Evaluation of the Effect of Pouteria Campechiana’s Fruit Powder and Ethanolic Extract on Aluminum-Chloride Induced Alzheimer’s Disease
Article Information
Joel Geradin Tueguem Tagne1, Anne Pascale Nouemsi Kengne1,2*, Hermine Tsafack Doungue1, Michel Pegui Kemtsop1, Julius Oben Enyong2
1Laboratory of Biochemistry, Medicinal Plants, Food Sciences and Nutrition, Department of Biochemistry, University of Dschang, Dschang, Cameroon
2Laboratory of Nutrition and Nutritional Biochemistry, Department of Biochemistry, University of Yaounde I, Yaounde, Cameroon
*Corresponding Author: Kengne Nouemsi Anne Pascale, Departmentnt of Biochemistry, University of Dschang, Dschang, Cameroon
Received: 26 March 2020; Accepted: 06 April 2020; Published: 20 May 2020
Citation: Joel Geradin Tueguem Tagne, Anne Pascale Nouemsi Kengne, Hermine Tsafack Doungue, Michel Pegui Kemtsop, Julius Oben Enyong. Evaluation of the Effect of Pouteria Campechiana’s Fruit Powder and Ethanolic Extract on Aluminum-Chloride Induced Alzheimer’s Disease. Journal of Food Science and Nutrition Research 3 (2020): 083-091.
Share at FacebookAbstract
Objective: The aimed of this work was to assess the protective effect of Pouteria campechiana’s (PC) fruit on neurological disorder and oxidative stress induced by aluminium chloride.
Methods: Alzheimer disease were induced using 100 mg/kg of AlCl3 orally twice/week during the 42 days. The behavioural assessment was performed using Water maze and eight arms maze tests. The activity of pouteria campechiana fruit were evaluated by administration of 200 mg/kg, 400 mg/kg fruit ethanolic extracts and 5% and 10% of PC’s fruit powders respectively. Wistar rats were treated for 42 days along with aluminium chloride. After treatment, Biochemical estimation of oxidative stress parameter and acetylcholine activity was done in brain also, the Brain histopathological examination was done by microscopic observations.
Results: Aluminium chloride treatment results in memory impairment and oxidative stress while treatment with powders and ethanolic extracts of PC showed an improvement on reduction of this oxidative stress by lower behavioural impairment, MDA content and the increased of total proteins, reductive glutathione, acetylcholinesterase and catalase, Histopathological examination revealed the presence of amyloid plaques and neurofibrillary tangles in the brain of negative control rats. For those treated with PC’s powders and extracts, few or no hallmarks were present.
Conclusions: PC’s fruit ethanolic extracts and powders revealed a protective effect against neurodegenerative effects of aluminum-induced Alzheimer’s disease (AD). PC fruit may treat AD.
Keywords
Pouteria campechiana, Neuroprotective activity, Rats, Alzheimer disease, Aluminum
Article Details
Abbreviations:
AD-Alzheimer disease; PC-Pouteria campechiana; Aβ-amyloid beta; NFT-neurofibrillary tangles
1. Introduction
Alzheimer disease is a neurodegenerative pathology that affects over 65 years old people. It manifests by some changes in behaviour, and by the appearance of some marks such as amyloid beta (Aβ) and neurofibrillary tangles (NFT) in the brain [1]. Recent researches estimate of about 50 millions of people diagnosed with AD in the world and this number would be approximately 132-150 million by the end of 2050, with the highest prevalence in emerging countries [2]. The commonest theory explaining causes of AD is oxidative stress (OS), which produces abundantly reactive oxygen species (ROS). The elevated production of ROS associated with age, reduces antioxidant defense mechanism by affecting neuronal activity [3, 4]. Previous research has reported Aluminum to be involved in AD through the mechanism of OS. It leads to progressive deterioration of mitochondrial function which culminates into excessive free radical generation eventually resulting in DNA damage, nitration of protein residues, and lipid peroxidation [5, 6]. Fruits and vegetables are shown to be good sources of bioactive compounds like ascorbic acid, tocopherols, β-carotene and phenolic compounds, flavonoids that may prevent from oxidative damage [7]. PC is a plant generally distributed in tropical regions in the world. The edible part of PC is its fruit and is used for many purposes. Recent studies have shown that the pulp of PC fruit is rich in bioactive components, namely phenolic compounds, flavonoids, vitamin C, tannins and saponins [8, 9]. This study was designed therefore to evaluate the effect of PC’s fruit in aluminum chloride-induced Alzheimer’s disease in albino rats.
2. Material and Methods
2.1 Plant material
PC fruits were collected in Mbanga in Littoral region of Cameroon and sent to national herbarium for identification. Once harvested, fruits were transported to the laboratory.
2.2 Animal material
Male and female albino rats of Wistar strain weighing 200 to 250 g and aged 4 months, were used for the experiment. They were acclimatized to the standard laboratory conditions and were fed with standard animal pellet feed ad libitum according to the protocol described by Telefo et al.
2.3 Extraction of plant material
After a few days for fruits to turn ripe, they were cleaned with tap water, peeled with an inoxidative sterilized knife. The pulp was cut into slight pieces and dried up for 72 hours at 45°C. Then it was coarsely powdered using a blender and the powder was mixed to ethanol 95% for extraction of bioactive compounds. The extract obtained was conserved in the freezer for further analysis.
2.4 Determination of phenolic content
Total phenolic content (TPC) was determined using the Folin-Ciocalteu method as described by Singleton and Rossi [10] with some slight modifications. The total flavonoid content (TFC) was determined using aluminum chloride method as described by Liu et al. [11].
2.5 Determination of extracts Antioxidant activities
DPPH radical scavenging activity was determined according to the method described by Mensor et al.; Ferric reducing antioxidant power was done by the method described by Hubert, and hydroxyl radical scavenging activity by the method described by Nagulendran et al.
2.6 Allotment of animals
Wistar rats were randomly divided into seven groups of six (3 males and 3 females). Group I served as normal control and received a normal diet (GC). Group II served as negative control and received normal pellet feed, with a solution of AlCl3 (G(-)). Group III received pellet formulated with 5% of PC’s powder (GP1). Group IV received pellet formulated with 10% of PC’s powder (GP2). Group V served as positive control and received normal pellet and vitamin C 200 mg/kg bw (G(+)). Group VI was administered normal pellet feed and PC’s ethanolic extract 200 mg/kg bw (GE1). Group VII was administered normal pellet feed and PC’s ethanolic extract 400 mg/kg bw (GE2). Every group was induced by a solution of AlCl3 100mg/kg bw twice/week except group I. The experiment was conducted in 6 weeks.
2.7 Behavioural assessment
Behavioral assessment was carried out for Eight arms Maze and Water maze on day 0 and 42.
2.8 Water maze
Water maze was performed according to the method described by Morris with slight modifications [12]. This test is based on the capacity of rat to escape a stressful and aversive environment. The water maze apparatus was 60 cm length and 180 cm width and filled with cold water at the 2/3. A platform was placed at the center of the apparatus at 1 cm below the surface of water, and was invisible by the rat. The memory activity was based on time spent by the animal to find the platform. When the animal spent more than 60s to find it, it was directed by the experimenter [13].
2.9 Elevated plus maze
The elevated plus maze consisted of eight opposite closed arms length of 50 cm each, connected to a central square of dimensions 10 × 10 cm. Animals were submitted on fasting for 24 hours before the experiment starts. Four arms of maze contained food (reward). Rats were placed individually at the central square. Behavioural assessment was done based on time spent by the animal to move from the central square to the eight closed arms (working memory), and on the number of arms containing food visited (reference memory). This was done on day 0 and day 42 [14].
2.10 Biochemical assessment
24 hours after the last administration, animals were sacrificed under chloroform vapour. The brains were removed, weighed and kept in a freezer. A 10% (w/v) brain tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4) and centrifuged at 3500 × g for 15 minutes at 4°C. The homogenate obtained was used for the assessment of biochemical parameters [15] such as acetylcholinesterase (AchE) (total protein, MDA, catalase, and reductive glutathione.
2.11 Histopathological examination
0.5 g of brain tissue was cut at the hippocampus region and immerged in formalin 10% for 72 hrs. The brains were washed in tap water and then dehydrated using serial dilutions of alcohol. Specimens were cleared in xylene and embedded in paraffin in a hot air oven at 56°C for 24 hrs. Paraffin beeswax blocks were prepared for sectioning at 4 mm using a microtome. The obtained tissue sections were collected on glass slide deparaffinized, stained with hematoxylin and eosin stains for histopathological examination using a light microscope [16].
2.12 Statistical analysis
The data were expressed as mean ± standard error mean (SEM) and were analyzed by One-way Analysis of variance (ANOVA) followed by Duncan test. The value of p<0.05 was considered statistically significant. Data were analyzed using Statistical Package for the Social Sciences (SPSS) software 22.0.
3. Results
3.1 Behavioral parameters
3.1.1 Water maze: On day 0, there was no significant difference between the different groups of rats at p=0.05. All the rats spent almost the same time to find the platform. The capacity of memorization was the same in rats. As the experiment went on till the 42nd day, there was a significant difference between (p<0.05) the +groups of rats. Those that were administered only AlCl3 took more time to find the platform. On the other hand, rats that were administered PC’s powder 5 g/100g and 10 g/100g were the fastest as shown in Table 1.
3.1.2 Elevated plus maze: The working memory and the reference memory were assessed. At the initial day (day 0), all the rats had significantly the same capacity of exploring the eight arms of the apparatus and of targeting arms containing food when submitted under fasting conditions. On the last day (42nd day), the working memory and reference memory differed significantly in all the groups. The negative control had the lowest memory capacity and the group administered with 400 mg/kg PC’s ethanolic extract the highest (Table 1).
3.2 Biochemical parameters
3.2.1 Total proteins: Assessment of total proteins content in the different groups showed that, negative control group had the lowest proteins content that was significantly different from that of groups treated with PC’s powders and extracts as illustrated in Table 2.
3.2.2 MDA: MDA content varied significantly in groups of rats. The negative control showed the highest content in MDA while the group treated with PC’s extract 400 mg/kg bw had the lowest content as shown in Table 2.
3.2.3 Reductive glutathione: Glutathione content was significantly different in the different groups of rats. The groups treated with PC’s powders 100 mg/100g and PC’s ethanol extract 400 mg/kg have the highest content and the lowest content was observed in the group that was administered only AlCl3 (Table 2).
3.2.4 Catalase activity: Activity of catalase was significantly higher in groups treated with PC’s powders and extracts compared to that of group which was administered only AlCl3 without any treatment. However, it was highest in group treated with PC’s powders 100 mg/100g (Table 2).
3.2.5 Acetylcholinesterase activity: AChE activity was significantly different in all the groups of rats. The lowest activity was observed in rats administered only with AlCl3, and the highest activity in rats treated with PC’s powders 10 g/100g and with PC’s ethanol extract 400 mg/kg bw (Table 2).
3.3 Brain histopathological examination
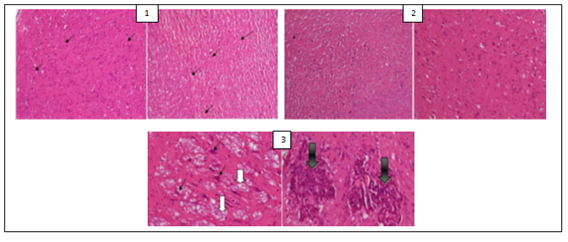
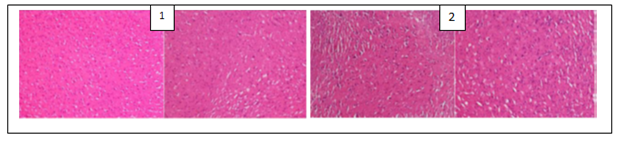
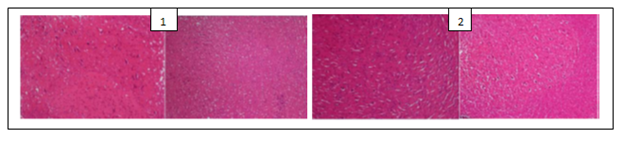
The histological examination of the brain of rats revealed the presence of some pathological hallmarks such as amyloid plaque and neurofibrillary tangles in negative control group. Tissue brain of rats that were treated by PC’s powders and extracts were more or less similar to that of normal control groups, with some areas of cell degeneration as shown in Figures 1, 2 and 3.
|
Groups |
Water maze |
Elevated plus maze (day 42) |
||
|
Day 0 (s) |
Dat 42 (s) |
WM |
RM |
|
|
GC |
20 ± 0.9 |
20.5 ± 3.90a |
1.88 ± 0.40d |
0.74 ± 0.16c |
|
G(-) |
18 ± 1.6 |
36.66 ± 5.68d |
0.76 ± 0.26a |
0.2 ± 0.0a |
|
GP1 |
21 ± 1.78 |
22.33 ± 3.78ab |
1.4 ± 0.2bc |
0.32 ± 0.18ab |
|
GP2 |
19 ± 0.72 |
22 ± 6.72b |
1.01 ± 0.14b |
0.46 ± 0.15b |
|
G(+) |
20 ± 0.78 |
19.5 ± 2.78a |
0.86 ± 0.05ab |
0.33 ± 0.05ab |
|
GE1 |
21 ± 1.73 |
30.33 ± 8.73c |
1.21 ± 0.02bc |
0.4 ± 0.0b |
|
GE2 |
20.5 ± 1.13 |
28.33 ± 5.13c |
1.5 ± 0.1c |
0.4 ± 0.05b |
Group I served as normal control and received normal diet (GC). Group II served as negative control and received normal pellet feed, with a solution of AlCl3 (G(-)). Group III received pellet formulated with 5% of PC’s powder (GP1). Group IV received pellet formulated with 10% of PC’s powder (GP2). Group V served as positive control and received normal pellet and vitamin C 200 mg/kg bw (G(+)). Group VI was administered normal pellet feed and PC’s ethanolic extract 200 mg/kg bw (GE1). Group VII was administered normal pellet feed and PC’s ethanolic extract 400 mg/kg bw (GE2). Values with different letters are significantly different at P<0.05
Table 1: Assessment of memory using water maze and elevated plus maze.
|
Groups |
Proteins (nm) |
MDA (mM) 10-3 |
Glutathione (nm) |
Cat (H2O2/min/mg protein) |
AChE (DO/min/g protein |
|
GC |
179.83 ± 3.01c |
4.47 ± 0.099a |
550.16 ± 52.32bc |
2.28 ± 0.67cd |
463.07 ± 5.74d |
|
G(-) |
153.16 ± 18.90a |
4.8 ± 0.095c |
289.33 ± 38.60a |
0, 53 ± 0.40a |
65.20 ± 18.45a |
|
GP1 |
173.16 ± 11.77bc |
4.55 ± 0.124ab |
567.66 ± 15.82bc |
1.32 ± 0.50b |
93.63 ± 3.81a |
|
GP2 |
170.33 ± 21.07b |
4.72 ± 0.60bc |
638.75 ± 112.30c |
1.60 ± 0.24bc |
278.26 ± 55.47c |
|
G(+) |
154.83 ± 5.34a |
4.63 ± 0.77b |
526.83 ± 116.79b |
1.99 ± 0.47c |
207.44 ± 22.36bc |
|
GE1 |
162.08 ± 1.70ab |
4.55 ± 0.74ab |
509.33 ± 69.54b |
1.06 ± 0.33ab |
183.55 ± 45.37b |
|
GE2 |
186.33 ± 7.58cd |
4.48 ± 0.25a |
600.83 ± 40.56c |
1.19 ± 0.61ab |
254.35 ± 47.03c |
Group I served as normal control and received normal diet (GC). Group II served as negative control and received normal pellet feed, with a solution of AlCl3 (G(-)). Group III received pellet formulated with 5% of PC’s powder (GP1). Group IV received pellet formulated with 10% of PC’s powder (GP2).
Table 2: Assessment of biochemical parameters.
Figure 1: (1) Hippocampus image of normal control rats (that were administered a normal chlorine solution 0.9%) with black arrows showing normal neurons; (2) Hippocampus image of positive control rats (that were administered AlCl3 100 mg/kg + vitamin C 200 mg/kg) showing a structure similar to that of normal control group; (3) Hippocampus image of negative control rats (that were administered only AlCl3 100 mg/kg) with white arrows showing neurofibrillary tangles and black arrows amyloid plaques.
4. Discussion
The evaluation of neuroprotective effect of PC’s fruit on aluminum-induced AD was investigated. For this purpose, powders and ethanol extracts of PC were made based on the fact that the PC fruit contains bioactive compounds like polyphenols and flavonoids that might have protective effects on AD [17]. The general observation made in this study is that the administration of AlCl3 without any treatment led to cognitive impairment in rats and those that were treated with PC’s fruit had a memory capacity similar to that of normal control rats. Al is a heavy metal that has been proven by several review to be involved in neurodegenerative processes through OS, reason why it is used as a model of induction AD which is the most common neurodegenerative disease [18].
Assessment of memory through water maze and elevated plus maze revealed cognitive impairment in negative control rats. Constant exposition to high concentrations in Al may trigger the generation of free radicals in the brain that may destroy neurons and lead to neurological deficit [6]. Rats treated with PC’s fruit powders and extracts instead, cognitive impairment was not observed because of the fact that PC’s fruit contained some bioactive compounds that may counteract the neurodegenerative action of Al [17]. Biochemical analysis of brain homogenates revealed a remarkable increase in MDA in the negative control group, contrary to groups treated with powders and extracts of PC’s fruit. Excess of Al in the brain may cause oxidation of polyunsaturated fatty acids followed by the generation of final products like MDA [18].
In the groups treated by PC’s fruit powders and extracts, the lower amount of MDA produced compared to the non-treated was probably due to the protective action of fruit bioactive compounds mainly phenolic compounds. Administration of AlCl3 to rats markedly reduced total proteins, catalase, glutathione and AChE activities. Meanwhile, the administration of PC’s fruit powders and extracts after aluminum chloride to rats increased the concentration in total proteins, catalase, glutathione and AChE. This might be attributed to bioactive compounds like phenolic compounds, flavonoids and vitamin C that contained PC’s fruit. One mechanism that uses Al to act is by degrading biological molecules like proteins [19]. This might explain the high reduction in proteins in negative control groups compared to the treated ones. The significant increase in glutathione in treated groups compared to non-treated, was probably due to the fact that Al inhibited gammaglutamyl synthase, which is an important enzyme involved in OS by catalyzing the production of glutathione. AChE is known to be one of the most important biomarkers involved in AD. Its level usually slows down in cases of constant destruction in OS conditions, leading to cognitive impairment [6]. This might explain why the negative control group showed a lower AChE activity than the treated groups.
Microscopic observations of hippocampus tissues revealed some neurodegenerative markers such as amyloid plaques and NFT in negative control rats as compared to groups treated with fruit whose brains were similar to those of normal control group. As Al accumulates into the brain, it triggers brain cell death with deposition of senile markers [16].
5. Conclusion
This work was aimed at assessing effect of PC’s fruit on a model of aluminum-chloride induced AD. From our findings, it was observed that constant administration of AlCl3 in rats led to cognitive impairment. Meanwhile, oral administration of powders and ethanol extracts of PC’s fruit to rats coupled to AlCl3 showed a reduction in cognitive impairment. The treated groups GP2 and GE2 showed the best neuroprotective effect than GP1 and GE1 and this was probably due to higher concentration of bioactive compounds. PC’s fruit in both forms (powder and extract) has a neuroprotective effect on AD with the best activity in the range of 10g of powder/100g of food and 400 mg of ethanol extract/kg bw. Further investigation on use of PC’s fruit in food formulation as a mean of prevention of AD might help to understand its clear mechanism of action.
Conflict of Interest
All contributors declare that they have no conflict of interest.
References
- Tramutola A, Lanzillotta C, Perluigi M, et al. Oxidative stress, protein modification and Alzheimer disease. BrainRes Bull 133 (2017): 88-96.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement 11(2015): 332-384.
- Onyango IG, Khan SM, Bennett Jr. Mitochondria in the pathophysiology of Alzheimer's and Parkinson's diseases. Front Biosci 22 (2017): 854-872.
- Singh SK, Castellani R, Perry G. Oxidative Stress and Alzheimer’s Disease. In Eds.: Bondy SC, Campbell A. Inflammation, Aging, and Oxidative Stress. Springer International Publishing, New York, USA (2016): 189-198.
- Adebayo Adekunle Buraimoh, Samuel Adeniyi Ojo, Joseph Olajide Hambolu, et al. Effects of aluminium chloride exposure on the histology of the liver of adult wistar rats. Journal of Pharmacy 2 (2009): 525-533.
- Russell Blaylock L. Aluminum induced immunoexcitotoxicity in neurodevelopmental and neurodegenerative disorders. Current Inorganic Chemistry 2 (2018): 1.
- Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. The American journal of clinical nutrition 72 (2000): 1424-1435.
- Silva CAM, Simeoni LA, Silveira D. Genus Pouteria: Chemistry and biological activity. Brazilian Journal of Pharmacology 19 (2009): 501-509.
- Candelario Rodriguez, Armando A, Durant-Archibold Ana Santana. Analysis of volatile components of P. sapota (Sapote Mamey) fruit by HS-SPME-GC-MS. Natural products communications 13 (2018): 1027-1030.
- Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticul 16 (1965): 144-158.
- Liu H, Qiu N, Ding H, et al. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Int 41 (2008): 363-370.
- Morris MC, Evans DA, Tangney CC, et al. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 67 (2006): 1370-1376.
- Hermine Tsafack Doungue, Anna Pascale Nouemsi Kengne, Dieudonné Kuate. Neuroprotective effect and antioxidant activity of Passiflora edulis fruit flavonoid fraction, aqueous extract , and juice in aluminum chloride-induced Alzheimer-s disease rats. Nutire 48 (2018): 23.
- Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. Journal of experimental psychology: Animal behavior processes 2 (1976): 97-116.
- Nade VS, Kawale LA, Shendye NV, et al. Protective effect of nebivolol on aluminium-induced neurobehavioral and biochemical alterations in rats. Int J Pharm Pharm Sci 6 (2014): 386-391.
- Bhagyasree and Kalyani. Neuroprotective effect of Anacardium occidentale (cashew apple fruit) against aluminum toxicity: an experimental study on cognitive dysfunction and biochemical alterations in rats. Asian Journal of Pharmaceutical and clinical Research 10 (2017): 164-169.
- Kong KW, Khoo HE, PrASAd NK, et al. Total phenolics and antioxidant activities of Pouteria campechiana fruit parts. Sains Malaysiana 42 (2013): 123-127.
- Birhane Alem Berihu, Mekbeb Afwerk, Yared Godefa Debeb, et al. Review on histological and functional effect of aluminium chloride on cerebral cortex of the Brain. International Journal of Pharmacology Sciences and Research 6 (2015): 1105-1116.
- Kowalczyk E, Kopff A, Kowalczyk E, et al. Effect of Long-Term Term T Aluminium Chloride Intoxication on Selected Biochemical Parameters and Oxidative-Antioxidative Balance in Experimental Animals. Polish Journal of Environmental Studies 13 (2003): 41-43.