hence, the occlusion of left circumflex (LCX) branch is beneficial for regenerative cardiology owing to the similarity in the compensatory mechanism as in LAD occlusion, minimal invasiveness, and minimal mortality rate. Also, LCX occlusion MI swine model is rare in the literature. Based on this background, we developed a modified minimally invasive acute MI model in Yucatan miniswine by catheter-driven controlled occlusion of LCX for regenerative cardiology.
2. Methodology
2.1 Animal Maintenance and Treatment
The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of Western University of Health Sciences, Pomona, California, USA. Three Yucatan miniswine (Sus scorfa, from Sinclair bioresources) 4-6 months of age, and average weight of ~26 Kgs were recruited to the study. The animals were fed with normal feed twice daily, given uninterrupted access to water and maintained in 12/12 light-dark cycle throughout the study. The surgical procedures were performed after an acclimatization of 7 days.
2.2 Anesthesia and Preoperative Preparations
The animals were fasted overnight prior to the surgery and sedated with Telazole (2.5-5 mg/Kg, IM) and Ketamine (1-2 mg/Kg, IM) cocktail. Anesthesia was maintained by isoflurane in oxygen 3-4% using a precision vaporizer via face mask prior to endotracheal intubation. The hypothermia was prevented by covering the animals with circulating warm water blanket. Vital signs were closely monitored for SPO2, end tidal CO2 (ETCO2), mucous membrane color, capillary refill time (CRT), heart rate, EKG, invasive blood pressure (IBP), and rectal temperature until full recovery. The antibiotic cephazolin (3 mg/Kg) and the analgesic carprofen (2.2 mg/Kg) were prophylactically administered intramuscularly. Venous lines were inserted in the ear veins for emergency drug administration and for fluid resuscitation in case of hypovolemia and low perfusion states. The animals were placed in sternal recumbence position and intubated with cuffed endotracheal (ET) tubes of 5.5- 6 mm diameter following our previous report [16]. The anesthesia was maintained by isoflurane in oxygen 1-2% through the endotracheal tube. Mechanical ventilation was maintained with the tidal volume of 10-15 ml/Kg and the respiratory rate of 12-20 breath/min respectively. The prophylactic antiarrhythmic drug amiodarone (5-10 mg/Kg) and anticoagulant heparin (200 IU/Kg) were administered intravenously. The animals were kept in dorsal position, the legs were loosely fixed, the hair was clipped, and the skin on the ventral side was cleaned with povidone iodine and isopropanol prior to sterile scrubbing.
2.3 MI Induction
All procedures were performed under sterile conditions. Prior to surgical procedures in each animal, scrubbing with gauze using povidone iodine and isopropanol was performed, , and the intervention site was cleaned, and the animal draped. The intervention site was covered by sterile OPSITE film to prevent infection. Femoral artery for catheterization was accessed by ultrasound guided percutaneous puncture using 6F sheath introducer (REF PHR6F11035SC, Merit Medical) following our previous report [16]. Then, 6F (AR2, CGC6100AR2, MERITMEDICAL) angiographic guiding catheter was introduced and advanced to the coronary artery using 0.035mm guide (REF IQ35F210J1O5, Merit Medical) wire. Fluoroscopic evaluation was performed by injecting non-ionic contrast (NDC 0407-1414-93, Omnipaque) media through the guiding catheter into the coronary arteries and angiographic recordings were performed using C-arm (REF Image Intensifier, GE Healthcare) connected to a computer and monitors. A fine guidewire (0.014 mm diameter, REF 1011840, Abbott) was advanced through the guiding catheter into the coronary arteries under fluoroscopic guidance halting at the branch of LCX. Then, a balloon catheter (REF 1012269-06, Abbott) (1.5 mm diameter) was advanced through the guide wire to attain the branch of LCX followed by the inflation of balloon to block the blood flow. The blockage was continued for 10-15 minutes to induce ischemia, and the infarction was verified by the ECG changes. The balloon was then deflated, the arrhythmia, hypotension, acidosis if any were managed by medications until the animal was stabilized. The emergency medicine and procedures included: Atropine 0.05 mg/Kg, Epinephrine 0.01ml/Kg, Diphenylhydramine 50mg, Phenylephrine 20mg/Kg, KCl 0.5 mEq/Kg, Protamine 1.5 mg, NaHCO3 1 mEq/Kg, Buprenorphine 0.3 mg/Kg and Mg2SO4 25%, CPR (Cardiopulmonary resuscitation), and electric shock using an AED (automated external defibrillator). Upon recovery animals were administered with pain medications and were closely monitored until sacrificed after 4-7 days.
2.4 Electrocardiogram (ECG)
Twelve lead ECG tracing (Philips Medical Systems) (4 limb leads and 6 chest leads, v1-v6) was performed following standard protocol to confirm MI induction which was compared with the pre-MI ECG. The ECG was repeated prior to sacrifice in a similar manner.
2.5 Echocardiography
Transthoracic echocardiography (Philips) was performed following standard protocol to confirm MI induction which was compared with the pre-MI echocardiography. The echocardiography was repeated prior to sacrifice in a similar manner. The left ventricular ejection fraction was calculated using Philips Q lab software.
2.6 Blood Analysis
Blood was collected in anticoagulated tubes from the ear vein and the plasma was separated using centrifugation. The plasma levels of myocardial enzymes such as high sensitivity Troponin I (Trop I), Creatine Kinase (CK) and lactate dehydrogenase (LDH) were examined using standard protocols in commercially available platform (IDDEX Bioanalytics, USA). The blood parameters were compared between pre-MI (baseline control), post-MI and 7days post MI.
2.7 Histology
The animals were sacrificed within a week post-MI and the left ventricular tissues were harvested, fixed in formalin, and processed for H&E staining following our previous protocols [17]. Ventricular tissue harvested from normal pigs of the same colony was used as control for comparison.
2.8 Statistical Analysis
The results are expressed as mean ± SEM and the statistical significance was evaluated by one way ANOVA following two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli employing GraphPad Prism software. P<0.05 was set for all experiments for statistical significance.
3. Results
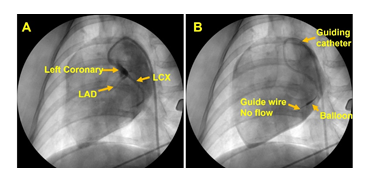
None of the three animals showed any adverse responses to anesthesia. The animals were under close observation and allowed to recover normally from anesthesia following the procedure. The animals exhibited normal preoperative responses and anesthetic induction periods. Utmost care was taken to prevent pain and distress during the surgical procedure. The branch of LCX was accessed for each animal using the guiding catheter under close monitoring of the vitals and ECG; however, two animals developed ventricular fibrillation due to the smaller size of LCX upon introducing the guiding catheter even without inflating the balloon. Interestingly, in the third animal the balloon catheter was successfully positioned and inflated for 10-15 min blocking the blood flow to induce ischemia and MI which were confirmed with a drastic increase the heart rate and ECG changes. The blockage of blood flow downstream the balloon was confirmed using angiogram (Figure 1). Continuous CPR and DC shock were given until the animal stabilizes. The post-MI pigs exhibited no adversities, recovered normally within 3-4 hrs, pain medication was administered and were closely observed for 48 hrs.
3.1 Electrocardiogram (ECG)
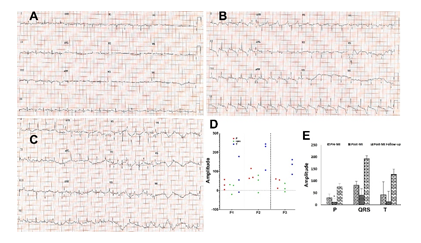
Twelve lead ECG tracing was performed to confirm MI induction which was compared with the pre-MI and follow-up ECG. Post-MI and follow-up animals displayed abnormal ECG patterns reflecting the myocardial ischemia and infarction (Figures 2A, 2B and 2C). The elevation of ST segments, failure of R wave progression and development of abnormal Q wave was predominant in post-MI compared to the control (Figures 2A and 2B). Inversion of T wave and further deeper Q wave were observed in follow-up ECG compared with immediate post-MI induction and control (Figures 2A, 2B and 2C). Additionally, the amplitudes of P, QRS and T waves were increased in the follow-up EKG compared to immediate post-MI (Figure 2A-2E). The pre-MI (control) ECGs displayed normal pattern (Figure 2A).
Figure 2: Representative cardiac EKG displaying the alterations in the patterns in (A) control, (B) post-MI and (C) follow-up MI. (D) Scatter plot showing the distribution of the amplitudes of P, QRS and T waves based on individual pigs (P1, P2 and P3) and (E) representing the amplitudes of P, QRS and T in pre-MI, post-MI, and follow-up EKGs. The statistical significance based on one-way ANOVA test is represented in the figure (* P<0.05, and unlabeled are non-significant).
3.2 Echocardiography
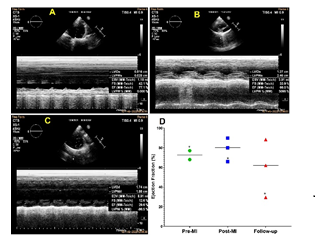
Echocardiography was performed to assess the left ventricular ejection fraction which was compared with the pre-MI, immediate post-MI, and follow-up groups. Follow-up animals (4 days post MI) displayed reduction in ejection fraction reflecting the myocardial ischemia and infarction compared to the pre-MI group. Interestingly, the average value for ejection fraction displayed an increase immediately following the MI induction (post-MI group) (Figures 3A-3D). However, the animal which successfully underwent and sustained the LCX occlusion displayed ejection fraction of 77.1%, 66%, and 29.6% respectively for pre-MI, immediate post-MI, and follow-up (Figure 3D).
Figure 3: Representative echocardiography displaying the alterations in the ejection fraction (A) control, (B) post-MI and (C) follow-up MI. (D) Scatter plot showing the distribution of the ejection fractions based on individual pigs with respect to the mean in pre-MI, post-MI, and follow-up ultrasound. *The pig which successfully underwent occlusion.
3.3 Blood Biomarkers
The high-sensitive Trop-I was significantly increased in the immediate post-MI and follow-up compared to the pre-MI control. Also, the level of Trop-I was significantly decreased in follow-up when compared to the immediate post-MI group (Figure 4A). Similarly, LDH was increased in immediate post-MI and follow-up groups; however, the follow-up group displayed decreased level than the immediate post-MI group (Figure 4B). The level of CCK was increased in immediate post-MI group than the control and was further increased in follow-up group compared to the control (Figure 4C). However, the increase in LDH and CCK were statistically not significant.
3.4 Histology
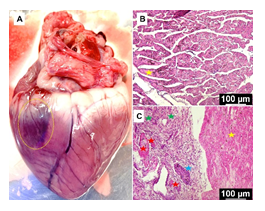
The postmortem heart displayed the discoloration suggestive of scarring representing the infarct zone (Figure 5A). Histology unveiled increased disorganization of cardiac ECM at the infarct zone, angiogenesis, hypertrophy, inflammation, and necrotic lesions when compared to the normal cardiac tissue. In addition, the non-infarcted areas of cardiac tissue in MI group displayed normal histology similar to the control (Figures 5B and 5C).
Figure 5: (A) The postmortem heart displaying the infarct zone. Representative images of the histology examination of formalin fixed sections of MI tissue showing the control (B) and MI group (C): yellow star represent normal ECM, blue star points ECM disorganization, red star shows blood vessels, and the green star displays inflammation. The images were acquired at 20x magnification.
4. Discussion
The inherently poor regenerative potential of adult heart results in the progressive deterioration of cardiac function subsequently leading to HF following an acute MI. Therefore, the modern cardiovascular medicine has a dire demand for effective regenerative approaches to repair the infarcted myocardium from the detrimental effects of non-contractile scar tissue, adverse remodeling, and ventricular dilatation with reduced ejection fraction following the ischemic episode associated with MI [18]. The current knowledge in regenerative cardiology largely owes to the small animal models; however, the translational success is limited due to the inherent differences with human heart demanding translationally worthwhile large animal models. Additionally, the simulation of local inflammatory milieu, fibrotic events, wound healing responses, transient scaring, neomusculogenesis and neovascularization are necessary for replicating the human MI in animal models for regenerative cardiology [19].
Interestingly, most pre-clinical models relied on the direct ligation of coronary artery where the thoracotomy and ligation procedures deviate the natural pathophysiology resulting in the increased mortality and unreproducible outcomes [19,20]. Importantly, the close similarities with human cardiovascular anatomy and physiology upgrade the swine models to be ideal for investigating the cardiac regeneration [21]. Hence, we simulated MI in miniswine following minimally invasive approaches. Even though the LAD MI accounts for more clinical registry and severity, the LCX MI presents similar molecular pathology [22]. However, the LAD MI is superior in LV remodeling with reduced systolic function compared to LCX MI as evident from human data. Additionally, the LAD occlusion results in apical infarction whereas LCX MI displays lateral and basal dominancy with considerable scar size in both cases [23]. Moreover, the compensatory increase in ejection phase shortening has been previously reported to be more in LCX ischemia compared to LAD MI [24]. Also, the LCX MI displays increased sphericity index along with preserved systolic function [25] suggesting the suitability to explore regenerative responses as the severe myocardial damage in LAD MI results in excessive fibrotic remodeling which favors excessive scarring.
Ideally, the less severe acute MI pathology is effective to understand the underlying inherent regenerative responses. Hence, we focus to develop LCX MI which possesses the additional advantage of accessing the infarct area for minimally invasive injectable regenerative approaches especially via transepicardial route using ultrasound or thoracoscopy guided fine needle injections. MI induction by minimally invasive catheter based LCX occlusion was successful in all the three pigs. However, two pigs developed ventricular fibrillation while advancing the guiding catheter owing to the smaller size of coronary artery which prevented us from advancing the balloon catheter. Interestingly, the third pig withstood the catheterization where the MI was induced by inflating the balloon at the distal branches of LCX. Despite the procedural alterations, all the three pigs developed classical features of clinical MI as evident form the ECG changes, ejection fraction, blood biomarkers and histology suggesting the induction of acute MI displaying reproducible outcomes. However, further fine tuning has been warranted for optimizing the technique.
The management of ventricular fibrillation was challenging; however, followed standard emergency medications and CPR along with DC shock administration simulating the clinical procedures. Also, the ECG presentation was closely monitored to assess the status of cardiac performance following the MI induction. The animals were under pain medication, and completely recovered from anesthesia in 4-5 h. Our prior approach to induce LAD infarction in Yucatan micropigs were not successful owing to interactable ventricular fibrillation and cardiac arrest. Information on the increased mortality rate in LAD occlusion, presenting <30% ejection fraction [26], led us to choose LCX occlusion for MI induction for improving the post-MI survival rate. Also, our focus was to induce an infarction with moderate severity with >40% ejection fraction. In addition, the coronary access through femoral artery using appropriate angiographic techniques and guiding catheters is helpful for locating the distal LCX branches suitable for occlusion. As expected, vasospasm was observed; however, was effectively managed by prophylactic administration of nitroglycerine locally by injecting through the catheter. The balloon catheter was inflated ~1.2 times the diameter of the artery which was calculated by optical coherence tomography during our pilot experiment (data not shown in this article). The occlusion of blood flow was confirmed by coronary angiogram and the occlusion was continued for 10-15 minutes to observe remarkable change in ECG pattern. Contrastingly, more than one hour of LAD occlusion has been reported to be effective for inducing severe transmural infarction in the minipigs [27]. However, the onset of ventricular fibrillation presented by the pigs in our study following 10-15 mins of occlusion suggests that the prolonged occlusion was lethal. Importantly, the post sacrifice myocardial tissues displayed classical histological features of acute MI further confirming the model.
Owing to the identical pathological and regenerative mechanisms underlying the infarct zone following the LAD and LCX occlusion, the present model of MI is ideal for developing cardiac regenerative strategies. Moreover, our model possesses the advantage of lower mortality, simulating the pathological features of MI and the suitability for injectable regenerative strategies suggesting the translational significance in regenerative cardiology. However, this pilot study displays limitations including lesser sample size, relatively younger age of pigs, and lack of hyperlipidemic phenotype (as hyperlipemia is a major risk factor to MI) warranting further investigation. Although our model has rectifiable deficiencies, we have successfully demonstrated a minimally invasive MI model by occluding LCX which offers immense translational opportunities in regenerative cardiology.
5. Conclusion
MI was successfully induced in three Yucatan mini pigs by angiography guided occlusion of LCX for 10-15 minutes using a balloon catheter which was confirmed by the pathological alterations in ECG, and reduction in ejection fraction in immediate post-MI and follow-up groups compared to the baseline. Additionally, the highly sensitive MI biomarker Troponin I, LDH and CCK were increased in immediate post-MI and follow-up groups compared to the baseline. The postmortem infarct zone tissue displayed the classical features of MI including ECM disorganization, hypertrophy, inflammation, and angiogenesis confirming the MI at tissue level. The present model possesses the advantage of minimal mortality, simulating the pathological features of MI and the suitability for injectable regenerative strategies suggesting the translational significance in regenerative cardiology.
Acknowledgements
The authors thank Dr. Willie Bidot, Ms. Emily Shadler and Ms. Sydney Davis for their help during the surgery and animal care.
Ethical Statement
The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of Western University of Health Sciences, Pomona, California, USA.
Authors’ Contributions
FT – conceptualization, experimental design and planning, data generation and analysis, manuscript preparation and edits, resources and funding; MR - conceptualization, experimental design and planning, data generation and analysis, manuscript preparation and edits; AK – experiments, data generation and manuscript edits; MA – experiments, data generation and manuscript edits; TR – experiments, data generation and manuscript edits; DKA – conceptualization, experimental design and planning, data analysis, manuscript edits, and resources.
Funding
The research work of FGT was supported by the startup research funds from Western University of Health Sciences. DK Agrawal is supported by research grants R01 HL144125 and R01HL147662 from the National Institutes of Health, USA.
Data Availability
Data with detailed information will be available upon request from the authors through proper channels.
Conflict of Interest Statement
The authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest. The authors declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
References
- Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 143 (2021): e254-743.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation (2022).
- Naqvi N, Iismaa SE, Graham RM, et al. Mechanism-Based Cardiac Regeneration Strategies in Mammals. Front Cell Dev Biol (2021).
- Finosh GT, Jayabalan M. Regenerative therapy and tissue engineering for the treatment of end-stage cardiac failure. Biomatter 2 (2012): 1-14.
- Tang Y, Liu Y, Fan Y, et al. To develop a novel animal model of myocardial infarction: A research imperative. Anim Models Exp Med 1 (2018): 36-39.
- Wang J, Bo H, Meng X, et al. A Simple and Fast Experimental Model of Myocardial Infarction in the Mouse. Tex Heart Inst J 33 (2006): 290-293.
- Shofti R, Zaretzki A, Cohen E, et al. The sheep as a model for coronary artery bypass surgery. Lab Anim 38 (2004): 149-157.
- Mawhinney JA, Mounsey CA, Taggart DP. The potential role of external venous supports in coronary artery bypass graft surgery†. Eur J Cardiothorac Surg 53 (2018): 1127-1134.
- Wan S, Arifi AA, Chan MCW, et al. Differential, time-dependent effects of perivenous application of fibrin glue on medial thickening in porcine saphenous vein grafts?. Eur J Cardiothorac Surg 29 (2006): 742-746.
- Gross GJ, Lockwood SF. Acute and chronic administration of disodium disuccinate astaxanthin (Cardax) produces marked cardioprotection in dog hearts. Mol Cell Biochem 272 (2005): 221-227.
- Schachner T, Bonaros N, Ruttmann E, et al. Training Models for Coronary Surgery. Heart Surg Forum 10 (2007): E248-250.
- Hocum Stone LL, Swingen C, Holley C, et al. Magnetic resonance imaging assessment of cardiac function in a swine model of hibernating myocardium 3 months following bypass surgery. J Thorac Cardiovasc Surg 153 (2017): 582-590.
- Hocum Stone L, Butterick TA, Duffy C, et al. Cardiac Strain in a Swine Model of Regional Hibernating Myocardium: Effects of CoQ10 on Contractile Reserve Following Bypass Surgery. J Cardiovasc Transl Res 9 (2016): 368-373.
- Litten-Brown JC, Corson AM, Clarke L. Porcine models for the metabolic syndrome, digestive and bone disorders: a general overview. Animal 4 (2010): 899-920.
- McCall FC, Telukuntla KS, Karantalis V, et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc 7 (2012): 1479-1496.
- Radwan MM, Siddique A, Thankam FG, et al. Translational model of vein graft failure following coronary artery bypass graft in atherosclerotic microswine. Gen Thorac Cardiovasc Surg (2021).
- Thankam FG, Dilisio MF, Dietz NE, et al. TREM-1, HMGB1 and RAGE in the Shoulder Tendon: Dual Mechanisms for Inflammation Based on the Coincidence of Glenohumeral Arthritis. PLoS ONE 11 (2016).
- Spannbauer A, Mester-Tonczar J, Traxler D, et al. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules 10 (2020): 1392.
- Price EL, Vieira JM, Riley PR. Model organisms at the heart of regeneration. Dis Model Mech 12 (2019): dmm040691.
- Shin HS, Shin HH, Shudo Y. Current Status and Limitations of Myocardial Infarction Large Animal Models in Cardiovascular Translational Research. Front Bioeng Biotechnol (2021).
- Tohyama S, Kobayashi E. Age-Appropriateness of Porcine Models Used for Cell Transplantation. Cell Transplant 28 (2019): 224-228.
- Kim SS, Choi HS, Jeong MH, et al. Clinical outcomes of acute myocardial infarction with occluded left circumflex artery. J Cardiol 57 (2011): 290-296.
- Ishikawa K, Aguero J, Tilemann L, et al. Characterizing preclinical models of ischemic heart failure: differences between LAD and LCx infarctions. Am J Physiol - Heart Circ Physiol 307 (2014): H1478–1486.
- Hoit BD, Lew WY. Functional consequences of acute anterior vs. posterior wall ischemia in canine left ventricles. Am J Physiol 254 (1988): H1065-1073.
- Hees PS, Fleg JL, Lakatta EG, et al. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol 90 (2002): 1231-1236.
- Entezarjou A, Mohammad MA, Andell P, et al. Culprit vessel: impact on short-term and long-term prognosis in patients with ST-elevation myocardial infarction. Open Heart 5 (2018): e000852.
- Crisóstomo V, Sun F, Maynar M, et al. Common swine models of cardiovascular disease for research and training. Lab Anim 45 (2016): 67-74.