Fetal Imaging Findings in a Case of Schinzel Giedion Syndrome
Article Information
Julliet Ogu1 and Thierry A.G.M. Huisman1*
1Edward B. Singleton Department of Radiology, Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA
*Corresponding Author: Thierry A.G.M. Huisman. Edward B. Singleton Department of Radiology, Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas, USA
Received: 28 April 2023; Accepted: 05 May 2023; Published: 19 May 2023
Citation: Julliet Ogu, Thierry A.G.M. Huisman. Fetal Imaging Findings in a Case of Schinzel Giedion Syndrome. Archives of Clinical and Medical Case Reports. 7 (2023): 212-215.
Share at FacebookAbstract
Schinzel Giedion Syndrome (SGS) is an extremely rare congenital malformation syndrome caused by de novo mutations in the SETBP1 gene. This condition is characterized by distinctive craniofacial dysmorphisms such as midface retraction, severe intellectual disability, increased risk of embryonal tumors, and multiple congenital anomalies, particularly cardiac, renal, and urogenital defects. Characteristic abnormalities can be identified in the prenatal period and confirmed using molecular genetics. We present the prenatal and matching postnatal imaging findings in a case of confirmed Schinzel Giedion Syndrome.
Keywords
Schinzel Giedion Syndrome; Prenatal imaging; Ultrasound; MRI
Article Details
1. Introduction
Schinzel Giedion Syndrome (SGS) is an extremely rare congenital malformation syndrome caused by de novo mutations in the SETBP1 gene [1]. To date, fewer than 100 cases have been reported worldwide. This condition is characterized by distinctive craniofacial dysmorphisms, severe intellectual disability, increased risk of embryonal tumors, and multiple congenital anomalies, particularly cardiac, renal, and urogenital defects. SGS is a progressive and fatal syndrome with most affected patients dying in early childhood. Major causes of morbidity and mortality include refractory seizures, recurrent infections, feeding difficulties and respiratory failure [2]. Diagnosis is typically based on the clinical identification of characteristic symptoms, followed by confirmation through genetic testing. Some prenatal abnormalities in patients with SGS can be identified during routine prenatal ultrasound (US) screenings, though these findings are seldom correlated with SGS given the rarity of this condition. The prenatal detection of SGS offers the potential for optimized management of the affected patient, as well as early preparation for severe clinical issues that may arise during the neonatal phase and beyond. In this report, we will present fetal MRI and U/S findings as well as matching postnatal imaging in a patient with confirmed Schinzel Giedion Syndrome.
2. Case Report
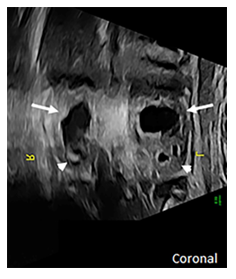
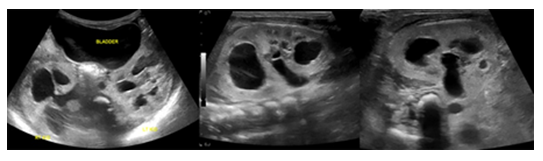
At 27 weeks of gestation, a 32-year-old primigravida received a routine fetal US which identified multiple fetal abnormalities, including an absent nasal bone, abnormal ears, bilateral rocker bottom feet, and bilateral dilatation of the upper renal poles consistent with duplicated renal systems (Figure 1).
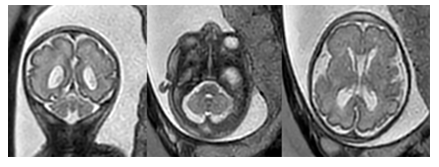
Additionally, the fetus was found to have ambiguous external genitalia although its’ internal genitalia suggested the presence of a uterus and vagina. The pregnancy was also complicated by intrauterine growth restriction and polyhydramnios. Family history was notable for the mother and the child’s father being third cousins. Following referral for genetic counseling, an amniocentesis was performed which revealed a normal 46 XX karyotype with normal results on FISH, CMA, and negative infectious panel. Whole exome sequencing results were pending at the time but would later confirm that the fetus was heterozygous for a pathogenic variant of SETBP1 which is consistent with a diagnosis of Schinzel Giedion Syndrome. The fetal ultrasound findings were confirmed on a fetal MRI performed at 30 weeks (Figure 2).
Additional findings on fetal MRI included urine within the fetal vagina concerning for vesicoureteral reflux or abnormal insertion of the ureter(s) into the vagina. The fetus was also noted to have folded ears and thickening of the skin over the forehead. Furthermore, the cerebellar vermis and horns, as well as the pons, were small for gestational age.
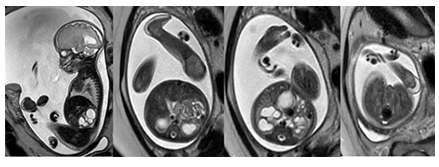
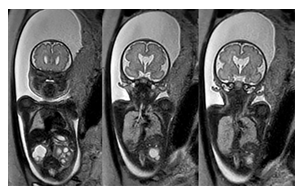
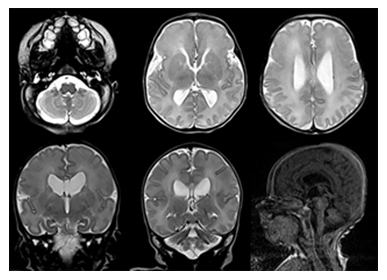
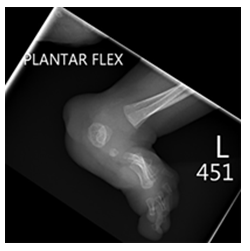
At 37 weeks, the mother was scheduled for induction of labor and delivered a female infant via vaginal delivery with initial apgar scores of 8 and 9 at one and five minutes, respectively. The early neonatal period was complicated by respiratory distress necessitating placement on 4L high-flow nasal cannula. On physical examination, the infant exhibited several notable features, including ocular hypertelorism, a retracted midface with a prominent forehead and absent nasal bridge, a high-arched cleft palate, low-set and posteriorly rotated ears with folded helixes, and hypoplastic labia majora with prominent labia minora. Additionally, the patient was found to have significant external rotation of both feet and bilateral talipes calcaneovalgus. An MRI brain (Figure 3) performed at one week of age depicted hypoplasia of the pons and cerebellum and a postnatal renal ultrasound (Figure 4) noted bilateral hydroureteronephrosis, both findings consistent with the original prenatal imaging findings. Furthermore, a voiding cysto-urethrogram was performed which excluded vesico-ureteral reflux as well as a skeletal survey which assessed the patient’s foot deformity (Figure 5).
3. Discussion
Schinzel Geidion Syndrome, first described in 1978 by Albert Schinzel and Andreas Giedion, is characterized by distinctive craniofacial deformities such as severe midface retraction, multisystem congenital abnormalities, an increased risk of neuroepithelial tumors, seizures, and global developmental delay [3, 4]. SGS is caused by heterozygous de novo mutations in the SETBP1 gene located on chromosome 18. The physiological function of the SETBP1 gene remains incompletely understood. However, the disease mechanism is believed to be secondary to a gain of function mutation that leads to the accumulation of the SETBP1 protein. The protein product has demonstrated involvement in gene expression regulation and exhibits ubiquitous expression, including disruption of the normal embryonic development of multiple organs suggesting a correlation with the multisystemic effects associated with the condition [5].
Prior to the discovery of the SETBP1 gene as the genetic cause for this condition, the diagnosis of SGS was based on the presence of key clinical features. Lehman et al proposed clinical criteria for diagnosis based on the presence of developmental delay and a distinctive facial phenotype (midface retraction, prominent forehead, nasal hypoplasia, dysplastic ears, and hypertelorism) associated with hydronephrosis and skeletal/limb malformations [3, 6]. Patients with SGS also commonly suffer from cardiac and genital abnormalities, seizures, severe intellectual disability, progressive neurodegeneration and difficulties with swallowing and breathing [3, 7-10]. Other symptoms may include vision and hearing problems, sleep apnea, choanal stenosis, macroglossia, abnormal dentition and hypotonia. Individuals affected by this condition typically do not survive past early childhood (18 months-4 years), with feeding difficulties, respiratory failure, recurrent infections, and refractory seizures being the most common causes of death [7-10]. SGS is a severe and progressive syndrome. The goals of treatment are focused on addressing the patient’s symptoms and improving their quality of life.
With the introduction of high-resolution prenatal US and MRI, characteristic prenatal abnormalities can be identified on fetal imaging and confirmed through molecular genetics. This report presents the prenatal imaging findings of a patient with SGS, revealing characteristic features such as hydronephrosis, skeletal/ limb abnormalities, nasal hypoplasia, a prominent forehead, dysplastic ears, and ambiguous genitalia. These findings were then confirmed on postnatal imaging. Given the rarity of SGS, clinical suspicion may be low in patients with multiple congenital anomalies on fetal imaging. When these characteristic fetal imaging findings are observed, Schinzel Giedion Syndrome should be considered as a potential diagnosis.
Conflicts of Interest
We have no conflicts of interest to disclose.
References
- A Hoischen et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet 42 (2010): 483-5
- A Takeuchi et al. Progressive brain atrophy in Schinzel-Giedion syndrome with a SETBP1 mutation. Eur. J. Med. Genet 58 (2015): 369-371.
- A M Lehman, D McFadden, D Pugash, et al. Schinzel-Giedion syndrome: Report of splenopancreatic fusion and proposed diagnostic criteria. Am. J. Med. Genet. A 146 (2008): 1299-1306.
- L J Anyanwu et al. Schinzel-Giedion syndrome: a case with sacrococcygeal teratoma and cor-triatriatum dexter. Pan Afr. Med. J 26 (2017).
- A Hoischen et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet 42 (2010): 483-5.
- Y Herenger et al. Long term follow up of two independent patients with Schinzel-Giedion carrying SETBP1 mutations. Eur. J. Med. Genet 58 (2015): 479-487.
- Bulut, Z Ince, U Altunoglu, et al. Schinzel-Giedion Syndrome with Congenital Megacalycosis in a Turkish Patient: Report of SETBP1 Mutation and Literature Review of the Clinical Features. Case Rep. Genet 4 (2017): 1-4
- P Labrune et al. Three new cases of the Schinzel-Giedion syndrome and review of the literature. Am. J. Med. Genet 50 (1994): 90-93.
- R Acuna-Hidalgo et al. Overlapping SETBP1 gain-of-function mutations in Schinzel-Giedion syndrome and hematologic malignancies. PLoS Genet 13 (2017): e1006683.
- J M Ko et al. Distinct neurological features in a patient with Schinzel-Giedion syndrome caused by a recurrent SETBP1 mutation. Childs Nerv. Syst 29 (2013): 525-529.