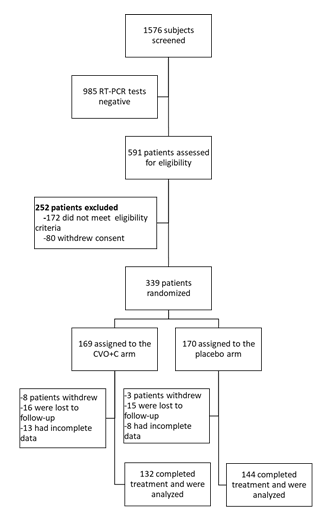
Efficacy and Safety of CVO PLUS CURATIF Capsules, Malagasy Improved Traditional Medication for Treating COVID-19: A Randomized, Double- Blind, Placebo-Controlled Trial
Article Information
Rianasoambolanoro Rakotosaona1,5*, Sedera A. Mioramalala1,3, Malala Arinomenjanahary Rakotoarisoa1,3, Antsa Rakotondrandriana1, Emmanuel Randrianarivo1, Felana Rabetokotany9, Fanomezantsoa Rakoto7, Dominique Razafimandimby7, Arsène Ravelo10, Fridolin Maminiaina4,6, Rabenja Rapelanoro3, Zely Randriamanantany2, Rivo Andry Rakotoarivelo2, Olivat Rakoto Alson3,8, Claude Arsène Ratsimbasoa1,2
1Centre National d’Application de Recherches Pharmaceutiques, Madagascar
2Faculty of Medicine, University of Fianarantsoa, Madagascar
3Faculty of Medicine, University of Antananarivo, Madagascar
4Faculty of Science, University of Antananarivo, Madagascar
5Ecole Supérieure Polytechnique, University of Antananarivo, Madagascar
6Institut Malgache des Vaccins Vétérinaires, Madagascar
7Military Hospital of Antananarivo, Madagascar
8HJRA Hospital, Antananarivo, Madagascar
9Centre d'Infectiologie Charles Mérieux Antananarivo, Madagascar
10Institut National de la Statistique, Madagascar
*Corresponding author: Rianasoambolanoro Rakotosaona, PhD, HDR, Centre National d’Application de Recherches Pharmaceutiques, Rue RP Rahajarizafy Ambodivoanjo BP 702, Antananarivo 101, Madagascar.
Received: 23 August 2022; Accepted: 06 September 2022; Published: 12 September 2022
Citation: Rianasoambolanoro Rakotosaona, Sedera A. Mioramalala, Malala Arinomenjanahary Rakotoarisoa, Antsa Rakotondrandriana, Emmanuel Randrianarivo, Felana Rabetokotany, Fanomezantsoa Rakoto, Dominique Razafimandimby, Arsène Ravelo, Fridolin Maminiaina, Rabenja Rapelanoro, Zely Randriamanantany, Rivo Andry Rakotoarivelo, Olivat Rakoto Alson, Claude Arsène Ratsimbasoa. Efficacy and Safety of CVO PLUS CURATIF Capsules, Malagasy Improved Traditional Medication for Treating COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial. Archives of Clinical and Biomedical Research 6 (2022): 817-825.
Share at FacebookAbstract
Background: Only a few drugs are currently approved to treat COVID-19. CVO PLUS CURATIF (CVO+C) is a capsule formulation of artemisinin and 1,8-cineole, two plant-derived compounds with anti-inflammatory and antiviral properties in vitro. These compounds have been repurposed for potential use as an oral COVID-19 treatment.
Methods: We performed a phase 3 randomized clinical trial on patients over the age of 18 years with SARS-CoV-2 infection and mild-tomoderate symptoms. Patients were randomly assigned to receive CVO+C (3 capsules per day) or placebo. The primary outcomes were the proportion of patients testing negative for SARS-CoV-2 on day 28 and the absence of serious adverse events. Secondary outcomes included recovery time and biological markers.
Results: In total, 1,576 individuals were screened, 339 of whom met the inclusion criteria. After data cleaning, 132 were from the CVO+C arm and 144 from the placebo arm. Treatment efficacy differed significantly (p = 0.011) between the CVO+C arm (87.1%, 95% CI: 81.3%-92.9%, with 70.45% of patients cured by day 14) and the placebo arm (75.0%, 95% CI: 67.8%-82.1%), with an OR of 2.25. The median time to recovery was 14 days for the CVO+C group and 21 days for the placebo group. There were 72 mild and moderate adverse events, 14 severe adverse events, and no serious adverse events in either group.
Conclusions: CVO+C was effective for the treatment of mild-to-moderate COVID-19. None of the patients in the CVO+C arm developed the severe form of COVID-19. Patients’ liver, kidney, and metabolic functions were preserved.
Keywords
Artemisinin; COVID-19; CVO Plus Curatif; Oxygenotherapy
Artemisinin articles; COVID-19 articles; CVO Plus Curatif articles; Oxygenotherapy articles
Article Details
1. Introduction
The virus that causes coronavirus disease 2019 (COVID-19), called SARS-CoV-2, was found for the first time in Wuhan, China, in 2019 [1]. On March 19, 2020, Madagascar identified its first case of SARS-CoV-2, which was brought into the country by an incoming traveler [2]. The disease has widespread health, social, and economic consequences around the world. Several therapeutic agents have been evaluated for use in the treatment of COVID-19, but very few have been found to have positive clinical effects and only a handful have been approved. Dexamethasone has been shown to be beneficial for patients on ventilators and to reduce mortality (25.7% in the usual care group vs. 22.9% in the dexamethasone group) [3]. Remdesivir has been reported to shorten recovery time (median of 10 days in the remdesivir group vs. 15 days in the placebo group) [4]. Some studies have reported treatment with hydroxychloroquine to be effective due to a significant decrease in viral load when used in combination with azithromycin [5], or a shortening of the time until the viral RNA becomes undetectable [6]. Due to limited resources, particularly the excessive cost of approved antivirals such as Remdesivir, managing COVID-19 in Madagascar and Africa is difficult. The development of low-cost, safe, and effective COVID-19 therapies remains a matter of priority. Artemisinin is the starting material for the latest antimalarial drugs (ACTs: Artemisinin Combined Therapies), which is obtained from a plant that has been used in traditional Chinese medicine for centuries: Artemisia annua L. [7]. In addition, artemisinin has been shown to have significant potential for cancer and antiviral therapy [8,9]. Artemisinin has been shown to have antiviral activity in vitro against SARS-CoV-2 in the monkey cell line Vero E6 [10,11]. In silico studies have suggested that artemisinin interacts with the Lys353 and Lys31 binding hotspots of the SARS COV-2 spike protein [12]. It also has anti-inflammatory activities [13,14]. The incidence of COVID-19 and COVID-19-related mortality rates have been reported to be very low in countries in which artemisinin is regularly used to treat malaria [15]. The combined antiviral and anti-inflammatory activities of artemisinin and its excellent safety profile in humans identify this drug as a potential candidate treatment for COVID-19 in patients [14,16]. Another molecule, 1,8-cineole, is a terpene oxide and is the major compound present in many essential oils, those extracted from Eucalyptus species and particularly in essential oil from leaves of Cinnamomum camphora L. grown in Madagascar[17-20]. 1,8-cineole, also known as eucalyptol, is often used in drug formulations for its expectorant and antitussive effects [21], [22]. 1,8-cineole has been shown to inhibit the Mpro proteinase of SARS-CoV-2, which is required for the reproduction of the virus [23]. In vitro studies have highlighted the anti-inflammatory properties of 1.8-cineole, which inhibits the production of IL-1β (interleukin-1-beta) and TNF-α by acting on the NF-κB signaling pathway [24] and improves respiratory function when used to treat asthma or bronchial inflammation [25]. CVO+C is a capsule for oral administration containing Artemisia annua L. extract (150 mg artemisinin and specific flavonoid and terpenic fractions) and an essential oil from the leaves of Cinnamomum camphora L. from Madagascar chemotype 1.8-cineole (7.1 mg 1,8-cineole). To evaluate the clinical efficacy and safety of CVO+C as treatment for COVID-19, we conducted a randomized, placebo-controlled clinical trial in adults with COVID-19.
2. Methods
2.1 Study Design
2.1.1 Study Design: This study was a phase 3 randomized, double-blind clinical trial evaluating the efficacy and safety of CVO+C for the treatment of COVID-19.
2.1.2 Study Site: We performed the study in the Analamanga region of Madagascar. Subjects were recruited from Voara village in Andohatapenaka, Antananarivo, Madagascar, and from three secondary healthcare centers in Antananarivo. Follow-up was conducted at the health center of the CNARP (Centre National d’Application de Recherches Pharmaceutiques).
2.2 Patients
Subjects with positive RT-PCR test results for SARS-CoV-2 were asked to participate in the study. They were included if they met the inclusion criteria for the study: adult male or non-pregnant (checked with a pregnancy test) female patient capable of understanding the information given, providing written informed consent (including consent for the collection of oropharyngeal swabs and venous blood in accordance with the protocol), with laboratory-confirmed (RT-PCR on nasopharyngeal/throat swabs) SARS-CoV-2 infection. The illness could be of any duration, but the patients had to display infiltrates on radiological imaging (chest X-ray or CT-scan) or clinical signs on examination (rales/crackles). Patients had to have a creatinine concentration ≤ 110 µmol/L, a creatinine clearance rate (EGFR) ≥ 60 mL/min /1.73 m2, ASAT and ALAT levels no higher than five times the upper limit of the normal range (ULN), and TBIL levels no higher than twice the ULN. In addition, patients had to have a normal baseline ECG for inclusion in this study, and ECG results had to remain normal throughout the study. Patients were excluded if their ALAT/ASAT levels were more than five times the ULN, if they were pregnant or breast-feeding, had severe (stage 4) chronic kidney disease or required dialysis (eGFR<30), were expected to be transferred to another non-participating hospital site within 72 hours, were allergic to any of the study drugs, had shortness of breath, had known prolonged QT syndrome or were using other drugs known to prolong the QT/QTc interval. We also excluded patients with other types of viral pneumonia.
2.3 Randomization and Blinding
The patients were randomly assigned to double-blind treatment or placebo, in a 1:1 ratio. One of the investigators provided codes following simple randomization procedures using a computer-generated randomization. The research pharmacist delivered 15 days of medicine using the code. Patients and physicians were kept blind to the allocation.
2.4 Study Medication
The patients were randomized to two arms. The patients in the treatment arm received CVO+C (Pharmalagasy, batch no. 1923-001) for oral administration, and were required to take three capsules per day for 15 days. The patients in the control arm received a placebo (Pharmalagasy, beta cyclodextrin and magnesium stearate, batch no. 1919-001) under the same conditions (3 capsules per day for 15 days) as the study treatment. The study treatment and the placebo were identical in physical appearance and taste.
2.5 Follow-up
The patients enrolled in the study were required to attend follow-up visits on days 7, 14, 21 and 28. At each visit, any adverse events or serious adverse events were recorded, viral load was determined (CT method for the ORF1ab and N genes of the virus), and clinical, hematological, and biological parameters (liver and kidney function) were assessed.
2.6 Outcomes
The primary outcome was the proportion of patients with complete clearance of the SARS-CoV-2 virus from oropharyngeal samples on day 28 and the absence of severe and serious adverse events. The secondary outcomes were recovery time, hematological parameters (leukocytes, lymphocytes and ESR), and biomarkers of liver function (ASAT and ALAT), renal function (creatinine levels) and blood sugar levels on days 7, 14, 21, and 28. Safety outcomes included adverse events occurring during treatment, serious adverse events, and premature discontinuation of treatment. If a severe or serious adverse event occurred, the participant stopped the treatment and received the official standard of care.
2.7 Statistical Analysis
2.7.1 Required Sample Size: Assuming a treatment efficacy of 40% for the placebo arm and 60% for the CVO+C arm, with equal numbers of patients assigned to each arm, a margin of error of 0.03, a power of 80% and an alpha risk of 5%, we would need to include 306 patients, with 153 patients per arm, to detect a significant difference between the two arms. Assuming an attrition rate of 10% due to deaths and withdrawals from the study, a total sample size of 338 patients would be required.
2.7.2 Statistical Analysis: A per-protocol analysis was performed by the endpoint analysis method. If a subject was lost to follow-up, the last known value was used for quantitative endpoints and dichotomous qualitative endpoints that were measured at regular intervals. The criteria for treatment efficacy were a negative RT-PCR test for SARS-CoV-2 at the end of the study, with no adverse events or with only mild (grade 1) or moderate (grade 2) adverse events. Regardless of the RT-PCR result at the end of the study (positive or negative), treatment was considered to have failed if the patient died, displayed serious adverse events (grade 3 or 4), was hospitalized with or without oxygenotherapy (via a mask, nasal prongs, high-flow oxygen treatment, intubation and mechanical ventilation with or without additional organ support, such as renal replacement therapy, or extracorporeal membrane oxygenation) or had been released from the hospital but was unable to perform everyday activities normally. Adverse events were considered mild (grade 1) if they were transient, required little or no treatment and did not interfere with everyday activities. They were considered moderate (grade 2) if they interfered with everyday activities, causing discomfort but no significant or permanent risk of harm to the subject, and were alleviated by additional treatment. We considered two grades of serious adverse events. Grade 3 severe adverse events were defined as events disrupting everyday activities or significantly affecting clinical status, incapacitating the subject, and possibly requiring intensive treatment interventions. Grade 4 serious adverse events were defined as potentially life-threatening. We performed statistical analyses with STATA® 13.1 (Copyright 1985–2013 StataCorp LP, Statistics/Data Analysis, StataCorp, 4905 Lakeway Drive, College Station, Texas 77845, USA). Qualitative variables are expressed as percentages or composition ratios for descriptive statistical analysis. They were analyzed with χ² tests, Fisher’s exact tests, and Wilcoxon Mann-Whitney tests. Quantitative variables are expressed as medians and interquartile ranges. Normally distributed quantitative variables were analyzed with t-tests, whereas Wilcoxon rank-sum tests were used to compare the two groups for non-normally distributed variables. All tests were one-tailed, and a p-value < 0.05 was considered significant. The odds ratio (OR) corresponding 95% confidence intervals (CI) were calculated in patients treated with CVO+C compared with placebo. The overall probability of conversion to negativity for RT-PCR tests was estimated by determining the time taken for viral RNA levels to become undetectable. This analysis was performed by the Kaplan-Meier method, with log-rank tests used for comparisons.
3. Results
3.1 Patients
Between January 18, 2021, and May 4, 2021, 1,576 underwent RT-PCR tests for SARS-CoV-2. The eligibility criteria were checked for the 591 patients who tested positive. We included 339 patients, 276 of whom were included in the final analysis. There were 132 patients in the CVO+C group and 144 in the placebo group (Figure 1). Baseline characteristics of the groups are presented in Table 1. At the start of the study, there were no significant differences between the two groups of patients in terms of sociodemographic, clinical, or biological characteristics.
|
Variables |
Placebo |
CVO+ C |
p-value |
|
Age (years) median (IQR) |
34.3 (27.3 – 43.2) |
34.4 (28.8 – 42.0) |
0.9a |
|
Sex |
|||
|
Male |
76 (52.8) |
79 (59.9) |
0.2b |
|
Female |
68 (47.2) |
53 (40.1) |
|
|
Sex-ratio |
1.1 |
1.4 |
|
|
General signs |
|||
|
FR med (IQR) |
19 (18 - 22) |
19 (18 – 22) |
0.4a |
|
FC med (IQR) |
78 (70 – 89) |
78 (69 – 87) |
0.6a |
|
TAS med (IQR) |
110 (100 – 120) |
110 (100 – 120) |
0.3c |
|
TAD med (IQR) |
70 (60 – 80) |
70 (60 – 80) |
0.2c |
|
Clinical signs |
|||
|
Dyspnea |
8 (5.6) |
10 (7.6) |
0.4b |
|
Fever |
29 (20.1) |
31 (23.5) |
0.5 b |
|
Cough |
70 (48.6) |
50 (37.9) |
0.07 b |
|
Asthenia |
67 (46.5) |
53 (40.1) |
0.2 b |
|
Aching |
42 (29.8) |
41 (31.0) |
0.7 b |
|
Odynophagia |
9 (6.2) |
5 (3.8) |
0.3 b |
|
Chest pain |
21 (14.6) |
25 (18.9) |
0.3 b |
|
Diarrhea |
12 (8.3) |
14 (10.6) |
0.5 b |
|
Agueusia |
37 (25.7) |
21 (15.9) |
0.04 b |
|
Anosmia |
59 (40.9) |
43 (32.6) |
0.1 b |
Table 1: Socio-demographic and clinical characteristics of the patients at baseline.
a: Wilcoxon test (Mann-Whitney) ; b: Chi2 test ; c: Student’s test
3.2 Outcomes
3.2.1 Primary outcome: Treatment success rates were significantly higher for the CVO+C group than for the control group (CVO+C: 87.1% [95% CI 81.3%-92.9%], control: 75.0% [95% CI 67.8%–82.1%]; p = 0.011), with an odds ratio of 2.25 [95% CI 1.19–4.24] (table 2).
|
Group |
Success |
Failure |
Total |
p-value* |
|
|
n (%) |
95% CI |
n (%) |
N (%) |
||
|
Placebo |
108 (75.0) |
67.8 – 82.1 |
36 (25.0) |
144 (100.0) |
0.011 |
|
CVO+C |
115 (87.1) |
81.3 - 92.9 |
17 (12.9) |
132 (100.0) |
|
|
Total |
223 (80.8) |
77.1 – 86.2 |
53 (19.2) |
276 (100.0) |
|
Table 2: Treatment Efficacy.
Treatment Failure
Treatment failure was observed in 53 patients, but there were no deaths during the study period. Treatment failure was virological in 39 patients: 11 patients from the CVO+C group and 28 patients from the placebo group. No cases of biological treatment failure were reported. Clinical treatment failure was reported in 14 patients who experienced severe adverse events and stopped treatment early (6 patients in the CVO+C group and 8 patients in the placebo group).
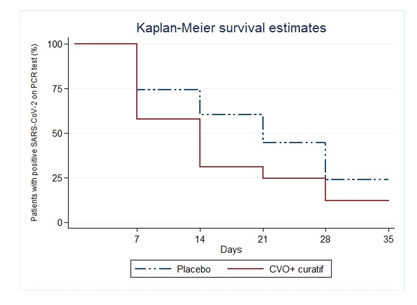
3.2.2 Secondary Outcomes: The percentages of patients for whom SARS-CoV-2 was undetectable on days 7, 14, 21, and 28 differed significantly (p<0.0001, log-rank test) between the CVO+C group (42.42%, 70.45%, 76.52%, and 89.39%, respectively) and the control group (27.08%, 39.85%, 40.97%, 56.94%, and 77.78%, respectively) (figure 2). The median recovery time was 14 days for the CVO+C group and 21 days for the control group.
Leukocyte and lymphocyte levels were normal throughout the study in most patients, and there was no clear difference between the groups. Median ESR was above normal values in both groups and appeared to be slightly higher in the control group. In general, aminotransferase levels increased at D7, gradually decreasing thereafter to reach normal values by D28. A similar pattern of change in aminotransferase levels was observed in both groups. Blood sugar and creatinine levels were normal in most patients, with no marked differences between groups. (Table 3)
|
Placebo |
CVO+C |
|||||||||
|
Biological Parameters |
D0 |
D7 |
D14 |
D21 |
D28 |
D0 |
D7 |
D14 |
D21 |
D28 |
|
Hematology |
||||||||||
|
Leukocytes (g/L) |
||||||||||
|
Mean ± SD |
3.5 ± 3.9 |
3.0 ± 1.3 |
2.7 ± 1.2 |
2.8 ± 1.2 |
2.8 ± 1.4 |
3.0 ±1.3 |
3.5 ± 1.6 |
3.2 ± 1.6 |
3.3 ± 2.7 |
3.0 ± 1.5 |
|
Median |
3.1 |
2.8 |
2.6 |
2.6 |
2.5 |
3.1 |
3.2 |
2.7 |
2.8 |
2.7 |
|
Q1 – Q3 |
2.1 – 3.7 |
2.0 – 3.8 |
2.0 – 3.3 |
2.1 – 3.3 |
1.8 – 3.5 |
1.7 – 3.9 |
2.2 – 5.1 |
2.0 – 3.9 |
1.9 – 3.7 |
1.9 – 3.7 |
|
Reference Range |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
2.0 – 7.5 |
|
Lymphocytes (g/L) |
||||||||||
|
Mean ± SD |
1.9 ± 0.5 |
1.6 ± 0.5 |
1.8 ± 0.7 |
1.8 ± 0.4 |
1.6 ± 0.4 |
1.9 ± 0.6 |
1.8 ± 0.6 |
1.8 ± 0.7 |
1.7 ± 0.6 |
1.9 ± 0.5 |
|
Median |
1.8 |
1.5 |
1.6 |
1.7 |
1.6 |
1.9 |
1.6 |
1.6 |
1.6 |
1.8 |
|
Q1 – Q3 |
1.5 – 2.3 |
1.1 – 2.0 |
1.5 – 1.8 |
1.5 – 2.0 |
1.4 – 1.9 |
1.3 – 2.4 |
1.4 – 2.3 |
1.3 – 2.1 |
1.3 – 2.0 |
1.6 – 2.0 |
|
Reference Range |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
1.0 – 4.8 |
|
Erythrocyte sedimentation (mm) |
||||||||||
|
Mean ± SD |
27.2 ± 26.1 |
22.5 ± 19.5 |
19.1 ± 18.1 |
27.0 ± 25.6 |
28.6 ± 27.3 |
23.0 ± 23.4 |
15.8 ± 13.0 |
19.8 ± 20.8 |
17.2 ± 15.1 |
20.6 ± 30.5 |
|
Median |
21 |
18 |
13.5 |
20 |
24 |
12 |
10 |
11.5 |
13.5 |
11 |
|
Q1 – Q3 |
10 – 33 |
10 – 27 |
6 – 28 |
10 – 33 |
10 – 38 |
4.5 – 28.5 |
8 – 20 |
5.5 – 29 |
5 – 25 |
5 – 23 |
|
Reference Range |
<10 |
<10 |
<10 |
<10 |
<10 |
<10 |
<10 |
<10 |
<10 |
<10 |
|
Hepatic function |
||||||||||
|
Aspartate aminotransferase (U/L) |
||||||||||
|
Mean ± SD |
44.6 ± 18.6 |
41.9 ± 17.0 |
40.1 ± 14.1 |
38.6 ± 14.1 |
39.9 ± 14.9 |
44.3 ± 15.7 |
42.6 ± 17.6 |
45.9 ± 22.1 |
44.9 ± 18.9 |
39.6 ± 13.7 |
|
Median |
40 |
41 |
38 |
35 |
35 |
41 |
40 |
42.5 |
42 |
37 |
|
Q1 – Q3 |
32 – 52 |
31 – 50 |
31 – 47.5 |
30 – 44 |
31 – 45 |
35 – 51 |
33 – 50 |
34 – 50 |
35 – 50 |
31 – 45 |
|
Reference Range |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
|
Alanine aminotransferase (U/L) |
||||||||||
|
Mean ± SD |
31.5 ± 24.9 |
32.7 ± 27.3 |
32.1 ± 27.8 |
27.6 ± 20.8 |
26.4 ± 19.1 |
30.7 ± 28.4 |
27.6 ± 15.5 |
32.1 ± 23.9 |
26.3 ± 18.1 |
22.9 ± 18.1 |
|
Median |
23.5 |
25 |
22 |
20.5 |
20 |
24 |
24 |
24 |
21 |
17 |
|
Q1 – Q3 |
17 – 39 |
17 – 37 |
14 – 40 |
13.5 – 35.5 |
14 – 31 |
16 – 32 |
16 – 34 |
18 – 38 |
17 – 29 |
14 – 26 |
|
Reference Range |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
<40 |
|
Kydney function |
||||||||||
|
Creatininemia for man (µmol/L) |
||||||||||
|
Mean ± SD |
93.9 ± 15.1 |
92.1 ± 15.1 |
91.9 ± 16.6 |
91.0 ± 14.9 |
90.4 ± 13.8 |
97.3 ± 21.1 |
93.8 ± 13.7 |
92.0 ± 16.0 |
88.5 ± 11.8 |
92.1 ± 12.7 |
|
Median |
90 |
92 |
90 |
89 |
92 |
97 |
93 |
91 |
87 |
91 |
|
Q1 – Q3 |
85 – 103 |
79.5 – |
82 – 99 |
82 – 100 |
82.5 – 100 |
82.5 – 107.5 |
85 – 103 |
80 – 101 |
80 – 98 |
82 – 100 |
|
103 |
||||||||||
|
Reference Range |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
53 – 115 |
|
Creatininemia for woman (µmol/L) |
||||||||||
|
Mean ± SD |
74.4 ± 16.1 |
75.6 ± 14.1 |
74.8 ± 15.7 |
72.9 ± 12.0 |
74.5 ± 12.4 |
78.3 ± 13.1 |
73.9 ± 15.5 |
73.9 ± 15.5 |
72.7 ± 12.3 |
71.6 ± 10.6 |
|
Median |
70.5 |
75 |
72 |
72.5 |
72.5 |
78 |
79.5 |
69 |
69.5 |
67 |
|
Q1 – Q3 |
63 – 80.5 |
65 – 86 |
64 – 81 |
64 – 80 |
65 – 83 |
69 – 89 |
67 – 87.5 |
62 – 82 |
64 – 78 |
64 – 77 |
|
Reference Range |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
44 – 105 |
|
Glycemia (mmol/L) |
||||||||||
|
Mean ± SD |
5.1 ± 0.8 |
5.4 ± 0.9 |
5.2 ± 1.0 |
5.3 ± 0.9 |
5.1 ± 1.0 |
4.9 ± 0.8 |
5.1 ± 0.9 |
5.4 ± 0.9 |
5.4 ± 0.8 |
5.2 ± 0.8 |
|
Median |
5.1 |
5.2 |
5.2 |
5.1 |
5.1 |
4.9 |
5.2 |
5.3 |
5.3 |
5.1 |
|
Q1 – Q3 |
4.6 – 5.6 |
4.7 – 5.9 |
4.7 – 5.8 |
4.7 – 5.5 |
4.5 – 5.4 |
4.5 – 5.4 |
4.6 – 5.7 |
4.9 – 5.8 |
4.9 – 5.8 |
4.8 – 5.5 |
|
Reference Range |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
4.10 – 5.90 |
Table 3: Change in hematology, biochemical markers of liver and renal functions over time.
3.2.3 Drug Safety: Adverse Events- A total of 72 incidences of mild and moderate adverse events, 14 severe adverse events, and no serious adverse events were observed in both groups. Some patients experienced more than one adverse event (range: 1 to 4). Generally, the frequency was higher among placebo recipients than among CVO+C recipients (29.1% vs. 22.7%), with similar frequencies of severe adverse events (5.6% vs. 4.6%). The percentages of patients experiencing adverse events on days 7, 14, 21, and 28 were 12.9%, 5.3%, 1.5%, and 3.0%, respectively, for the CVO+C group, and 9.7%, 7.6%, 8.3%, and 3.5%, respectively, for the placebo group. The mild and moderate adverse events observed included somatic, pulmonary, neurological, muscular, digestive, and cutaneous events. Digestive adverse events were the most frequently reported (30 incidences, 12 in CVO+C and in placebo). Adverse events resolved progressively after the end of treatment.
Severe Adverse Events- Severe adverse events led to the discontinuation of treatment. Severe asthenia was reported in two participants in the CVO+C group. Dyspnea was reported in four participants, with two in the CVO+C group, and two in the control group. Cardiac disturbance was reported by three participants in the control group. Digestive severe adverse events were reported in three patients, one in the placebo group, and the in the CVO+C group. And two patients in the placebo group progressed in severe form. None of the patients in the CVO+C group presented progression to the severe form of COVID-19.
4. Discussion
When CVO+C capsules were used to treat SARS-CoV-2 for 15 days, the success rate was much higher, and it requires less time for the virus to become undetectable than when the placebo was used. No serious (grade 3 or 4) adverse reactions were observed. In the CVO+C group, no abnormalities in the lab showed signs of drug-related systemic or organ-specific toxicity. The CVO+C capsules were therefore considered to have a satisfactory safety profile. None of the patients treated with CVO+C displayed progression to severe COVID-19. Confirmed COVID-19 can cause a wide range of symptoms, from asymptomatic to severe respiratory distress, and some patients have a high risk of death [26]. No specific treatment for SARS-CoV-2 infection has yet to be available. Patient management is therefore based essentially on the treatment of symptoms and supportive care [27]. In patients progressing to severe COVID-19, clinical deterioration results largely from a cytokine release syndrome (CRS) [28,29] induced by several pro-inflammatory cytokines, including interleukin-6 (IL6), tumor necrosis factor-alpha (TNF-α), and transforming growth factor-beta (TGF-β) [30,31]. Many studies have described the anti-inflammatory and immunomodulatory effects of artemisinin and its derivatives, which, by inhibiting the pro-inflammatory nuclear factor kappa B (NF-κB) signaling pathway, reduce TNF-α and IL-6 levels and attenuate signaling via the Smad2/3-dependent TGF-β pathway [32-34]. Ex vivo and in vitro studies have also shown that 1.8-cineole can change how the immune system works by stopping monocytes and macrophages from releasing pro-inflammatory cytokines [25,35]. The tea version of CVO (Covid Organics) was first introduced in Madagascar at the onset of the Covid-19 outbreak [36]. Peter Seeberger’s research team at the German-based Max Planck Institute showed in vitro efficacy of Covid Organics against SARS CoV-2 with an EC50 of 7.73% and a selectivity index of 5.28 [37]. This first step led to the development of CVO+C formulations. The CVO+C is based on standardized formulations of Artemisinin and 1,8-cineole with a much higher percentage of active compounds than the tea form CVO. Artemisinin and 1.8-cineole may have helped patients in the CVO+C group get better faster because they reduce inflammation and change the immune system. Treatments reducing the release or activity of pro-inflammatory mediators might also be able to prevent or reverse the cytokine storm, thereby improving the condition of patients who would otherwise progress to severe COVID-19, but this remains to be demonstrated for CVO+C. Mild, moderate, and severe adverse events that occurred in this study are similar to the symptoms and signs of the COVID-19. Digestive symptoms were reported as the third symptom after fever and cough [38-40]. Asthenia and dyspnea are cited among the specific signs of COVID-19 in hospitalized patients [41]. Nevertheless, for ethical reasons and for the security of the patients, treatment of the 14 participants who developed severe adverse events was stopped. These adverse events may be unrelated to the treatment, but this remains to be confirmed with a larger scale study. Our findings indicate that CVO+C capsules are safe and could be recommended for patients with mild-to-moderate COVID-19. However, this study has several limitations. The main limitation was the poor knowledge about the disease. The study was conducted a year into the pandemic. Despite efforts and scientific advances, the state of the medical knowledge of COVID-19 is still limited [42,43]. In addition, we recruited 339 participants, but we lost more than expected (18.58%), and 276 were included in the final analysis (instead of 306), and because of the urgent circumstances in which the study was conducted, we ended the study. Furthermore, the study was facing a lack of funding. We had planned to perform CT scans, variant searches, and immunological studies for more understanding of the primary outcomes.
5. Conclusion
In summary, the efficacy of CVO+C for the treatment of non-severe COVID-19 was 87.1% [CI: 95%: 81.3%-92.9%] and 70.45% of the patients were cured by day 14. None of the patients treated with CVO+C progressed to the severe form, and all patients had preserved liver, kidney, and metabolic functions. Based on how well CVO+C capsules worked and how safe they were in this study; this treatment could be an option for people with mild to moderate COVID-19.
Declarations
Ethics Approval and Consent to Participate
This trial is registered with the Pan African Clinical Trials Registry (No. PACTR202103601407640; date of approval: 24/03/2021) and was approved by the ethics committee of the Ministry of Public Health of Madagascar (approval no. 216 MINSANP/SG/AGMED/CERBM, 17/12/2020). The trial was done in line with the Declaration of Helsinki, and all the methods were done according to the rules and guidelines. Informed consent was obtained from the subjects studied for all tests and treatments.
Author Contributions
RR, ORA, and CAR wrote the manuscript with input, edits, and oversight from all authors. SAM, AnR, ArR, DR, and FM analyzed and interpreted the patient data. MAR, ER, and FeR performed the biological examination. FaR, RaR, ZR, RAR, and CAR supervised and revised the article.
Consent for Publication
All authors have agreed to the submission of this manuscript for publication.
Availability of Data and Materials
The data is available from the Centre National d’Application de Recherches Pharmaceutiques.
Competing Interests
The authors declare that they have no other conflicts of interest.
Funding
This work was supported by Pharmalagasy, the Ministry of Health, the Ministry of High-Level Education and Scientific Research, and the World Health Organization (WHO).
Acknowledgments
We thank the World Health Organization (WHO) for logistics and technical advice and Bionexx, who, along with Pharmalagasy and CNARP, developed the CVO+C formulation.
References
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine 382 (2020): 727-733.
- Randremanana RV, Andriamandimby S, Rakotondramanga JM, et al. The COVID-19 epidemic in Madagascar: clinical description and laboratory results of the first wave, march-september 2020. Influenza and Other Respiratory Viruses 15 (2021): 457-468.
- RECOVERY Collaborative Group, Peter Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 384 (2021): 693-704.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. New England Journal of Medicine 383 (2020): 1813-1826.
- Gautret P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents 56 (2020): 105949.
- Huang M, Li M, Xiao F, et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. National Science Review 7 (2020): 1428-1436.
- Tu Y. From Artemisia annua L. to Artemisinins: The Discovery and Development of Artemisinins and Antimalarial Agents, 1st edition. Academic Press (2017).
- Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy », in Seminars in cancer biology 46 (2017): 65-83.
- Efferth T, Romero MR, Wolf DG, et al. The antiviral activities of artemisinin and artesunate. Clinical Infectious Diseases 47 (2008): 804-811.
- Gilmore K, et al. In vitro efficacy of Artemisinin-based treatments against SARS-CoV-2. Sci Rep (2020).
- Cao R, et al. Anti-SARS-CoV-2 Potential of Artemisinins In Vitro. ACS Infect. Dis 6 (2020): 2524-2531.
- Sehailia M, Chemat S. Antimalarial-agent artemisinin and derivatives portray more potent binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein than hydroxychloroquine: potential repurposing of artenimol for COVID-19. J Biomol Struct Dyn (2020): 1-11.
- Li G, Li Y, Li Z, et al. Chapter 6: artemisinin and derivatives: clinical studies. Artemisinin-based and other Antimalarials: detailed account of studies by Chinese scientists who discovered and developed them (2018): 353-413.
- Uckun FM, Saund S, Windlas H, et al. Repurposing Anti-Malaria Phytomedicine Artemisinin as a COVID-19 Drug. Pharmacol (2021).
- Kangbai JB, Babawo LS, Kaitibi D, et al. Re-reading ACT, BCG, and Low COVID-19 in Africa. SN Compr. Clin. Med 3 (2021): 11-15.
- Firestone TM, Oyewole OO, Reid SP, et al. Repurposing Quinoline and Artemisinin Antimalarials as Therapeutics for SARS-CoV-2: Rationale and Implications », ACS Pharmacol. Transl. Sci 4 (2021): 613-623.
- Giamakis A, Kretsi O, Chinou I, et al. Eucalyptus camaldulensis: volatiles from immature flowers and high production of 1, 8-cineole and β-pinene by in vitro cultures », Phytochemistry 58 (2001): 351-355.
- Vilela GR, Almeida GS, RegitanoD'Arce MAB, et al. Activity of essential oil and its major compound, 1, 8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. Journal of Stored Products Research 45 (2009): 108-111.
- Möllenbeck S, König T, Schreier P, et al. Chemical Composition and Analyses of Enantiomers of Essential Oils from Madagascar », Flavour and Fragrance Journal 12 (1997): 63-69.
- De Medici D, Pieretti S, Salvatore G, et al. Chemical analysis of essential oils of malagasy medicinal plants by gas chromatography and NMR spectroscopy. Flavour and Fragrance Journal 7 (1992): 275-281.
- Laude EA, Morice AH, Grattan TJ. The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulmonary pharmacology 7 (1994): 179-184.
- Juergens LJ, Worth H, Juergens UR. New Perspectives for Mucolytic, Anti-inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv Ther 37 (2020): 1737-1753.
- Sharma AD, Kaur I. Molecular docking and pharmacokinetic screening of eucalyptol (1,8 cineole) from eucalyptus essential oil against SARS-CoV-2. Notulae Scientia Biologicae 12 (2020).
- Feuillet V, Canard B, Trautmann A. Combining Antivirals and Immunomodulators to Fight COVID-19. Trends Immunol 42 (2021): 31-44.
- Juergens UR, Dethlefsen U, Steinkamp G, et al. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respiratory Medicine 97 (2003): 250-256.
- da Rosa Mesquita R, Silva Junior LCF, Santana FMS, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr (2020): 1-6.
- Stasi C, Fallani S, Voller F, et al. Treatment for COVID-19: An overview. Eur J Pharmacol 889 (2020): 173644.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine 180 (2020): 934-943.
- Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. Journal of infection 81 (2020): e16-e25.
- Uckun FM. Prognostic factors associated with high-risk for fatal ARDS in COVID-19 and potential role for precision medicines as part of COVID-19 supportive care algorithms. Ann. Pulm. Crit. Care Med 3 (2020): 1-4.
- Uckun FM. Reducing the fatality rate of COVID-19 by applying clinical insights from immuno-oncology and lung transplantation. Frontiers in pharmacology 11 (2020): 796.
- Zhang H, Qi S, Song Y, et al. Artemisinin attenuates early renal damage on diabetic nephropathy rats through suppressing TGF-β1 regulator and activating the Nrf2 signaling pathway », Life Sciences 256 (2020): 117966.
- Aldieri E et al. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-kB activation. FEBS letters 552 (2003): 141-144.
- Wu X, Zhang W, Shi X, et al. Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms », Acta Biochim Biophys Sin 42 (2010): 916-923.
- Sadlon AE, Lamson DW. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Alternative medicine review 15 (2010): 33-43.
- Peter O, Aliyu IJ, Temitope FO, et al. Comparative pharmacognostic and chromatographic assessment of COVID-ORGANICS (CVO) herbal product and Artemisia annua L. Journal of Pharmacognosy and Phytotherapy 13 (2021): 91-107.
- Nie C, Trimpert J, Moon S, et al. In vitro efficacy of Artemisia extracts against SARS-CoV-2. Virol J 18 (2021): 182.
- Yasuhara J, Kuno T, Takagi H, et al. Clinical characteristics of COVID-19 in children: a systematic review. Pediatric pulmonology 55 (2020): 2565-2575.
- Kharoud HK, Asim R, Siegel L, et al. Review of clinical characteristics and laboratory findings of COVID-19 in children-Systematic review and Meta-analysis. medRxiv (2020).
- Christophers B, Marin BG, Oliva R, et al. Trends in clinical presentation of children with COVID-19: a systematic review of individual participant data. Pediatric Research (2020): 1-8.
- Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database of Systematic Reviews 2021 (2021).
- Khan M, Adil SF, Alkhathlan HZ, et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules 26 (2021): 39.
- Peng Y, Tao H, Satyanarayanan SK, et al. A Comprehensive Summary of the Knowledge on COVID-19 Treatment. Aging Dis 12 (2021): 155-191.