Fibrin Hydrogel Aided Cardiac Progenitor Cell Delivery Enhances Regenerative Tendency in Myocardial Infarct Model
Article Information
Subha Sa, Sachin J Shenoyb, Arya Anilc, Sabareeswaran Ad, Deepthi ANe, Lissy K Krishnana*
aDivision of Thrombosis Research, Department of Applied Biology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695012, Kerala, India
bDivision of In vivo Models and Testing, Department of Medical Devices Engineering, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695012, Kerala, India
cDivision of Laboratory Animal Science, Department of Applied Biology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695012, Kerala, India
dDivision of Experimental Pathology, Department of Applied Biology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695012,Kerala, India
eDepartment of Pathology, Hospital Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695011, Kerala, India
*Corresponding author: Lissy K Krishnan, Senior Grade Scientist G, Division of Thrombosis Research, Department of Applied Biology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, 695012, Kerala, India
Received: 28 September 2020; Accepted: 08 October 2020; Published: 16 October 2020
Citation: Subha S, Sachin J Shenoy, Arya Anil, Sabareeswaran A, Deepthi AN, Lissy K Krishnan. Fibrin Hydrogel Aided Cardiac Progenitor Cell Delivery Enhances Regenerative Tendency in Myocardial Infarct Model. Archives of Clinical and Biomedical Research 4 (2020): 513-541.
Share at FacebookAbstract
Attempts to regenerate myocardial infarct (MI) using stem cells have been achieving random success rates. This study considered delivering cardiac progenitors (rCPs), developed from rat adipose-derived mesenchymal stem cells (rADMSCs), into rat MI tissue. The study aimed to employ fibrin-based insoluble matrix as a niche for in vitro differentiation of rADMSCs to rCPs and specific composition of fibrin hydrogel to deliver the cells into MI tissue. PKH26 tracker dye labelled-rCPs/rADMSCs suspended in fibrinogen/thrombin was injected to peri-infarct zone in rat heart created by coronary artery ligation. The MI tissues were harvested after 28d, imaged to locate transplanted cells, and analyzed to detect markers of differentiation, angiogenesis, and immune response. Co-localization of Connexin 43/TroponinT with PKH26 indicated differentiation of transplanted progenitors into cardiomyocyte like cells. Additionally, the presence of CD31+ve cells, fewer CD68+ve cells, and reduced collagen deposition are appreciable in tissues transplanted with rCP in fibrin, as compared to those that received rADMSC. The results obtained indicate that fibrin acts as a favorable niche for in vitro priming of rADMSCs into transplantable lineagecommitted rCPs. The in situ clottable fibrin aided the retention and differentiation of transplanted rCPs into cardiomyocytes. Also by 28d, the tissues showed better angiogenic/ immunomodulatory effects and reduced the calcification tendency.
Keywords
Adipose-tissue derived mesenchymal stem cells; Cardiac progenitor; Cell delivery; Fibrin hydrogel; Myocardial infarction; Regenerative tendency
Adipose-tissue derived mesenchymal stem cells articles; Cardiac progenitor articles; Cell delivery articles; Fibrin hydrogel articles; Myocardial infarction articles; Regenerative tendency articles
Article Details
Abbreviations
ABAM: Antibiotic-antimycotic; cDNA: complementary Deoxyribonucleic acid; CPCSEA: Committee for the purpose of control and supervision of experiments on animals; DAPI: 4, 6, diamidino-2- phenylindole; DMEM-LG: Dulbecco’s modified Eagle’s Medium – Low glucose; DPX: Dibutyl phthalate Polystyrene Xylene; EDTA: Ethylene diamine tetra-acetic acid; ESCs: Embryonic stem cells; FBS: Fetal bovine serum; Fib: Fibrin; GAPDH: Glyceraldehyde -3- phosphate dehydrogenase; GJA 1: gap junctional protein alpha, 1/connexin 43; H & E: Harris’s hematoxylin and eosin; HBSS: Hank’s balanced salt solution; IAEC: Institutional Animal Ethics Committee; IGF-1: Insulin-like growth factor -1; iPSCs: Induced pluripotent stem cells; IS: Injured tissue site. IVIS: In vivo imaging system; LV: Left ventricular; MI: Myocardial Infarction/ infarct; mRNA: messenger RNA; MSCs: Mesenchymal stem cells; NS: Native tissue site; P3: Passage 3; PBS: Phosphate buffered saline; PGF: Platelet growth factor; qRT –PCR: real-time quantitative reverse – transcriptase-polymerase chain reaction; rADMSCs: rat adipose tissue derived mesenchymal stem cells; rCPs: rat Cardiac progenitor cells; RNA: Ribonucleic acid; SFM: Serum-free medium; TCPS: Tissue culture polystyrene; TNNT 2: cardiac Troponin T type 2.
Introduction
Myocardial infarction (MI), leading to irreversible death of millions of cardiomyocytes accounts for one of the highest causes of mortality worldwide. The subsequent cascade of cardiac remodelling events results in non-contractile scar tissue formation and ventricular wall thinning, gradually causing unsatisfactory cardiac functions [1]. Though replacement surgeries and assistive devices improve the cardiac function, regeneration of the damaged myocardium remains a major challenge [2,3]. Reports of animal studies and clinical trials have highlighted the advantages of stem cell transplantation strategies for the regeneration of damaged myocardium. Application of various cell types including skeletal myoblasts, endothelial progenitor cells (EPCs), bone marrow mononuclear fraction, and mesenchymal stem cells (MSCs) have been explored [4]. However, studies have observed significant intra-myocardial calcifications upon transplantation of unselected bone marrow-derived MSCs in acutely injured myocardial tissue, due to its multipotent nature and undesired lineage commitment [5]. Out of adipose derived mesenchymal stem cells (ADMSCs) and bone marrow-derived mesenchymal stem cells (BMMSCs) transplanted in porcine models of MI, the former showed better engraftment and left ventricular ejection fraction. Therefore, ADMSCs may be considered a better candidate for the regeneration of MI. Other advantages of ADMSCs include ease of collection, autologous cell availability, and multipotency. However, the multipotent nature of MSCs is like a double-edged sword; with the ability to form both desirable and undesirable cell lineages. Calcified and ossified myocardial tissue generation has been considered to be a major drawback of stem cell-based therapies [6]. Also, malignant tumor formation in the cardiac tissue within 4-8 weeks of transplantation has been reported [7], suggesting uncontrolled lineage specifications and growth. ADMSC differentiation to cardiomyocytes is hardly evidenced but seems to have a minor contribution to the improvement of angiogenesis [8]. Therefore, priming ADMSCs in vitro into cardiac progenitors, consisting of both cardiomyocyte and endothelial lineage cells, is likely to prevent undesirable cell differentiation post-transplantation, if the in vivo signals are also favorable.
Several researchers have reported the differentiation of either embryonic stem cells (ESCs) or MSCs in vitro into cardiomyocyte phenotype using various strategies including the use of chemical agents like 5-azacytidine [9], co-culture with rat neonatal cardiomyocytes [10] or by induction with cytokines and growth factors (GFs) [11]. Also, GFs like Insulin-like growth factor-1 (IGF-1) has been reported to promote differentiation of ESCs upon transplantation into MI [12]. Fibrin based niche comprising specific GFs assisting lineage commitment of ADMSCs to ectodermal cells like keratinocytes and neural cells has been reported [13,14]. Therefore, fibrin niche constituted with specific GFs could be a promising strategy to derive cardiac progenitors from ADMSCs.
When the in vivo response to transplanted cells is considered, the hostile microenvironment present in the myocardial injury site, contributed by ischemia and inflammation, limits the attachment and survival of the MSCs delivered in the injured heart. In effect, the therapeutic effects of MSC transplantation have been reported to be unsatisfactory due to the poor survival of these cells in acute heart injury [15]. To increase sufficient grafting of transplanted cells, several delivery methods have been attempted by different investigators including intravenous or intracoronary injection and trans-endocardial delivery with reported advantages/disadvantages [16]. Further, the use of delivery vehicles such as polymeric scaffolds, collagen, various hydrogels, etc., were also attempted to improve the transplantation outcome in cell-based regenerative therapy [6,17]. Contribution of fibrin in enhancing tissue angiogenesis inthe treatment of cardiovascular diseases has been described in the literature [18]. It has been suggested that retention of the protein factors within the 3-dimensional hydrogel and the specific binding sites for GF to the matrix could be responsible for the angiogenic potential of fibrin when used as a cell delivery matrix.
Despite all the limitations described in the literature, ADMSCs transplantation dominates over other cell types because of differentiation potential and immunomodulatory effects and the release of paracrine factors. Cardiac tissue engineering attempts showed promising progress in the last 2 decades; however, an important challenge is the poor integration of in vitro engineered tissue with the native tissue [19]. The importance of microvasculature for successful regeneration of MI or integration of in vitro engineered tissue upon implantation or transplantation of stem cells has been well recognized. To circumvent the problems attempts to generate vascularized cardiac patches are in progress [20]. The pro-angiogenic properties of MSCs are also well described in the literature [21]. It has been reported that ADMSCs transplantation contributes mainly to paracrine-mediated cardio-protection and angiogenesis. The angiogenesis was also shown to be improved in 28 days upon transplantation of ADMSCs [6]. This specific property of MSCs advocates the advantage of therapeutic stem cell transplantation for promoting the angiogenesis in MI, apart from their contribution towards cardiomyocyte regeneration. However, no study has reported the outcome of transplantation of rCPs derived from ADMSCs.
Based on this background, the present study aimed to take benefit of ADMSCs and fibrin to stimulate the chances of MI regeneration. The study postulated that the derivation of rCPs from rADMSCs in vitro can be achieved using fibrin-based niche which may be made available during and after in vivo transplantation to attain progressive differentiation of progenitors and to assist angiogenesis. Immunomodulatory effects of cells and fibrin were also analyzed in tissues transplanted with both rADMSCs and rCPs to assess the benefit of both cell types and chose the best combination for future studies.
Materials and Methods
Isolation of rADMSCs and in vitro priming to Cardiac lineage
Rat adipose tissue was collected upon tracheostomy, with the approval of Institutional Animal Ethics Committee and Institutional Committee for Stem cell research (SCT/IAEC-266/FEBRUARY/2018/95, SCT/IC-SCR/49/September 2018) from male Sprague –Dawley rats weighing > 250g. Rat ADMSCs (rADMSCs) were isolated as described previously [22]. Please see supplementary file for a detailed procedure for preparing lineage-committed cells for transplantation.
Animal Study plan
All experiments conformed to the guidelines issued by the CPCSEA (Committee for the purpose of control and supervision of experiments on animals, Government of India) committee and approved by the Institutional Animal Ethics Committee (IAEC-SCTIMST). Twenty four (adult male) inbred colony of Sprague-Dawley rats (body weight: 250-400g each) obtained from the Division of Laboratory Animal Sciences, SCTIMST, India were used for the experiment. The animals were kept under standard animal care conditions and were given free access to food and water throughout the study. Experimental animals were randomly assigned to one of the following six study groups as indicated in table 1, before surgery with at least 4 animals included in each group.
|
Group I |
Serum-free medium injected control (C-SFM) |
|
Group II |
Fibrin injected control (C-Fib) |
|
Group III |
rADMSCs transplanted in serum-free medium as the delivery vehicle (rADMSC-SFM) |
|
Group IV |
rADMSCs transplanted in fibrin as the delivery vehicle (rADMSC-Fib) |
|
Group V |
rCPs transplanted in serum-free medium as the delivery vehicle (rCP-SFM) |
|
Group VI |
rCPs transplanted in fibrin as the delivery vehicle (rCP-Fib) |
Table 1: Description of different animal study groups
Experimental Surgery
The development of regional myocardial ischemia is described in the supplementary file. The cells were transplanted at the injury site 15 - 20 min after the MI using a 1ml syringe. 106 cells/animal suspended in fibrinogen were injected along with thrombin at the site of injury for test animals. Fibrinogen at a concentration of 20mg/ml and thrombin at a concentration of 1IU/ml were used. In Group I, 200ul of SFM alone was injected whereas, in Group II, fibrin alone was injected. Soon after transplantation/injection, the chest wall was closed after maintaining negative pressure inside the thoracic cavity by gentle suction. Postoperative care was given to the animals by the administration of antibiotics and painkillers for 5 days after the development of MI and cell transplantation. Animals were euthanized humanely after 28 days post-transplantation. For histopathology evaluation, perfusion fixation was done initially with Ringer’s lactate solution to wash the red blood cells followed by 10% neutral buffered formalin. The heart was dissected out and stored in 10% neutral buffered formalin before routine tissue processing.
Tracking transplanted cells
Standard necropsy was carried out by pathologist and experimental hearts were collected on 28th day in phosphate-buffered saline (PBS). The gross coronal tissue slices were then used for imaging (IVIS Spectrum Preclinical in vivo imaging system, Perkin Elmer, USA). The excitation wavelength used was 500nm and the emission was measured at 600nm. The retention of rADMSC and rCP in the transplanted site was assessed by fluorescent imaging of the explanted tissue area. Following fluorescent imaging, the heart tissue was cryoprotected using liquid nitrogen. The frozen heart tissue was then embedded in freezing medium (Jung, Lecia Microsystems, Germany) and cut into 14 µm sections using a cryostat (Leica, Germany). The sections were then washed with PBS and permeated with 0.2% Triton-X 100 for 5min, and blocked with 3% bovine serum albumin (BSA; Sigma, USA) for 30 minutes. The sections were incubated with the primary antibodies against cardiomyocyte marker Troponin T (TNNT 2; Abcam, UK), gap junction protein Connexin 43 (GJA 1; Thermo Scientific, USA) and pro-inflammatory marker CD68 (Abcam, UK) overnight at 4°C. The corresponding secondary antibody conjugated with Alexa Fluor 488 (Abcam, UK) was incubated for one hour at room temperature to develop the antigens. Nuclear staining was done using 4, 6, diamidino-2-phenylindole (DAPI, Invitrogen, USA). To identify the presence of lineage-committed transplanted rCPs, dual-labeled cells (PKH26 and cardiac marker) were analyzed.
Histopathology evaluation
Formalin-fixed, paraffin-embedded rat heart tissue sections were stained for analysis of the injury site formed by ischemia of the heart wall. For H & E staining, formalin-fixed sections were processed in 70% alcohol overnight, followed by three changes of acetone (20min each), two changes of xylene (10 and 15min respectively), and two changes of paraffin wax (60min each). Then the tissues were embedded in paraffin wax. Transverse tissue sections were obtained using a microtome (Leica RM 2255, Germany). Using xylene, paraffin was cleared from sections (3 changes, 15min each), rehydrated in descending grades of isopropanol (100% for 3min, 95% for 3 min, 80% for 3min and 70% for 3min) and washed in tap water. The rehydrated sections were stained using Harris’s hematoxylin and eosin (H & E). The tissue sections were submerged in Harris’s hematoxylin for 15min, washed in tap water for
5min, differentiated in 1% acid-alcohol (1-2 fast dip), and blued with 0.2% ammonia water for 1min. The slides were washed with tap water for 5min, counter-stained with 1% eosin Y for 5min. Tissue sections were dehydrated in isopropanol (95% for 5min, 2 changes in 100% ethanol for 5min each), cleared in xylene (2 changes, 10min each), mounted in DPX (Dibutylphthalate Polystyrene Xylene) and viewed under transmitted light microscope (Nikon Eclipse Ni-E, Japan). Microphotographs of lesion areas were captured using the camera (Nikon DsR1, Japan) attached to the microscope.
Identification of Cell Fate
Immunohistochemical staining was done by standard protocol. Briefly, 4μm formalin-fixed paraffin embedded sections were de-paraffinized for 30 minutes in the oven at 70°C and in xylene for 10 minutes, followed by dehydration in graded isopropanol. The antigen retrieval was performed using Tris EDTA buffer (Catalog # PS009, Pathn Situ Biotechnologies, India) in a microwave oven at 100°C for 20 minutes. The endogenous peroxidase activity was blocked using 3% hydrogen peroxide followed by washing with immuno wash buffer (Catalog #PS006, Pathn Situ Biotechnologies) for 5 minutes twice and non-specific background staining was blocked using protein block (Catalog # X0909, Agilent Dako, Santa Clara, CA, USA). The slides were incubated with mouse monoclonal anti-human CD31 primary antibody (Clone JC70A, ready-to-use, Code IR610, Agilent Dako) for one hour and washed with immuno wash buffer for 5 minutes twice. The sections were visualized using horseradish peroxidase labeled anti-Rabbit secondary antibody system (Dako REAL EnVision, Catalog # K5007, Agilent Dako) for 30 minutes, washed with immuno wash buffer for 5 minutes twice followed by incubation with DAB chromogen system (Dako REAL EnVision, Catalog # K5007) for 10 minutes. The sections were finally washed with distilled water, counterstained with hematoxylin, dried, and mounted using DPX (Dibutylphthalate Polystyrene Xylene).
Masson’s trichrome staining was carried out in formalin-fixed tissue sections to assess the area of fibrosis. The integrated area of fibrosis was calculated using magnified images ofthe trichrome stained sections using Image J software (NIH, Maryland, USA).
Statistical analysis
All graphical data are presented as the mean ± standard error obtained from three independent experiments. Statistical differences between groups were assessed using Student’s t test. Values of p ≤ 0.05 were considered significant.
Results
In vitro priming of rADMSCs to rCPs and transplantation into MI model
The isolated rADMSCs were plastic-adherent and appeared to have fibroblastic spindle-shaped morphology. Distinct morphological features were observed in primed cells as compared to the control/undifferentiated rADMSCs from day 5 of induction. The qRT-PCR results indicating the upregulation of lineage-specific markers are also described in detail in the supplementary file (Supplementary Figure S1). The development of MI was confirmed visually by observing the blanching of the myocardium distal to the ligation and the results obtained in this section are described in the supplementary file. (Supplementary Figure S2).
Fluorescence emission by transplanted cells
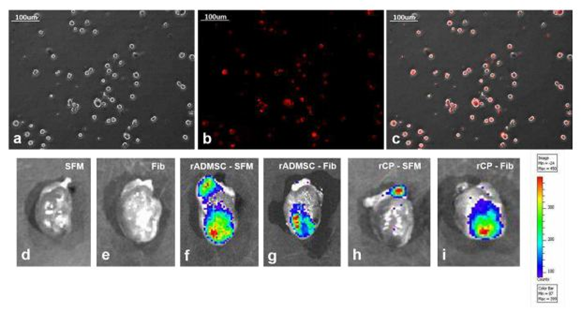
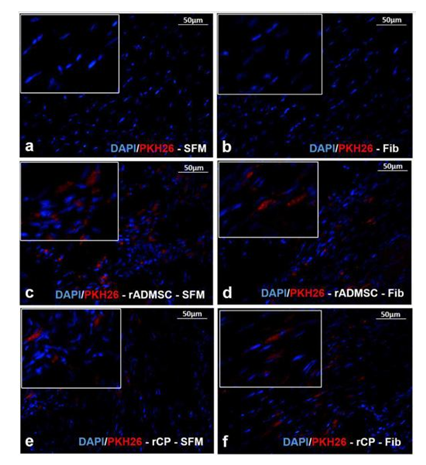
The red fluorescence of both rADMSCs and rCPs (harvested from 6-8d cultures) labeled with PKH26- lipophilic dye was confirmed before transplantation (Supplementary Figure S3a-c). Post-transplantation, the IVIS images of isolated hearts harvested on the 28th day showed distinctly different fluorescence, in each experiment group. No autofluorescence from the control heart tissues was detected in IVIS images. The primed rCPs delivered in fibrin showed more localized red fluorescence in the transplanted site than the heart tissues in which rCPs were transplanted in SFM. This indicated that though an equal number of cells were transplanted in all animals, the tissues in which fibrin were used as cell delivery vehicle retained more cells in the site of injury. Both the cell types; rADMSCs and rCPs delivered in SFM also were retained, however, the cells seemed to have migrated away from the injection site. So the results confirmed that irrespective of the cell type, transplantation using fibrin as the delivery vehicle was remarkably efficient in containing the cells within the infarct area (Supplementary figure S3d-i). The microscopy of tissue cryo-sections detected red fluorescence of PKH26+ve cells confirming that the cells transplanted in both the SFM and fibrin survived for 28d and the observation was analogous to the whole heart tissue IVIS imaging results. Analysis of the controls: Group I (SFM injected) and Group II (Fibrin injected) tissue sections did not reveal any red fluorescence, confirming that the red fluorescence emitted from test tissues correspond to the transplanted cells. Upon viewing different fields, intensely emitting fluorescence was more frequent in the sections of tissues in which cells were delivered in fibrin (Supplementary Figure S4).
The fate of transplanted cells in vivo
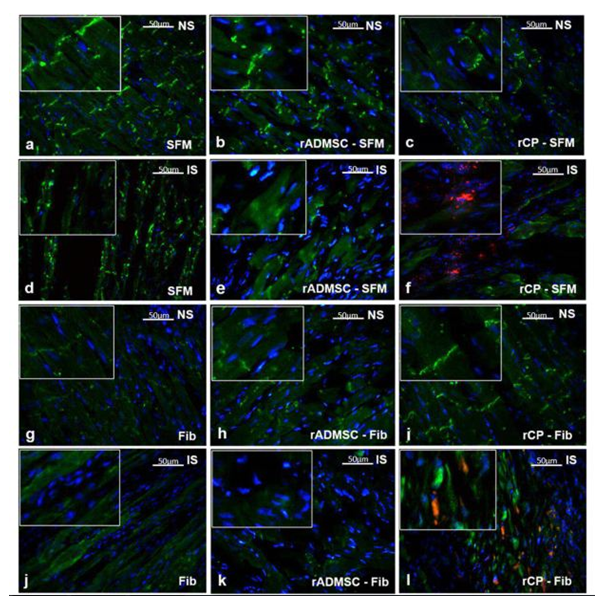
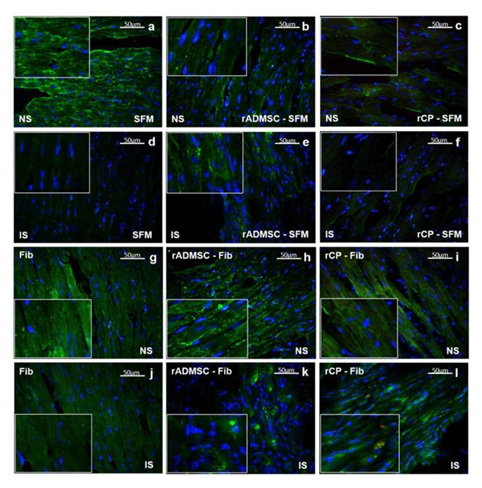
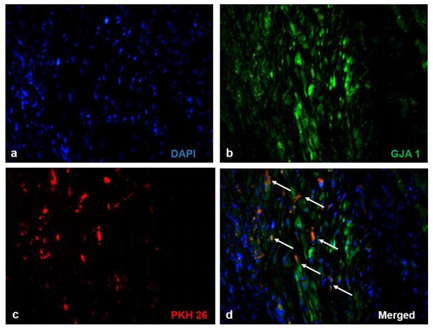
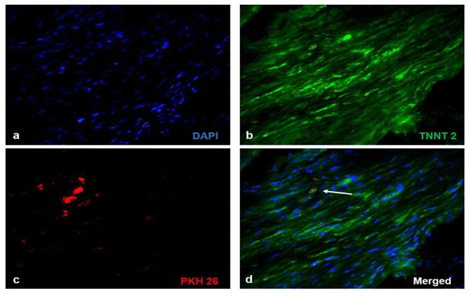
Significant numbers of cells, dual positive for PKH26- Connexin 43 (Figure 1) and PKH26 – cardiac Troponin T2 (Figure 2), were observed in the sections of rCP-Fib transplanted tissues. The individual panels of fluorescence indicating nuclear stain, red fluorescence of PKH26, and green fluorescence of cardiac-specific markers Connexin 43 and TNNT2 in rCP- Fib transplanted tissues is represented in the supplementary file (Supplementary figure S5 and S6, respectively). The results specify the survival and differentiation of the transplanted rCPs in the MI tissue when fibrin was used as the delivery vehicle. Though PKH26+ cells were observed in other tissue cryo-sections, the typical orange color, corresponding to the co-localized cardiac-specific markers and PKH26, was seen only when cells were delivered using fibrin (Figure 1l & Figure 2l). The fluorescence emitted from different planes indicates that differentiated cells are distributed in the 3-D tissue.
Figure1: Representative Fluorescence micrographs of Co-localization of PKH26 and Connexin 43 in tissue sections.1a, b, c, g, h, and i indicating merged images of native tissue site (NS) of Groups I to VI, respectively. 1d, e, f, j, k, and l indicating injured tissue site (IS) of Groups I to VI stained for Connexin 43/GJA 1. The nuclear stain is indicated by DAPI and Connexin 43/GJA 1 is stained green and PHK26 is stained red. Co-localized area of both red and green showing orange color is magnified and represented as an inset in figure 1l of animal group transplanted with rCP-Fib. (Magnification –40x).
Figure 2: Representative Fluorescence micrographs of Co-localization of PKH26 and TNNT 2 in tissue sections. 2a, b, c, g, h, and i indicating merged images of native tissue site (NS) of Groups I to VI, respectively. 2d, e, f, j, k, and l indicating injured tissue site (IS) of Groups I to VI stained for cardiac Troponin T. Nuclear stain is indicated by DAPI and Troponin T is stained green and PHK26 is stained red. Co-localized area of both red and green showing orange color is magnified and represented as an inset in figure 2l of animal group transplanted with rCP-Fib. (Magnification – 40x).
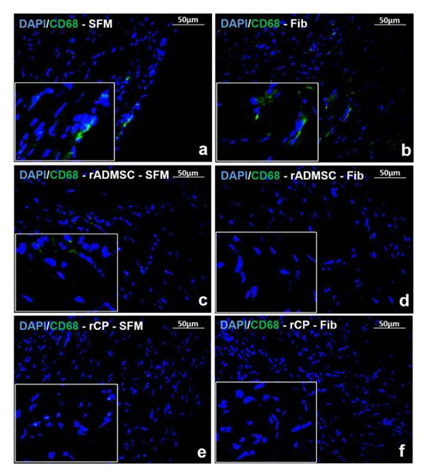
Identification of pro-inflammatory marker
Immunostaining for pro-inflammatory marker CD68 in tissue cryo-sections indicated that the animal tissues injected with rADMSC - Fib, rCP - SFM and rCP - Fib did not express CD68, 28 days post transplantation while, tissue sections immunostained from other treated animal tissues (injected with SFM, Fib, and rADMSC – SFM) comprised CD68 positive cells indicating an inflammatory response in the heart tissues 28 days post-transplantation (Figure 3). The results indicate that the transplantation of rCP is immunomodulatory in the presence and absence of fibrin; whereas, ADMSCs were immunomodulatory in the presence of fibrin only. This may be because only when ADMSCs were transplanted with fibrin, the cells were retained in the MI site; those transplanted in SFM was translocated to other area. However, no immunomodulation was observed in fibrin control tissues.
Figure 3: Immunostaining of Inflammatory marker CD68. Representative Fluorescent micrograph of tissue sections of a) SFM, b) Fib, and c) rADMSC - SFM transplanted groups showed CD68 positive cells represented by green fluorescence. CD68 positive cells were absent in tissue sections of d) rADMSC-Fib, e) rCP-SFM, and f) rCP-Fib transplanted groups indicated by the absence of green fluorescence. The nucleus is stained with DAPI (magnification – 40x).
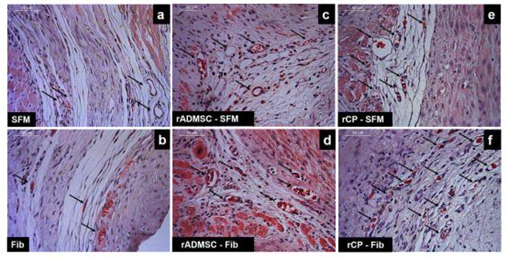
Histological appearance of 28th day MI tissue
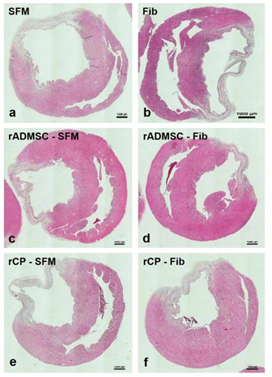
Tissue sections of infarcted rat hearts stained with hematoxylin and eosin confirmed the development of MI as evidenced by the thinning of the LV wall (Supplementary Figure S7). The high power field of H & E stained sections indicated the structures like wall thinning, fibrotic areas, new blood vessels, regions without myocytes, and rich with atypical nuclei (Figure 4). In C-SFM tissues (Figure 4a), infarct area was wide and the rarely seen blood vessels were restricted to the peri-infarct area. In both SFM-rADMSC (Figure 4c) and SFM-rCP (Figure 4e) injected tissues, infarct area was narrower as compared to C-SFM injected tissues and blood vessels were seen more frequently. The angiogenic effect may be attributed to the paracrine effect of the injected cells; even though, many cells migrated to other region, few cells may have adhered to the peripheral zone of the injured tissue making a difference. In C-Fib tissues (Figure 4b) infarct region was narrower and more blood vessels were seen. In Fib-rADMSC (Figure 4d) tissues, the infarct area was much reduced and the blood vessels were more frequently seen indicating that the paracrine effects of well restrained cells (Supplemnary Figure 3g) made a major difference in angiogenesis. In the rCP-Fib tissues (Figure 4f) the infarct area was completely covered with granulation tissue and more numbers of blood vessels were seen. Therefore, the pre-differentiated cells make significant effect on regeneration in terms of granulation tissue formation and angiogenesis.
Figure 4: Representative higher magnification bright-field images of H and E staining of tissue sections. a) Group I (SFM transplanted), b) Group II (fibrin transplanted), c) Group III (rADMSC-SFM), d) Group IV (rADMSC-Fib), e) Group V (rCP-SFM) and f) Group VI (rCP-Fib). LV wall thinning is evident from H & E stained tissue sections in specifically cell transplanted tissues. Arrows indicate new blood capillaries in tissue sections (magnification – 40x).
Evidence for Angiogenic response in MI tissue by 28th day
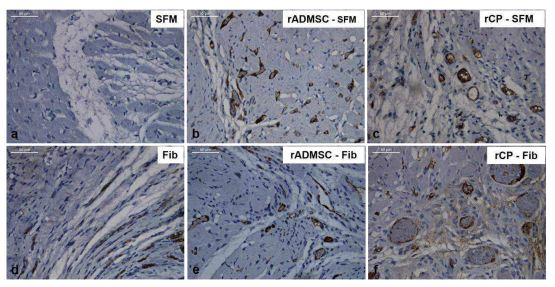
The immunohistochemistry of tissue sections with CD31 confirmed the observation of H&E stained sections. In C-SFM (Figure 5a) injected tissue there was no CD31 stained blood vessel seen; whereas, in C-Fib delivered tissues (Figure 5d) presence of blood vessels was evident. Peri-infarct zones with CD31 stained blood vessels were seen in rADMSC-SFM (Figure 5b), rADMSCs-Fib (Figure 5e) and rCP-SFM (Figure 5c) injected tissues. Distribution of large capillary-like structures was remarkable in rCP-Fib (Figure 5f) injected tissues, without discriminating the MI zone.Thus clearly, both fibin and rADMSCs are effective for inducing angiogenesis; but rCPs and fibrin together makes a major difference in terms of granulating the infarct tissue along with angiogenesis.
Figure 5: Representative bright-field images of CD 31 immunostaining. Prominent DAB stained large capillary-like structures were visible in rCP-Fib and rCP- SFM groups compared to rADMSC-SFM and rADMSC-Fib groups in the border areas of infarct zones indicating angiogenesis. CD 31 staining was not prominent in SFM transplanted groups and fibrin alone transplanted groups (magnification – 40x).
The reduced fibrotic response of MI
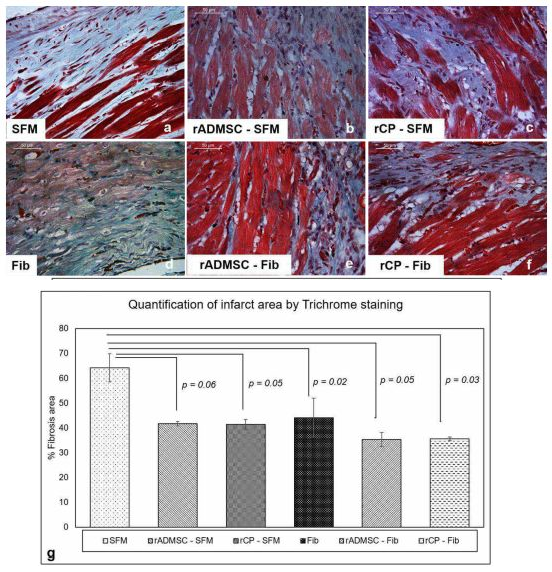
Masson’s trichrome staining revealed that the fibrotic area was wider in C-SFM (Figure 6a) tissues and rADMSC-SFM injected tissues (Figure 6b). Upon quantification (Figure 6g) the average area was less in rADMSC-SFM injected tissues as compared to the SFM injected tissues; however, the difference was not significant (p=0.06) (Figure 6g). In SFM-rCP injected tissues also, even though the quantification showed p=0.05 (Figure 6g), visually the collagen was distributed similar to that in SFM-rADMSC tissues (Figure 6b). The quantified reduction from SFM indicates that some of the transplanted rADMSCs/rCPs may have some effects in the injected area; however, since most of the cells migrated away from the injection site (Supplementary Figure 3f & 3h) the paracrine effect of injected cell was less significant. A significant reduction in the percentage of the fibrotic area was observed in all other tissues, including the fibrin alone injected tissues (Figure 6 d-f), when compared to SFM injected sham control. Overall, it is established that more than rADMSC/rCP, fibrin is responsible reducing the fibrotic tissue.
Figure 6: Representative Micrographs of Masson’s trichrome staining of border zones of the infarct. (a-f), Masson’s Trichrome staining for identifying the fibrotic area in the border zones of LV myocardium among six groups. (Magnification – 40x). (g), Quantification of collagen deposition represented as the percentage of fibrosis area in the border zones of MI was done using Image J software using the high power field of the border zones of all six groups. Error bars represent mean ± Standard error. Statistical analysis using Student’s t-test and the respective p values obtained is represented in the graph (n=3).
Discussion
Different cell types have been used by various researchers for transplantation therapy, aiming to regenerate/repair the damaged myocardium. But the efficacy of cell-based regenerative strategy are inadequate due to the hurdles in: i) selection of appropriate cell source [23]; ii) multipotency of transplanted cells causing undesirable lineage commitment and iii) poor retention of transplanted cells in the infarcted heart due to a hostile environment [15]. Despite the better outcome, bone marrow mesenchymal cell transplantation is cumbersome for clinical translation [24]. Transplantation of both undifferentiated stem cells and functional beating heart cells with electrophysiological properties caused pro-arrhythmic events [25,26]. Reports from previous studies have also revealed the formation of severe calcification and ossification in the infarcted myocardium in transgenic mice models upon transplantation of unfractionated bone marrow or undifferentiated MSCs [27]. The paracrine effect of MSC is unlikely to regenerate MI tissue, unless adequate cell homing occurs [28]. Several methods were adopted to improve cell homing. While the autologous ADMSCs transplantation with platelet-rich fibrin significantly improved LV function, effective remodeling of the MI tissue was not evident [29]. Ryu and colleagues have reported that the injection of bone marrow mononuclear cells and fibrin gel into the infarcted myocardium enhanced neovascularization by improving the microvessel density. Delivery of cells with fibrin gel exhibited more viable cells and less fibrous tissue compared to the cell-alone injection in other media [30]. This kind of promising response suggests that delivery of a suitable lineage committed cell using an injectable matrix that support further differentiation could be benficial. Many studies used transplantation of ADMSCs; however, multipotency of cells and poor support from niche have been identified as reasons for promoting undesirable/no differentiation. Therefore, in this proof-of-concept study, in vitro priming of rADMSCs to rCPs was considered, aiming to promote post-transplant differentiation of these cells into cardiomyocyte like cells and for the development of blood vessels, respectively. In the present study, a combination of fibrin-based matrix and growth factors was found to effectively induce pre-differentiation of rADMSCs into cardiac lineage cells as evidenced by the expression of cardiomyocyte structural marker, cardiac troponin T mRNA, in vitro till 16d of culture. Also, mRNA of the major gap junctional protein present in the myocardium, connexin 43 (GJA 1) was expressed by the rCPs indicating that the priming of rADMSCs with a combination of fibrin matrix and growth factors resulted in the maintenance of cardiomyocyte phenotype till 16 days in culture. In addition, expression of Flk-1 and CD31 indicated some of the ADMSCs are committed to endothelial progenitor cells (EPCs). The cells for transplantation were harvested between 6-8 days of induction, which may contain a heterogeneous population of a pool of cells comprising rADMSCs, rCPs and rEPCs. By continuing in vitro culture for 16 days, the lineage stability and further differentiation potential of the in vitro primed cells were confirmed.
Transfer of cells from one milieu to another one can produce a shock to the cells, and more intensely, when the new one is a hostile environment. Therefore, a novel concept of continued supply of fibrin niche in vivo was explored in our study, envisaging better cell survival and progression of differentiation. There are two important aspects to be considered when fibrin is used as a delivery vehicle. The high concentration of fibrinogen as used in hemostatic fibrin adhesive can result in a very tight network of fibrin strands causing stress to the cells. The major reasons of stress could be poor oxygen/medium supply and reduced flexibility for cells to migrate and grow. Previous reports have shown that increased concentration of thrombin has pro-apoptotic effects on cultured cardiomyocytes and endothelial cells [31]. Another important design criterionis optimization of the concentrations of components (fibrinogen and thrombin) to ensure in situ clotting upon injection. Therefore, bare minimum concentration of thrombin (1IU/ml) along with dilute fibrinogen was used to regulate clotting time and fibrin fiber density/thickness.
Appropriate controls were used to establish the effect of fibrin as a delivery vehicle and also to prove the pre-differentiation strategy to be superior for achieving desirable post-transplant differentiation. To confirm the effect of pre-differentiation, rADMSCs was used as a control to rCPs. To confirm the effect of fibrin as a delivery vehicle, SFM was used as a control for delivering both cell types. Fibrin control was included to see if the delivery vehicle alone has any effect. Thus, with sufficient groups of animals as controls, we have tried to answer if fibrin is a promising cell delivery vehicle and if pre-differentiation is a good strategy. Both transplantation of rCP and the use of fibrin are found to benefit when the data is analyzed against respective controls.
Previous studies have shown that ADMSC transplantation improves cardiac function mainly via paracrine action promoting angiogenesis [8]; however, no study has reported effect of cardiac progenitor transplantation. The cells harvested on the 6th to 8th day of induction were harvested for transplantation because TNNT 2 and GJA 1 mRNA expression were noticed around this time. In the in vitro culture, no protein level expression of cardiac markers was detected on the 8th day of induction/priming, indicating that the cells were lineage-committed without detectable level of functional proteins. But by the 28th day post-transplantation, proteins were expressed in corresponding cells in tissue sections, evidenced by the co-localization with tracker dye, indicating that the differentiation of transplanted cells occurred in the injured tissue site. Gap junction protein Connexin 43 was more prominently observed as compared to TNNT 2. Even with 14 µm thick cryosections, considerable numbers of differentiated cells were detected. Intracellular TNNT 2 molecules could have been more evident in 5 µm sections, which was not attempted in this study. Such post-transplant differentiation of rADMSCs embedded and delivered in fibrin was not seen. So progression into cardiomyocytes upon delivery using fibrin niche may be attributed to the cardiac lineage specificity of transplanted cells and the support from fibrin hydrogel niche.
In the case of angiogenesis also, as compared to rADMSCs transplanted in fibrin, the rCPs transplanted in fibrin was effective in developing blood vessels. This may be because of the presence of endothelial lineage-committed cells in the in vitro pre-differentiated rCPs at the time of cell transplantation. Therefore, fibrin does play a role in the development of blood vessels in the injured tissue; but fibrin alone is not able to induce significant angiogenesis in the injured heart tissue, as similar to the response seen in other soft tissues like skin [32]. Since only a portion of the cells were lineage-committed, the paracrine effects of ADMSCs also may be available when the mixed cell population is transplanted. The transmigration potential of ADMSCs has been already reported [33]. A similar response is seen in this study also. Both undifferentiated rADMSCs and rCPs injected using SFM were seen migrating when tissues were imaged for detecting tracker dye. The rCPs may contain a pool of undifferentiated rADMSCs in addition to the lineage-committed cells,which may have migrated to other locations more readily. Despite a significant pool of cells (rADMSCs/rCPs) migrating away when injected in SFM, few/undetectable (by imaging) numbers of transplanted cells may be residing in the injection site and causing a reduction in infarct area/collagen deposition and increase in angiogenesis.
Bioactive materials with appropriate physical strength and degradation kinetics that promote cell-matrix interactions may be an ideal cell delivery vehicle for the treatment of MI [34]. Fibrin gels are reported to exhibit excellent biocompatibility, promote cell attachment, and have a controlled biodegradable nature. It acts as a protective cell carrier safeguarding the cells from the forces during application and cell delivery processes and is also reported to enhance cell viability and tissue regeneration [29]. Improvement in wall thickness was observed in the infarcted rat hearts upon delivery of a mixture of fibrin with myoblasts five weeks post-implantation [35]. In this study also wall thinning action was less in C-Fib as compared to C-SFM. Similarly,the estimated fibrotic area was significantly less in C-Fib as compared to SFM. The advantage of fibrin as an excellent biomaterial for cell delivery relates to the feasibility of embedding cells in the liquid stage. Lack of angiogenesis in the Fib-control injected tissue suggests that the homing of stem/progenitor cells from the native heart tissue does not happen easily as it happens in the other soft tissues such as skin or liver [36].
In a clinical scenario, stem cell transplantation to acute MI is not feasible because autologous cell preparation may take a few weeks. By the time the cells get ready for transplantation, many changes take place in the injured myocardium. Whereas, in this study, an acute injury was treated. Therefore, for clinical translation, it is important to study the transplantation outcome in atleast 4-6 weeks old myocardial injury, to establish the regenerative potential of pre-differentiated autologous ADMSCs in chronic MI and for translation of the strategy into clinics. The number of cells transplanted may also make a difference in transplantation outcome which needs to be addressed in future studies. In the long term studies, improvement in cardiac output in terms of left ventricular ejection fraction may be studied upon delivering rCPs into MI. Despite such limitations, this study demonstrates a reduction in both the wall thinning response to injury and fibrosis/macrophage infiltration upon rCP transplantation in fibrin. Improved angiogenesis is highly appreciated when rCPs and fibrin are delivered. In addition, favorable differentiation into cardiomyocyte like cells is another highlight of the study. In summary, both rADMSCs and rCPs are found to be effective in preventing adverse immune response to acute MI. Injected fibrin induces limited angiogenesis in the peri infarct area, in addition to preventing collagen deposition. However, effective angiogenesis in the infarct area and cardiomyocyte regeneration required rCPs, in addition to fibrin. The angiogenic response is remarkably high upon transplantation of rCPs, as compared to rADMSCs, indicating the benefit of pre-differentiation of ADMSCs into CPs before transplantation.
Conclusion
A tendency to regenerate cardiac tissue upon stem cell-based transplantation, assisted by fibrin, is established in experimental model. The study concludes that transplantation of rADMSC- derived rCPs, embedded in fibrin hydrogel niche is a promising approach for regenerative therapy. The conclusion is based on favourable angiogenesis/cardiomyocyte differentiation and minimal CD68 invasion/collagen in tissues upon transplantation of rCPs in fibrin, within 28d of experiment. The strategy of transplantation of higher numbers of rCP using fibrin as delivery medium may be studied in detail for understanding the long term regeneration potential of the experimental MI model.
Acknowledgments
The authors are grateful to the Director, SCTIMST, and the Head, Biomedical Technology Wing, SCTIMST for the facilities provided. The authors thank Ms. Priyanka A, Mr. Ranjith S, Mr.Anilkumar V, and Ms. Deepa S for providing the pharmacopeia grade human fibrinogen and thrombin. The authors are grateful to Mr. Manoj and Mr. Sunil for providing proper animal care during the entire procedure. The authors thank SCTIMST for providing internal research funds (Project No.6208) and fellowship to Subha S. The authors are grateful to Dr.R.S. Jayasree and her students, Division of Biophotonics, for extending the IVIS imaging facility.
Conflict of interest statement
The authors declare that they have no competing interests.
Ethical approval and consent to participate
All institutional and national guidelines for the care and use of laboratory animals were followed. All protocols in this study were approved by the Institutional Animal Ethics Committee (IAEC), SCTIMST (Reference number - SCT/IAEC-266/FEBRUARY/2018/95), and by the Institutional Committee for Stem Cell Research (IC-SCR), SCTIMST, Thiruvananthapuram, Kerala, India.( Reference number -SCT/ICSCR/49/September 2018).
- All authors listed have made substantial contributions as described in the section “Author’s contribution”
- Authors confirm no potential conflicts of interest (financial or non-financial)
- Research involving Animals were conducted after review and approval by the Institutional
- Animal Ethics Committee (IAEC) conforming to CPCSEA, Government of India, guidelines
- Informed consent is not applicable
Consent for publication
The submission of this manuscript has been approved by all authors. The Research and Publication Cell of SCTIMST reviewed & recommended publication of the manuscript and was approved by the Director of the Institute.
Availability of supporting data
All supporting data is included in the current manuscript. The supplementary file is attached separately. Any additional information required by the reader shall be shared upon request.
Funding
The research fund was received from an internal Institute project (No.6208) and is acknowledged.
Authors’ contributions
Subha S designed a niche for the in vitro priming of rADMSCs into rCPs, performed the molecular analysis, preparation of cells for transplantation and immunostaining of tissue cryosections, compiled data, and drafted the manuscript. Sachin J Shenoy performed the experimental surgery of the MI model and carried out the cell transplantation into the infarcted heart and the follow-up animal care. Arya Anil assisted during the animal procedure and the follow-up animal care. Sabareeswaran A performed the necropsy of animals and performed the H & E staining. Deepthi AN performed the antigen retrieval and immunostaining of CD 31 in formalin-fixed paraffin-embedded tissue sections and its analysis. Corresponding author (Lissy K Krishnan) has contributed to developing the concept and study design, critically revising for important intellectual content, analysis of data, approving and editing the final version of the manuscript to be published. All authors read and approved the final manuscript.
References
- Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. International Journal of Cardiology 168 (2013): 934-945.
- Furtado MB, Nim HT, Boyd SE, et al. View from the heart: cardiac fibroblasts in development, scarring and regeneration. Development 143 (2016): 387-397.
- Ma T, Sun J, Zhao Z, et al. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Research & Therapy 8 (2017): 124.
- Madigan M, Atoui R. Therapeutic use of stem cells for myocardial infarction. Bioengineering 5 (2018): 28.
- Yoon YS, Park JS, Tkebuchava T, et al. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 109 (2004): 3154-3157.
- Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015). Stem Cell Research & Therapy 7 (2016): 82.
- Jeong JO, Han JW, Kim JM, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circulation Research 108 (2011): 1340-1347.
- Yang D, Wang W, Li L, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PloS One 8 (2013): e59020.
- Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of Clinical Investigation 103 (1999): 697-705.
- He XQ, Chen MS, Li SH, et al. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Molecular and Cellular Biochemistry 339 (2010): 89-98.
- Guo X, Bai Y, Zhang L, et al. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications. Stem Cell Research & Therapy 9 (2018).
- Kofidis T, de Bruin JL, Yamane T, et al. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells 22 (2004): 1239-1245.
- Sivan U, Jayakumar K, Krishnan LK. Constitution of fibrin-based niche for in vitro differentiation of adipose-derived mesenchymal stem cells to keratinocytes. BioResearch Open Access 3 (2014): 339-347.
- Chandrababu K, Senan M, Krishnan LK. Exploitation of fibrin-based signaling niche for deriving progenitors from human adipose-derived mesenchymal stem cells towards potential neural engineering applications. Scientific Reports 10 (2020): 7116.
- Chen Z, Chen L, Zeng C, et al. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells International (2018).
- Katarzyna R. Adult stem cell therapy for cardiac repair in patients after acute myocardial infarction leading to ischemic heart failure: an overview of evidence from the recent clinical trials. Current Cardiology Reviews 13 (2017): 223-231.
- Huang NF, Lam A, Fang Q, et al. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regenerative Medicine 4 (2009): 527-538.
- Hadjipanayi E, Kuhn PH, Moog P, et al. The fibrin matrix regulates angiogenic responses within the hemostatic microenvironment through biochemical control. PloS One 10 (2015): e0135618.
- Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circulation Research 92 (2003): 1068-1078.
- Qian Z, Sharma D, Jia W, et al. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 9 (2019): 2143.
- Tao H, Han Z, Han ZC, et al. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells International (2016).
- Zuk PA, Zhu MI, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering 7 (2001): 211-228.
- Wang H, Zhou J, Liu Z, et al. Injectable cardiac tissue engineering for the treatment of myocardial infarction. Journal of Cellular and Molecular Medicine 14 (2010): 1044-1055.
- Fraser JK, Wulur I, Alfonso Z, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology 24 (2006): 150-154.
- Gillum N, Sarvazyan N. Adhesion proteins, stem cells, and arrhythmogenesis. Cardiovascular Toxicology 8 (2008): 1-3.
- Smit NW, Coronel R. Stem cells can form gap junctions with cardiac myocytes and exert pro-arrhythmic effects. Frontiers in Physiology 5 (2014): 419.
- Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood, The Journal of the American Society of Hematology 110 (2007): 1362-1369.
- Ja KM, Miao Q, Tee NG, et al. iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. Journal of Cellular and Molecular Medicine 20 (2016): 323-332.
- Chen YL, Sun CK, Tsai TH, et al. Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. American Journal of Translational Research 7 (2015): 781.
- Ryu JH, Kim IK, Cho SW, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials 26 (2005): 319-326.
- Raivio P, Lassila R, Petäjä J. Thrombin in myocardial ischemia-reperfusion during cardiac surgery. The Annals of Thoracic Surgery 88 (2009): 318-325.
- Feldman DS, Osborne S. Fibrin as a tissue adhesive and scaffold with an angiogenic agent (FGF-1) to enhance burn graft healing in vivo and clinically. Journal of Functional Biomaterials 9 (2018): 68.
- De Becker A, Van Riet I. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy?. World Journal of Stem Cells 8 (2016): 73.
- Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacology & Therapeutics 105 (2005): 151-163.
- Christman KL, Fok HH, Sievers RE, et al. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Engineering 10 (2004): 403-409.
- Rennert RC, Sorkin M, Garg RK, et al. Stem cell recruitment after injury: lessons for regenerative medicine. Regenerative Medicine 7 (2012): 833-850.
Overview of Supplementary File:
This is an extension document which describes the method of preparation of lineage-committed rat cardiac progenitors (rCPs) derived from rat adipose-derived mesenchymal stem cells (rADMSCs), details of experimental surgery carried out for creating myocardial infarct (MI) in the rat model and transplantation of rCPs into MI region and tracking the cell retention based on fluorescence of transplanted cells. The data presented include proof of lineage commitment of ADMSCs to cardiac progenitors in vitro and post-transplantation. Fluorescence images of the whole heart on the 28th day of transplantation demonstrate the advantage of fibrin in cell retention at the infarct tissue.
Isolation of Adipose-Derived Mesenchymal Stem Cells and In vitro priming to cardiac lineage Briefly, adipose tissue was thoroughly washed with sterile Hank’s Balanced Salt Solution (HBSS), minced and treated with 0.15PZ U/ml of Collagenase NB 4 Standard Grade enzyme (Serva Electrophoresis, Germany) for a period of 30- 45 minutes at 37°C with continuous shaking. The digested cell suspension was strained (70uM, BD Falcon, USA), centrifuged, washed twice and the cell pellet was re-suspended in low glucose Dulbecco’s modified Eagle’s medium (DMEM- LG; Gibco, USA) supplemented with 10% Fetal Bovine Serum (FBS; Gibco, USA) and Antibiotic- antimycotic solution (ABAM; Invitrogen, USA). The cells were seeded in a 10cm2 tissue- culture polystyrene dish (TCPS; Nunc, Denmark) and incubated at 37°C under 5% CO2. The medium was changed at 3-day intervals. Upon reaching ~ 80% confluence, cells were passaged by standard trypsinization protocol using 0.25% Trypsin- EDTA (Invitrogen, USA) for cell expansion. Passage 3 (P3) rADMSCs were primed into cardiac lineage in vitro using a cocktail of growth factors (GFs) and fibrin matrix. For rADMSC priming, TCPS dishes were coated with fibrin matrix as described earlier [1]. Briefly, clinical-grade fibrinogen concentrate was reconstituted in water. Diluted (5IU) thrombin was treated with TCPS and the excess enzyme was aspirated, the surface was air-dried and a thin layer of (12.5 ul per cm2) diluted fibrinogen (2mg ml-1) containing 20ug fibronectin and exogenous gelatin (0.2%; Sigma Chemicals, USA) was spread and allowed to clot on the surface, lyophilized (Modulyo 4K, Edwards, UK) in a sterile atmosphere, and stored at 4°C till used. rADMSCs were primed to cardiac lineage in fibrin coated culture dish with a combination of 1.2µg/ml in-house prepared platelet growth factor (PGF) [2], 50ng/ml of Insulin-like growth factor -1 (IGF-1, Cell Signalling Technology, USA) and 50 µg/ml of L- ascorbic acid (Sigma, USA) in 10% DMEM/F12 medium for a period of 48h followed by withdrawal of PGF and IGF-1. After 48h of growth factor priming, culture was continued in 10% DMEM/F12 supplemented with 50 µg/ml L-ascorbic acid till 8th/16th day of initiation, at 37°C under 5% CO2 with medium change on alternate days. The in vitro primed cells were characterized by quantitative reverse – transcriptase polymerase chain reaction (qRT-PCR) for the expression of cardiomyocyte-specific markers Connexin 43 (GJA 1) and Cardiac Troponin T (TNNT 2) and the endothelial-specific markers CD31 and Flk-1. RNA was isolated using TRIZOL reagent (Invitrogen, USA) based on the manufacturer’s protocol. RNA quantification was done using Nanodrop 1000 Spectrophotometer (Thermo Scientific, USA). 200ng of total RNA was converted to cDNA using the OrionX cDNA kit (Origin, India) in a thermal cycler (Master cycler; Eppendorf). qRT-PCR of cardiac and endothelial lineage markers was carried out in a volume of 15µl containing 20ng cDNA, 100 pmol of respective intron spanning forward and reverse primers, and 7.5 µl of OrionX 2X Real-time PCR master mix (Origin, India). Forty cycles of reaction were performed using the Bio-Rad iQ5 Real-time PCR detection system (Bio-Rad, USA) under the following conditions: Enzyme activation: 95°C for 15 minutes; denature: 95°C for 30 seconds; annealing: 52°C for 20 seconds; and extension: 72°C for 20 seconds. GAPDH was used as the house-keeping gene. Melt curve analysis was performed for each gene to confirm the specificity of each reaction. The relative gene expression was calculated using un-differentiated rADMSCs grown on bare TCPS for respective time periods and fold change in expression was calculated after normalization with GAPDH as housekeeping gene on each day of analysis using the formula 2-ΔΔCt. The characterized rCPs were used for transplantation into animal models of MI. The sequence of primers used for qRT-PCR is listed in Supplementary Table 1.
|
Gene |
Gene Bank No. |
Primer sequence (5’-3’) |
Amplicon size |
|
TNNT 2 |
NM_012676 |
Forward - GATGCTGAAGATGGTCCA Reverse - TCATCAAAGTCCACTCTCTC |
110bp |
|
GJA 1 |
NM_012567 |
Forward - TTGACTTCAGCCTCCAAG Reverse - GGCACCTCTCTTTCACTTA |
75bp |
|
CD 31 |
NM_031591.1 |
Forward - TTGCCGCCTTGATAGTTG Reverse – CCTTCTCACTGTTGGAGTTC |
117bp |
|
Flk -1 |
NM_013062.1 |
Forward - AGCATCAGCATAAGAAGATTGTA Reverse - GTCACACTGTCTATGGTCAAG |
97bp |
|
GAPDH |
NM_017008.4 |
Forward - GGCACAGTCAAGGCTGAGAATG Reverse - ATGGTGGTGAAGACGCCAGTA |
143bp |
Supplementary Table 1: List of primers used in the study
Preparation and labelling of cells for transplantation
All myocardial injuries received 106 cells except 2 control groups. Before transplantation, P3 rADMSCs (un-differentiated) cultured on bare TCPS or rCPs between 6th and 8th day of in vitro culture on fibrin based niche were labelled with fluorescent, membrane intercalating dye PKH26 (red fluorescence, Sigma, USA) according to the manufacturer’s instructions. Briefly, the cells were harvested by trypsinization and were washed in serum-free medium (SFM). The cells were then suspended in the given diluent (diluent C, 0.5ml), mixed with the dye solution in diluent (0.5ml), and incubated for 2.5min at room temperature with periodic mixing in dark. The reaction with dye was stopped by adding an equal volume of FBS to the cell suspension with dye for 1min, to bind with the excess dye and the suspension was centrifuged at 400g for 10min at 22°C. The cells were then washed with a 10% serum containing medium to remove the unbound dye and were used for transplantation.
Experimental Surgery
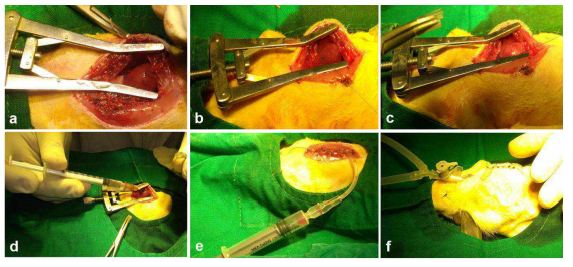
The rats were anesthetized with ketamine (80mg/kg body weight) and xylazine (10mg/kg body weight) and were clipped and sterilized with antiseptic betadine. Mechanical ventilation was achieved by tracheostomy by connecting the endotracheal tube to a ventilator with cycles of 40-60 breaths per minute and a tidal volume of 1.2ml per 100-gram body weight. Animals were then maintained under general anesthesia with 1.5% isoflurane. A left thoracotomy was performed between the 4th and 5th intercostal spaces and after retraction of the ribs, the heart was exteriorized. The left coronary artery was transiently ligated with 6-0- prolene suture approximately 2mm from its origin between the left atrium border and the pulmonary artery sulcus. The development of regional myocardial ischemia was observed by the blanching of the tissue over the anterior surface of the left ventricular wall and the rapid development of akinesia.
Cells were transplanted at the injury site 15 - 20 min after the MI using a 1ml syringe. 106 cells/animal suspended in fibrinogen were injected along with thrombin at the site of injury for test animals. Fibrinogen at a concentration of 20mg/ml and thrombin at a concentration of 1IU/ml were used. In Group I, 200ul of SFM alone was injected whereas, in Group II, fibrin alone was injected. Soon after transplantation/injection, the chest wall was closed after maintaining negative pressure inside the thoracic cavity by gentle suction. Postoperative care was given to the animals by the administration of antibiotics and painkillers for 5 days after the development of MI and cell transplantation. Animals were euthanized humanely after 28 days post-transplantation. For histopathology evaluation, perfusion fixation was done initially with Ringer’s lactate solution to wash the red blood cells followed by 10% neutral buffered formalin. The heart was dissected out and stored in 10% neutral buffered formalin before routine tissue processing.
Results
In vitro priming of rADMSC to rCPs
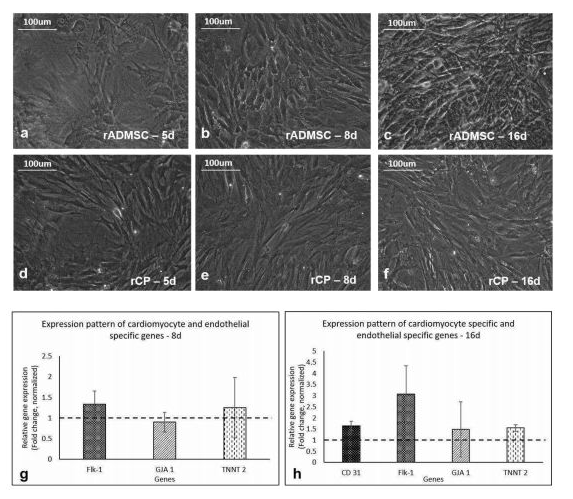
The isolated rADMSCs were plastic-adherent and appeared to have fibroblastic spindle-shaped morphology (Supplementary Figure S1). Distinct morphological features were observed in primed cells as compared to the control/undifferentiated rADMSCs from day 5 of induction. The elongation of cells and formation of tube-like structures was observed from the 5th day of GF treatment and the elongated morphology of primed cells was maintained until 16 days of culture. The appearance of phase-bright cells was also evident from the 5th day of priming in vitro. The qRT-PCR results indicated a 0.25-fold up-regulation of cardiac-specific markers Troponin T (TNNT2) by 8d and the expression of TNNT2 was almost consistent up to 16d (0.55-fold) of culture in vitro. The expression of Connexin 43 (GJA 1) mRNA, a predominant gap junctional protein present in the myocardium was also found to be expressed in primed rCPs till 16d of culture in vitro. A 0.34-fold up-regulation of Flk-1, the major vascular endothelial growth factor (VEGF) receptor, was observed in the in vitro primed cells by 8d and was further up-regulated to 2-fold by 16d as compared to the un-differentiated rADMSCs. However, CD31 mRNA expression was not observed in the in vitro primed rADMSCs onthe 8th day of culture, but by the 16th day of in vitro priming, its expression (0.64-fold) was observed in the rCPs.
Supplementary Figure S1 Representative images of the morphology of rADMSCs and in vitro primed rCPs:
1a,b,c, Phase contrast images of un-differentiated rADMSCs cultured on bare TCPS at 5d, 8d, and 16d of culture in vitro, respectively. 1d, e, and f, Phase contrast morphology of primed rCPs on fibrin matrix at 5d, 8d, and 16d of culture in vitro, respectively (Magnification -20x). 1g, Graphical representation of relative gene expression pattern of in vitro primed rCPs for the markers: Flk-1, GJA1, and TNNT 2 on 8d. 1h, the relative gene expression pattern of rCPs for the markers Flk-1, CD31, GJA1 and TNNT 2 at 16d of culture in vitro by qRT-PCR. Fold change is indicated relative to GAPDH expression on each day of analysis using the 2-δδCt method, upon normalization with results obtained from the un-differentiated rADMSCs cultured on bare TCPS. The dotted lines represent the basal level of gene expression in un-differentiated rADMSC cultures of the respective time periods. Error bars represent standard error (n=3).
Transplantation in MI model
The development of MI was confirmed visually by observing the blanching of the myocardium distal to the ligation. Following the development of the ischemic area in the myocardium, SFM, Fib, rADMSC-SFM, rADMSC-Fib, rCP-SFM, rCP-Fib were injected to the peri-infarct zone. Following transplantation, air from the thoracic cavity was removed by suction and the chest wall was closed. Animals recovered slowly with the help of a ventilator which was weaned after the restoration of spontaneous breathing (Supplementary figure S2).
Supplementary Figure S2 Representative Images depicting the MI model and transplantation Experiments:
(a) Parasternal thoracotomy was done followed by tracheostomy. (b) Surgical creation of MI by ligation of LAD by 6-0 prolene suture. (c) The development of MI was observed by the blanching of the tissue distal to ligation. (d) Transplantation was performed in the peri-infarct zone, (e) air from the thoracic cavity was removed by suction using a syringe and (f) chest wall was closed in layers and animals were ventilated till restoration of spontaneous breathing and allowed to survive for 28 days.
IVIS imaging of tissues for cell tracking
The red fluorescence of either rADMSCs or rCPs (harvested from 6-8d cultures) labeled with PKH26- lipophilic dye, was confirmed before transplantation (Supplementary Figure S3a-c). Post-transplantation, the IVIS images of isolated hearts harvested on the 28th day showed distinctly different fluorescence, based on the experiment. The primed rCPs delivered in fibrin showed more localized red fluorescence in the transplanted site than the heart tissues with rCPs transplanted in SFM. This indicated that though an equal number of cells were transplanted in all animal groups, the group in which fibrin was used as a cell delivery vehicle retained more cells in the site of injury. There was no autofluorescence from the control heart tissue in IVIS images (Supplementary Figure S3d, e). Though both cell types-rADMSCs (Supplementary Figure S3f) and rCPs (Supplementary Figure S3h) delivered in SFM also were retained in the heart tissue, the intensity of fluorescence was less in these as compared to the rADMSCs (Supplementary Figure S3g) and rCPs (Supplementary Figure S3i) delivered in fibrin, as evidenced by the migration of red fluorescence from the site of injections. So the results confirmed that retention of both rADMSCs and rCPs was better upon transplantation using fibrin as a cell delivery vehicle. Both cells – rADMSCs and rCPs seemed to have migrated from the site of injury to other areas, upon delivery with SFM.
Supplemetary Figure S3 PKH26 labeling of cells and IVIS imaging of whole heart tissue:
3a, Representative phase-contrast image of trypsinized cells for labeling and transplantation, 3b, a fluorescent micrograph of PKH26 labeled cells and 3c, merged micrograph of phase contrast and fluorescent fields, indicating the specificity of red fluorescence in labeled cells (Magnification – 20x). Absence of red fluorescence in (d) SFM, and (e) Fib indicating the absence of auto-fluorescence. Intensity of red fluorescence was higher in (g) rADMSC-Fib and (i) rCP-Fib as compared to (f) rADMSC-SFM and (h) rCP-SFM 28d post-transplantation.
Supplementary Figure S4 Fluorescent micrograph of tissue cryosections stained with DAPI:
Absence of red fluorescence of PKH26 in a) SFM and b) Fib transplanted groups stained for DAPI. Presence of red fluorescence along with the nuclear stain DAPI indicating the presence of transplanted cells in tissue cryo-sections of animal hearts in the groups – c) rADMSC-SFM, d) rADMSC-Fib, e) rCP-SFM and f) rCP-Fib. (Magnification – 40x).
Supplementary Figure S5 Fluorescent micrographs of individual panels of Connexin 43 immunostained sections:
Immunostaining of rCP- Fib (Group VI) transplanted groups representing a) Nuclear stain indicated by DAPI (blue), b) Connexin 43/ GJA1 (green), c) PKH26 (red) and d) merged images representing co-localization of fluorescent signals. The Co-localized area of both red and green showing orange color is represented as white arrows (Magnification – 40x).
Supplementary Figure S6 Fluorescent micrographs of individual panels of cardiac Troponin T2 immunostained sections:
Immunostaining of rCP- Fib (Group VI) transplanted groups representing a) Nuclear stain indicated by DAPI (blue), b) cardiac Troponin T/TNNT 2 (green), c) PKH26 (red) and d) merged images representing co-localization of fluorescent signals. The Co-localized area of both red and green showing orange color is represented as white arrows (Magnification – 40x).
Supplementary Figure S7 Representative bright-field images of H and E staining of tissue sections:
a) Group I (SFM transplanted), b) Group II (fibrin transplanted), c) Group III (rADMSC-SFM), d) Group IV (rADMSC-Fib), e) Group V (rCP-SFM) and f) Group VI (rCP-Fib). LV wall thinning was evident from H & E staining of tissue sections in all animal groups (magnification – 1x) (n=4).
References:
- Sreerekha PR, Divya P, Krishnan LK. Adult stem cell homing and differentiation in vitro on composite fibrin matrix. Cell Proliferation 39 (2006): 301-312.
- Resmi KR, Krishnan LK. Protease action and generation of β-thromboglobulin-like protein followed by platelet activation. Thrombosis Research 107 (2002): 23-29.