Evaluation of a Novel Method Using Computed Tomography to Predict New Onset of Atrial Fibrillation or Embolic Events after Transcatheter Aortic Valve Implantation: the Role of Hounsfield Unit Density Ratio in the Left Atrial Appendage
Article Information
Marco Angelillis1*, Giulia Costa1, Roberta Antonazzo Panico1, Chrysanthos Grigoratos2, Cristina Giannini1, Francesca Fiorelli1, Paolo Spontoni1, Laura Stazzoni1, Marco De Carlo1, Anna Sonia Petronio1, Roberto Pedrinelli1
1Cardiac Thoracic and Vascular Department, University of Pisa, Italy
2Fondazione Gabriele Monasterio, Pisa, Italy
*Corresponding Author: Marco Angelillis, Cardiac, Thoracic and Vascular Department, University of Pisa, Via Paradisa 2, 56121 Pisa, Italy
Received: 30 March 2019; Accepted: 09 April 2019; Published: 12 April 2019
Citation: Marco Angelillis, Giulia Costa, Roberta Antonazzo Panico, Chrysanthos Grigoratos, Cristina Giannini, et al. Evaluation of a Novel Method Using Computed Tomography to Predict New Onset of Atrial Fibrillation or Embolic Events after Transcatheter Aortic Valve Implantation: the Role of Hounsfield Unit Density Ratio in the Left Atrial Appendage. Cardiology and Cardiovascular Medicine 3 (2019): 050-058.
Share at FacebookAbstract
Backgrounds: Evaluation of left atrial appendage (LAA) with angio-computed tomography (CCTA) in order to predict new onset of atrial fibrillation (AF) or embolic events is a new upcoming topic. No previous reported studies are available in patients undergoing transcatheter aortic valve implantation (TAVI).
Methods: We analyzed pre-procedural CCTA scans of 325 patients who underwent TAVI performing a linear coefficient of attenuation analyses with Hounsfield units (HU) in LAA. HU in LAA distal and proximal was calculated, as well as the ratio. A sensibility and specificity analyses was conducted in order to identify the optimal cutoff to predict new onset AF or embolic events after TAVI.
Results: Patients were divided into 4 groups according to the presence of AF. Baseline clinical and echocardiographic features were similar except for a significantly higher STS score and mitral regurgitation severity in PRE-TAVI AF group (p=0.003 and p=0.002 respectively). HU analyses showed a statistical difference in measure performed in LAA distal and in the HU LAA distal/Proximal ratio, with the lowest value in patients with pre-TAVI AF (p<0.001 and p<0.001 respectively). The ROC analyses found 0.84 as the cut-off for to predict the composite endpoint of new AF or embolic events, with sensitivity of 51% and specificity of 52% (p=0.008).
Conclusion: In patients with aortic stenosis (AS), use of LAA assessment with CCTA to predict embolic events or new onset AF is no efficacy and cannot be substituted clinical indications for prevention and therapy of embolic events.
Keywords
Hounsfield unit; Atrial fibrillation; Stroke; Computed tomography
Article Details
1. Introduction
Atrial fibrillation (AF) is the most frequent cause of cardioembolic stroke [1], requiring preventive oral anticoagulant therapy. Currently, the most effective cardioembolic risk estimation is based on clinical scores, such as CHA2DS2-VASc score, although there are some evidences in the literature suggesting that LAA flow velocity estimation with TEE scan could reveal patient risk of stroke [2] and paroxysmal AF [3]. Unfortunately, TEE is an invasive method, and without solid evidence of benefits, it cannot be applied as screening in all patients with AF or at high risk for embolic events. Angio-cardiac computed tomography (CCTA) showed a high correlation with TEE in LAA scan, both for thrombus detection, (sensibility and specificity >90%), and for LAA patency assessment after a closure intervention [4]. Preliminary evidence based on three series of 19, 23 and 40 patients, undergoing TEE and CCTA after left atrial appendage closure (LAAC) [5-7], suggests that the quantitative assessment with Hounsfield Unit (HU) is as effective as TEE evaluation. Moreover, Yasuoka et al. [8] proposed a new LAA analysis method with CCTA, using the quantitative HU in LAA, which showed significant correlation with LAA peak velocity estimated by TEE. Postoperative atrial fibrillation, which occurs in up to 10-20% of patients [9], is a common complication after transcatheter aortic valve implantation (TAVI) and increases significantly the peri-operative patient morbidity causing hemodynamic instability and embolic events. The aim of our study was to evaluate if pre-operative LAA scan with CCTA in TAVI patients can predict the incidence of postoperative AF, long-term onset of AF and embolic events.
2. Materials and Methods
We retrospectively reviewed data about consecutive patients with aortic stenosis who were treated with TAVI in our center from 2007 to 2016. Clinical, procedural and follow-up data was obtained by the Italian ClinicalService® Project (Clinical Trial Registration NCT01007474), which is an on-going nation-based clinical data repository and medical care project for the analysis and improvement of the implantable devices use in the Italian clinical practice [10]. Clinical follow-up was conducted through outpatient clinics and telephone calls at 1 and 6 months and every 6 months afterwards.
2.1 CCTA protocol and analyses
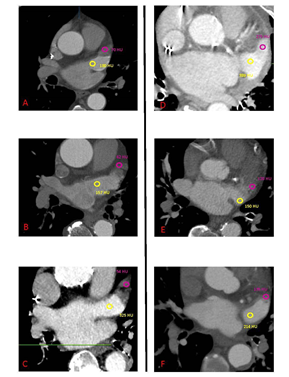
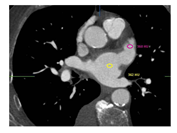
A multi-slice CT angiography ECG-gated study was performed pre-procedure with a GE Light Speed VCT 64 scanner using collimation of 0.6 mm at a fixed pitch of 0.2 with an injection of 100 cc of Isovue 370. A dedicated protocol was formulated, with kilovolt variable according patient’s BMI (80 kV with BMI <21 kg/mq, 100kV with BMI between 21 e 27 kg/mq,120 kV with BMI >27 kg/mq). Adjusting for body habitus, a standard convolution kernel of B35f was applied. The gantry rotation time was 330 ms. Image acquisition was performed throughout the cardiac cycle. The ECG at the time of acquisition was reviewed before reconstruction to select out ectopy. Images were reconstructed with slice thickness of 0.625 mm every 10% R-R interval through the heart and the aortic arch. Electrocardiogram adaptive scanning was employed with a higher dose in systole but a low overall radiation dose, achieving high-quality systolic scans in all patients. HU measurements were performed in distal and proximal LAA, using multiplanar reconstruction, as previously described in the literature [8]. A region of interest (ROI) of 2 mm diameter was used to evaluate HU in each part and optimal contrast opacification control was performed measuring the average HU in the whole LA (Figure 1-2). The LAAd/LAAp HU ratio (HU ratio) was then calculated and statistically assessed as a useful predictive index of AF new onset or embolic events. All angio-CT images were analyzed using GE Workstation (Volume Viewer version 12.3, General Electric Company). The population included in the analysis was divided into four groups:
- Group 1: Patients without AF
- Group 2: Patients with history of AF
- Group 3: Patients with peri-procedural new onset AF (between 2 and 30 days after TAVI).
- Group 4: Patients with AF or ischemic Stroke/TIA within 30 days after TAVI
All baselines, procedural, and follow-up data were analyzed according to the above defined 4 groups. The primary study endpoint was a composite endpoint of AF onset and ischemic stroke/TIA after TAVI. Events were defined according to the Valve Academic Research Consortium-2 (VARC 2) [11].
2.2 Statistics
Descriptive statistics were used to describe patients’ characteristics; mean with standard deviation and median value with interquartile range (IQR) were used for continuous variables and counts, and percentages for categorical variables. Comparisons between groups were assessed using Kruskal-Wallis’ Test for continuous variables, and Chi-square test or Fisher exact test for extreme proportions for categorical variables, as appropriate. Statistical tests were based on a two-sided significance level of 0.05. The analyses of time-to-the-first event were described by means of Kaplan-Meier curves and the Proportional Hazard assumption was verified, when comparing groups. The ROC analysis was used to evaluate Sensitivity/Specificity/VPN/VPP and to identify the best cut-off value of LAA distal/proximal HD, which can predict the Composite Endpoint. Statistical analyses were performed with the SPSS (IBM SPSS version 23.0, New York).
3. Results
Of 420 patients screened from 2007 to 2016, we included in our analysis only 325 patients with pre-procedural gated CCTA evaluable or available 1-year follow-up. Baseline clinical and echocardiographic patients’ characteristics are detailed in Table 1. 95 patients were excluded from the analysis because of non-gated CCTA or because lost in follow-up. The Final population included 325 patients. Baseline clinical and echocardiographic features were similar between the four groups with the exception of the STS risk score and mitral regurgitation severity which were significantly higher in Group 2 compared to the other groups, as expected considering the score algorithm and the mitral regurgitation pathophysiology in AF patients (p=0.003 and p=0.002 respectively). These aspects are both compatible with the presence of AF, which increase STS surgical risk evaluation and is associated with high grade of mitral regurgitation.
|
Variable |
All patients (N=325) |
GROUP 1 (N=227) |
GROUP 2 (N=52) |
GROUP 3 (N=9) |
GROUP 4 (N=37) |
p-value |
|
Age at procedure, years |
83 ± 5.0 |
83 ± 5.0 |
82 ± 5.0 |
84.0 ± 3.0 |
83.0 ± 6.0 |
0.57 |
|
Man |
43.1% (140) |
43.6% (99) |
42.3% (22) |
66.7% (6) |
35.1% (13) |
0.38 |
|
Body mass index, (Kg/m2) |
26.0 ± 4.0 |
25.6 ± 4.1 |
26.2 ± 4.3 |
27.3 ± 5.1 |
27.2 ± 5.5 |
0.19 |
|
Logistic Euroscore, (%) |
16.4 (10.8-25.0) |
15.9 (10.5-24.0) |
20.5 (11.8-30.1) |
22.0 (12.1-28.9) |
16.4 (11.8-20.0) |
0.66 |
|
STS PROM score, (%) |
5.1 (3.3-7.7) |
5.0 (3.2-7.1) |
6.5 (4.4-10.8) |
4.4 (3.2-6.4) |
4.3 (2.9-5.7) |
0.003 |
|
Arterial hypertension |
86.8% (28) |
85.9% (195) |
86.5% (45) |
88.9% (8) |
91.9% (34) |
0.79 |
|
Diabetes mellitus |
32.5% (105/323) |
32.6% (74/227) |
24.0% (12/50) |
33.3% (3/9) |
43.2% (16/37) |
0.308 |
|
Coronary artery disease* |
46.4% (149/321) |
46.4% (104/224) |
46.2% (24/52) |
44.4% (4/9) |
47.2% (17/36) |
0.999 |
|
Prior PCI |
28.0% (90/321) |
28.4% (64/225) |
17.6% (9/51) |
33.3% (3/9) |
38.9% (14/36) |
0.174 |
|
Prior stroke or TIA |
18.9% (34/180) |
20.5% (25/122) |
25.8% (8/31) |
0.0% (0/5) |
4.5% (1/22) |
0.152 |
|
Creatinine (mg/dl) |
1.1 (0.9-1.5) |
1.2 (0.9-1.5) |
1.2 (1.0-1.7) |
1.0 (0.7-1.1) |
1.0 (0.8-1.3) |
0.071 |
|
GFR<30 mL/min |
20.4% (66/324) |
21.1% (48/227) |
25.0% (13/52) |
0.0% (0/8) |
13.5% (5/37) |
0.274 |
|
Peripheral vascular disease |
20.4% (66/324) |
21.1% (48/227) |
25.0% (13/52) |
0.0% (0/8) |
13.5% (5/37) |
0.213 |
|
Chronic obstructive pulmonary disease? |
12.3% (40/325) |
11.5% (26/227) |
15.4% (8/52) |
33.3% (3/9) |
8.1% (3/37) |
0.179 |
|
New York Heart Association class III-IV |
60.0% (195/325) |
60.4% (137/227) |
67.3% (35/52) |
55.6% (5/9) |
48.6% (18/37) |
0.358 |
|
LV end diastolic volume, (ml) |
110.5 ± 40.2 |
111.9 ± 40.9 |
108.2 ± 39.1 |
104.8 ± 25.7 |
106.3 ± 40.7 |
0.75 |
|
LVEF, (%) |
50.2 ± 11.0 |
49.9 ± 11.3 |
50.1 ± 11.1 |
56.7 ± 7.6 |
50.8 ± 9.6 |
0.33 |
|
Mean transaortic valve gradient, (mmHg) |
48.8 ± 15.4 |
49.3 ± 15.6 |
45.3 ± 16.3 |
48.9 ± 15.8 |
50.8 ± 12.5 |
0.24 |
|
Moderate or severe aortic regurgitation |
22.6% (67/297) |
25.8% (54/209) |
16.7% (8/48) |
12.5% (1/8) |
12.5% (4/32) |
0.20 |
|
Moderate or severe mitral valve regurgitation |
46.6% (142/305) |
41.7% (88/211) |
65.3% (32/49) |
22.2% (2/9) |
55.6% (20/36) |
0.002 |
|
Peak systolic pressure in pulmonary artery (mmHg) |
5.4% (16/297) |
4.9% (10/204) |
9.8% (5/51) |
0.0% (0/8) |
2.9% (1/34) |
0.497 |
Data shown as % (n) and as means ± (SD) or median and interquartile range; STS PROM-STS Predicted Risk of Operative Mortality; PCI-percutaneous coronary intervention; TIA-transient ischemic attack; GFR-glomerular filtration rate; LVEF-left ventricular ejection fraction; TAVI-transcatheter aortic valve implantation; *Coronary artery disease is defined as the presence of at least one lesion ≥ 75%; ?referred to severe stage with FEV1/FVC 30-49%; Group 1: No atrial fibrillation; Group 2: Pre-TAVI atrial fibrillation; Group 3: peri-procedural atrial fibrillation; Group 4: post-TAVI atrial fibrillation or embolic events.
Table 1: Baseline clinical and echocardiography characteristics.
Data regarding CCTA analysis of LAA are detailed in Table 2. HU in distal LAA and distal/proximal ratio were found statistically different between four groups, with a lower value in patients with pre-TAVI AF compared to the other groups (p<0.001and p<0.001 respectively).
|
Variable |
All patients (N=325) |
GROUP 1 (N=227) |
GROUP 2 (N=52) |
GROUP 3 (N=9) |
GROUP 4 (N=37) |
p-value |
|
HU LAA Distal |
308.4 ± 145.8 |
330.8 ± 126.7 |
194.8 ± 159.1 |
315.3 ± 146.3 |
328.9 ± 166.4 |
<0.001 |
|
HU LAA Proximal |
402.8 ± 135.0 |
399.9 ± 130.2 |
402.3 ± 139.1 |
415.2 ± 101.9 |
418.5 ± 166.1 |
0.948 |
|
HU LAA Distal/Proximal |
0.79 ± 0.32 |
0.85 ± 0.26 |
0.52 ± 0.38 |
0.76 ± 0.29 |
0.82 ± 0.33 |
<0.001 |
|
HU Left atrial |
379.8 ± 142.5 |
371.4 ± 137.8 |
403.1 ± 148.8 |
393.0 ± 103.8 |
396.0 ± 167.5 |
0.515 |
HU-Hounsfield Unit; LAA-left atrial appendage; Group 1: No atrial fibrillation; Group 2: Pre-TAVI atrial fibrillation; Group 3: peri-procedural atrial fibrillation; Group 4: post-TAVI atrial fibrillation or embolic events.
Table 2: ANGIO-CT DATA.
Follow-up data are reported in Table 3. No statistical difference was observed among the groups, except for a higher rate of cerebrovascular events in group 4, as per protocol defined. Performing ROC analysis, the HU ratio cut-off value of 0.84 was found as the predictor of our primary endpoint (new onset AF or embolic events), unfortunately with poor sensitivity and specificity (respectively 51% and 52%, p=0.008) (Figure 1).
|
Variable |
All patients (N=325) |
GROUP 1 (N=227) |
GROUP 2 (N=52) |
GROUP 3 (N=9) |
GROUP 4 (N=37) |
p-value |
|
Death |
45.2% (147/325) |
41.9% (95/227) |
55.8% (29/52) |
55.6% (5/ 9) |
48.6% (18/37) |
0.268 |
|
Cardiovascular Death |
21.5% (70/325) |
20.7% (47/227) |
21.2% (11/52) |
33.3% (3/ 9) |
24.3% (9/37) |
0.799 |
|
Stroke/TIA |
5.8% (23/325) |
0% (0/227) |
9.6% (5/52) |
11.1% (1/ 9) |
35.1% (13/37) |
<0.001 |
|
Rehospitalization |
50.2% (163/325) |
47.1% (107/227) |
51.9% (27/52) |
55.6% (5/ 9) |
64.9% (24/37) |
0.241 |
TIA-transient ischemic attack; Group 1: No atrial fibrillation; Group 2: Pre-TAVI atrial fibrillation; Group 3: peri-procedural atrial fibrillation; Group 4: post-TAVI atrial fibrillation or embolic events.
Table 3: Follow-up data.
4. Discussion
This study represents the largest reported CCTA analyses of LAA in a real-world population. The major findings of our study are:
- HU analyses of LAA is feasible in all patients undergoing CCTA;
- In patients with AF, distal LAA HU value is significantly lower than the rest of the population;
- Currently, HU analyses alone cannot predict AF new onset or embolic events.
Over the last years, The CCTA use for LAA morphology and dimension assessment during the LAAC planning has increased. CCTA provides 3D reconstruction and a better assessment of ostium, neck and landing zones, improving the intra-operative positioning of the device with better LAA sealing. Recently, different authors reported the use of CCTA during follow-up after LAAC in order to assess residual patency. Jaguszewski [5] et Saw [6] reported a series of 19 and 23 patients, respectively, where, CCTA was performed after LAAC. Authors reported as using 100 HU as cut-off, discriminated LAA patency with high sensibility and specificity (using TEE as gold standard). Our group [7] confirmed this cut-off value for non-cardiac dedicated CCTA. Moreover, Cochet [12] reported a large series of 117 patients where cardiac dedicated CCTA found LAA patency after closure in 44% of cases using 100 HU as a cut-off. Considering the increasing use of CCTA for LAA assessment, Yasuoka et al. [8] demonstrated a correlation between the LAA flow velocities estimated with Doppler during TEE with the HU values at CCTA; considering the predictive power of the LAA flow velocities for new AF and embolic events, a correlation between HU values and these events could be supposed.
We found that in patients with history of AF (paroxysmal or persistent), HU value in distal LAA is statistically lower than the one measured in patients with no history of AF. This aspect reflects the LAA anatomy and function during AF. In fact, during atrial fibrillation the left atrium and the LAA have no coordinated contraction, resulting in a stunned chamber. This situation reduces the contrast agent diffusion into the LAA apex resulting in a lower HU value. With ROC analyses, we found that the HU ratio cut-off value of 0.84is the best to predict future events in patients with AS treated with TAVI. Nevertheless, we found low value for sensibility and specificity (51% and 52% respectively). These results show that the routinely use of LAA HU analyses in patients treated with TAVI cannot be suggested for prediction of post-operative events, in contrast with Yasuoka results. Some explanation can be found: firstly, Yasuoka compared two different techniques in a small number of patients. In fact, even if we can hypothetical consider evaluation of flow velocity and density of contrast, both influenced by LAA contractions, the two techniques are different in temporal aspect, as doppler assessment represents as instantaneously measure while HU assessment is influenced by the time for contrast diffusion in LAA. Secondly, our population is at high risk for thromboembolic events and new onset of AF, while the population in Yasouka’s study was younger and at lower risk.
5. Limitations
The retrospective nature of our investigation is one of the main limitations. Despite being, to our knowledge, the largest reported cohort of patients undergoing LAA CCTA assessment, our findings are based on specific and selected patients (aortic stenosis undergoing TAVI), thus, our conclusions cannot be applied to the general population. Elderly population with multiple comorbidities is, at high risk for AF and embolic events. CCTA scans were all dedicated to cardiac assessment, so this aspect reinforced the HU analyses performed.
6. Conclusions
In patients with AS screened with CCTA for TAVI procedure, routinize use of LAA assessment in order to predict embolic events or new onset AF is not reliable and cannot replace clinical indications for prevention and therapy. However, CCTA analyses of LAA represent a new interesting method for assessment of morphology, dimensions, and presence of slow blood flow in the LAA. The clinical significance of HU LAA assessment at CCTA requires further study in the general population.
Conflict of Interest
Petronio AS is a consultant for Medtronic. All other authors have no conflict of interest to declare.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 133 (2016): 447-454.
- He Y, Zhang B, Zhu F, et al. Transesophageal echocardiography measures left atrial appendage volume and function and predicts recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Echocardiography 35 (2018): 985-990.
- Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: a novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke 45 (2014): 1481-1484.
- Pathan F, Hecht H, Narula J, et al. Roles of Transesophageal Echocardiography and Cardiac Computed Tomography for Evaluation of Left Atrial Thrombus and Associated Pathology: A Review and Critical Analysis. JACC Cardiovasc Imaging 11 (2018): 616-627.
- Jaguszewski M, Manes C, Puippe G, et al. Cardiac CT and echocardiographic evaluation of peri-device flow after percutaneous left atrial appendage closure using the AMPLATZER cardiac plug device. Catheter Cardiovasc Interv 85 (2015): 306-312.
- Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging 16 (2015): 1198-1206.
- Angelillis M, Gargiulo G, Moschovitis A, et al. Computed tomography detection and quantification of left atrial appendage residual patency as collateral finding after percutaneous closure. Int J Cardiol 260 (2018): 42-46.
- Yasuoka R, Kurita T, Kotake Y, et al. A novel method to estimate blood flow velocity in the left atrial appendage using enhanced computed tomography: role of Hounsfield unit density ratio at two distinct points within the left atrial appendage. Heart Vessels 32 (2017): 893-901.
- Siontis GCM, Praz F, Lanz J, et al. New-onset arrhythmias following transcatheter aortic valve implantation: a systematic review and meta-analysis. Heart 104 (2017): 1208-1215.
- Ussia GP, Barbanti M, Petronio AS, et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J 33 (2012): 969-976.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 60 (2012): 1438-1454.
- Cochet H, Iriart X, Sridi S, et al. Left atrial appendage patency and device-related thrombus after percutaneous left atrial appendage occlusion: a computed tomography study. Eur Heart J Cardiovasc Imaging 19 (2018): 1351-1361.