Dynamic Changes in Neutrophil Counts and Neutrophil Granular Protein Levels in Convalescent COVID-19 Patients
Article Information
Anuradha Rajamanickam1, Nathella Pavan Kumar1, Arul Nancy P1, Nandhini Selvaraj1, Saravanan Munisankar1, Rachel Mariam Renji1, Vijayalakshmi V1, Manoj Murhekar2, Jeromie Wesley Vivian Thangaraj2, Muthusamy Santhosh Kumar2, CP Girish Kumar2, Tarun Bhatnagar2, Manickam Ponnaiah2, R Sabarinathan2, V Saravanakumar2, Subash Babu3
1ICMR-National Institute for Research in Tuberculosis-International Center for Excellence in Research, Chennai, India
2National Institute of Epidemiology (ICMR), Second Main Road, Tamil Nadu Housing Board, Ayapakkam, Near Ambattur, Chennai, India
3ICMR-NIRT-International Center for Excellence in Research, Chennai, India
*Corresponding author: Anuradha Rajamanickam, ICMR-National Institute for Research in Tuberculosis-International Center for Excellence in Research, Chennai, India.
Received: 10 March 2022; Accepted: 29 March 2022; Published: 12 April 2022
Citation:
Anuradha Rajamanickam, Nathella Pavan Kumar, Arul Nancy P, Nandhini Selvaraj, Saravanan Munisankar, Rachel Mariam Renji, Vijayalakshmi V, Manoj Murhekar, Jeromie Wesley Vivian Thangaraj, Muthusamy Santhosh Kumar, CP Girish Kumar, Tarun Bhatnagar, Manickam Ponnaiah, R Sabarinathan2, V Saravanakumar, Subash Babu. Dynamic Changes in Neutrophil Counts and Neutrophil Granular Protein Levels in Convalescent COVID-19 Patients. Archives of Clinical and Biomedical Research 6 (2022): 378-389.
Share at FacebookAbstract
Neutrophils play an important role in the pathophysiology of COVID-19. However, the dynamics of changes in neutrophil numbers and function in convalescent individuals of SARS-CoV2 infection has not been well explored. Hence, we aimed to determine absolute counts, percentages of neutrophils, Neutrophil-To-Lymphocyte Ratio (NLR) and the plasma levels of neutrophil elastase (NE), myeloperoxidase (MPO) and proteinase-3 (PTN-3) in seven groups of COVID-19 individuals, based on days since RT-PCR confirmation of SARS-CoV-2 infection. Our data demonstrated that both absolute counts and percentages of neutrophils, neutrophil-to-lymphocyte ratio (NLR), as well as the circulating levels of NE, MPO and PTN-3 decreased from Days 15-30 to Days 91-120 and plateaued thereafter. Severe COVID-19 patients exhibited increased levels of neutrophil counts and percentages, NL ratio and NE, MPO and PTN-3. Thus, our study provides an important insight into the kinetics of alterations in neutrophil counts, percentages, the NL ratio and the levels of neutrophil granular proteins in convalescent COVID-19 individuals.
Keywords
COVID-19; Myeloperoxidase; Neutrophils; Neutrophil elastase; Proteinase-3
COVID-19 articles; Myeloperoxidase articles; Neutrophils articles; Neutrophil elastase articles; Proteinase-3 articles
Article Details
1. Introduction
Coronavirus disease 2019 (COVID-19) is triggered by SARS-CoV-2. The COVID-19 symptoms spans a spectrum from no symptoms to shock, severe respiratory failure, and dysfunction of multi-organ systems [1]. Neutrophils migrate to the infection site following viral infection and initiate the antiviral response. Recent reports found that neutrophils are elevated in severe COVID-19 patients compared to mild patients [2-7]. COVID-19 patients were shown to have augmented numbers of neutrophils in the blood [2, 8]. Recent data suggests that Neutrophil-To-Lymphocyte Ratio (NLR) could be used as a systemic inflammation indicator for COVID-19 [9-11]. Moreover, in other diseases such as Chronic Obstructive Pulmonary Disease (COPD), pancreatitis and cardiovascular disease, NLR has been used as a good prognostic marker for clinical outcomes and disease development [11]. Neutrophils are the first responders and kill pathogens through oxidative burst and phagocytosis but damage the tissues [12]. Neutrophil granules encompass numerous serine proteases consisting of proteinase-3, Neutrophil Elastase, lysozyme, MPO, cathepsin G and lactoferrin, which has a role in bystander tissue destruction [13]. These enzymes and proteins, that are also present in neutrophil extracellular traps (NETs), can modify immunity to viral infection via induction of autoantigens and immune complexes [14]. In our study, we sought to study COVID-19 influence on neutrophil numbers and the circulating levels of neutrophil granular proteins in COVID-19 patients over time.
2. Materials and Methods
2.1. Study population
Acute COVID-19 (15-30 days from RT-PCR confirmation, n=46) and Convalescent COVID-19 individuals (classified by days after infection as 31-60, n=33; 61-90, n=38; 91-120, n=34; 121-150, n=32; 151-180, n= 37 and more than 180, n=40), residing in Chennai and Tiruvallur were enrolled in the study between November 2020 and December 2020 after taking informed consent from the enrolled study individuals [15, 16]. Those who had active COVID-19 infection under home isolation and recovered COVID-19 patients within 0-15 days of RT-PCR confirmation were excluded from the study. The age group ranged between 18-75 years. COVID-19 was confirmed by RT-PCR in Government approved laboratories and published elsewhere [15, 16].
2.2. Measurement of Hematological Parameters
Hematological parameters were measured from fresh venous EDTA blood samples on all individuals using an ACT 5 Diff. hematology analyzer (Beckman Coulter, Brea, CA, USA). The NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count. Demographic details and other clinical parameters are shown in Table 1 and the patient’s population was the same as described previously [15, 16].
|
Days after RT-PCR confirmation |
15-30 days |
31-60 days |
61-90 days |
91-120 days |
121-150 days |
151-180 days |
More than 180 days |
|
Subjects enrolled |
n=46 |
n=33 |
n=38 |
n=34 |
n=32 |
n=37 |
n=40 |
|
Median age (range) |
41.5(18- 70) |
36(25-68) |
45(19-59) |
45 (21-69) |
45.5(27-59) |
42 (23-58) |
38.5(21-78) |
|
Gender (M/F) |
27/19 |
17/18 |
22/15 |
22/12 |
14/18 |
23/16 |
26/14 |
|
Fever, no. (%) |
29 (67) |
22 (65) |
28 (74) |
23 (74) |
25 (83) |
23 (72) |
17 (47) |
|
Chills, no. (%) |
9 (21) |
5 (15) |
2 (5) |
7 (22) |
4 (13) |
1 (3) |
3 (8) |
|
Cough, no. (%) |
21 (49) |
20 (59) |
14 (37) |
15 (48 ) |
14 (47) |
17 (53) |
12 (33) |
|
Sore throat, no. (%) |
21 (49) |
12 (35) |
11 (29) |
12 (38) |
10 (33) |
16 (50) |
13 (36) |
|
Runny nose, no. (%) |
7 (16) |
6 (18) |
5 (13) |
NIL |
3 (10) |
6 (19) |
5 (14) |
|
Taste loss, no. (%) |
24 (55) |
14 (41) |
17 (44) |
12 (39) |
11 (37) |
20 (63) |
12 (33) |
|
Smell loss, no. (%) |
21 (49) |
14 (41) |
21 (55) |
9 (29) |
11 (37) |
16 (50) |
10 (28) |
|
Muscle aches, no. (%) |
23 (53) |
20 (59) |
29 (76) |
15 (48) |
18 (60) |
21 (66) |
13 (36) |
|
Joint pain, no. (%) |
21 (49) |
18 (53) |
20 (53) |
10 (32) |
18 (60) |
14 (44) |
9 (25) |
|
Abdominal pain, no. (%) |
3 (7) |
3 (9) |
4 (11) |
2 (6.5) |
3 (10) |
2 (7) |
3 (8) |
|
Vomit, no. (%) |
3 (7) |
4 (12) |
5 (13) |
4 (13) |
3 (10) |
5 (16) |
3 (8) |
|
Diarrhea, no. (%) |
10 (23) |
5 (15) |
4 (11) |
4 (13) |
6 (30) |
5 (16) |
2 (6) |
|
Seizures, no. (%) |
NIL |
1 (3) |
NIL |
NIL |
NIL |
NIL |
NIL |
|
Hypertension, no. (%) |
11 (26) |
7 (21) |
7 (18) |
7 (23) |
9 (30) |
9 (28) |
8 (22) |
|
Diabetes, no. (%) |
8 (19) |
7 (21) |
11 (30) |
9 (29) |
11 (37) |
8 (25) |
7 (19) |
|
Asthma, no. (%) |
2 (5) |
2 (6) |
1 (3) |
1 ( 3) |
NIL |
1 (3) |
NIL |
|
Chronic Kidney Disease, no. (%) |
NIL |
NIL |
NIL |
NIL |
1 (3) |
NIL |
1 (3) |
|
Neuro, no. (%) |
NIL |
NIL |
2 (5) |
NIL |
NIL |
NIL |
NIL |
|
Heart, no. (%) |
1 (6) |
2 (3) |
1 (3) |
NIL |
NIL |
1 (3) |
NIL |
|
Rheumatic fever, no. (%) |
NIL |
NIL |
1 (3) |
NIL |
NIL |
1 (3) |
NIL |
|
Corticosteroids, no. (%) |
4 (9) |
3 ( 9) |
2 (5) |
3 (10) |
1 (3) |
1 (3) |
NIL |
|
Antiviral drug, no. (%) |
4 (9) |
5 (15) |
2 (5) |
4 (13) |
NIL |
NIL |
NIL |
Table 1: Demographics and clinical parameters of the study population.
2.3. Measurement of Neutrophils Granular Proteins
Plasma levels of MPO, PTN-3 (R&D Systems, Minneapolis, MN, USA), NE (Cell Sciences Hycult Biotech, Canton, MA, USA) ELISA kits followed by the manufacturer’s protocol. The detection limits were as follows: MPO, 62.50–4,000 pg/ml; PTN-3, 15.6–1,000 pg/ml; NE, 0.4–25 ng/ml. We have assigned the lowest standard value to the samples that were below the threshold of detection.
3. Statistical Analysis:
GraphPad PRISM.9 was used to analyse the data (GraphPad Software, Inc., San Diego, CA, USA). Cross-sectional analysis of haematological parameters and the levels of neutrophil granular proteins were accomplished by means of polynomial model for best fit curve (either first order or second order model). Central tendency were measured using Geometric Means (GM). Kruskal-Wallis test with Dunn’s multiple comparisons was used for comparative analysis. Nonparametric Mann-Whitney U test was used for the comparison between mild versus severe COVID-19 patients. Spearman rank correlation matrix was done using JMP 16 (SAS) software.
3.1. Ethics statement
The study was approved by the Ethics Committees of ICMR-NIRT (NIRT-INo:2020047) and ICMR-NIE (NIE/IHEC/202008-01). Informed written consent was taken from all the recruited individuals. All the protocols were followed according to the relevant institutional ethical committee guidelines.
4. Results
4.1. Study population characteristics
The clinical and demographics data are illustrated in Table I. No considerable differences were seen among the study populations in their age or sex [15,16].
4.2. Decrease in numbers of Neutrophils and Neutrophil to Lymphocyte Ratio in convalescent COVID-19 individuals over a period of time
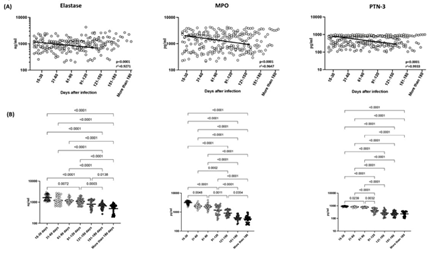
To establish the neutrophil percentage, neutrophils absolute numbers, neutrophil to Lymphocyte Ratio (NLR), in acute and convalescent COVID-19 individuals over time, we observed these parameters in seven groups of COVID-19 individuals. As shown in Figure 1A, cross sectional analysis revealed a significant reduction in the percentages of neutrophils (first order model polynomial model fit curve, R2=0.97 by Akaike’s Information Criterion), absolute numbers of neutrophils (first order model polynomial model fit curve, R2=0.95 by Akaike’s Information Criterion) and NLR (first order model polynomial model fit curve, R2=0.77 by Akaike’s Information Criterion) from day 15-30 till 91-120 days and plateaued thereafter. As shown in Figure 1B, the comparative analysis also exhibited significant differences between different time intervals. The percentages, absolute numbers and the NLR of neutrophils started decreasing from days 15-30 till 91-120 days. The 95% of confidence intervals are shown in Suppl.Table.1. Thus, the percentage and number and NLR levels decrease over time following COVID-19 infection.
(A)Analysis of absolute neutrophils counts, percentages and Neutrophil to Lymphocyte Ratio were shown using best fit curve model from acute and convalescent COVID-19 individuals classified as groups based on days since RT-PCR confirmation. Both absolute counts and percentages and Neutrophil to Lymphocyte Ratio were shown with preferred model for best fit curve and each dot represents single individuals. Thick black line represents best fit curve. (B) Analysis of absolute neutrophils counts, percentages and Neutrophil to Lymphocyte Ratio from acute and convalescent COVID-19 individuals classified as groups based on days since RT-PCR confirmation. The data are represented as scatter violin plots with each circle representing a single individual. p values were calculated using the Kruskal-Wallis test with Dunn’s post-hoc for multiple comparisons.
4.2. Decreased levels of Neutrophil granular proteins in convalescent COVID-19 individuals over a period of time
Next, we wanted to ascertain the levels of neutrophil granular proteins in convalescent COVID-19 individuals over time. As illustrated in Figure 2A, cross sectional analysis exhibited that the levels of NE (first order model polynomial model fit curve, R2=0.93 by Akaike’s Information Criterion), MPO (first order model polynomial model fit curve, R2=0.96 by Akaike’s Information Criterion) and PTN-3 (first order model polynomial model fit curve, R2=0.99 by Akaike’s Information Criterion) started declining from days 15-30 till 91-120 days. After 121 days of infection, the neutrophil granular proteins levels (NE, MPO and PTN-3) plateaued. As shown in Figure 2B, the comparative analysis also exhibited significant differences between different time intervals. The circulating levels of NE, MPO and PTN-3 began declining from days 15-30 till 91-120 days. The 95% of confidence intervals are shown in Suppl.Table.1. Thus, the neutrophil granular proteins levels decrease over the time following COVID-19 infection.
Analysis of neutrophil granular proteins; Neutrophil Elastase (NE), Myeloperoxidase (MPO) and Proteinase-3 (PTN-3) from acute and convalescent COVID-19 individuals classified as groups based on days since RT-PCR confirmation. The levels of neutrophil granular proteins are shown with preferred model for best fit curve and each dot represent single individuals. Thick black line represents best fit curve. (B) Analysis of neutrophil granular proteins; Neutrophil Elastase (NE), Myeloperoxidase (MPO) and Proteinase-3 (PTN-3) from acute and convalescent COVID-19 individuals classified as groups based on days since RT-PCR confirmation. The data are represented as scatter violin plots with each circle representing a single individual. p values were calculated using the Kruskal-Wallis test with Dunn’s post- hoc for multiple comparisons.
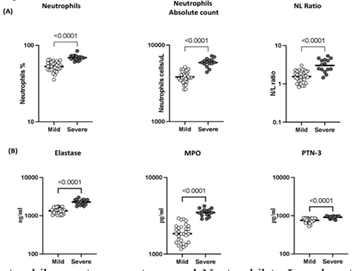
4.3. Severe COVID-19 disease is associated with elevated levels of neutrophils, NLR and neutrophil granular proteins
To examine the association between neutrophils and disease severity, we ascertained the absolute counts of neutrophils, percentage of neutrophils and NLR in COVID-19 patients with mild or severe disease. As illustrated in Figure 3A, the percentage of neutrophils (Geomean (GM) of 52.7% in mild, 68.8% in severe, p<0.0001), absolute count of neutrophils (GM of 3829 cells/ul in mild, 5898 cells/ul in severe, p<0.0001), NLR (GM of 1.55 cells/ul in mild, 3.02 cells/ul in severe, p<0.0001) were significantly elevated in severe COVID-19 patients compared with mild COVID-19 patients. Subsequently, we examined the levels of neutrophil granular proteins in mild and severely diseased COVID-19 patients. As shown in Figure 3B, the levels of NE (GM of 1341 ng/ml in mild, 2282 ng/ml in severe, p<0.0001), MPO (GM of 1850 pg/ml in mild, 3480 pg/ml in severe, p<0.0001) and PTN-3 (GM of 757 pg/ml in mild, 909 pg/ml in severe, p<0.0001) were significantly elevated in severe than the mild COVID-19 patients. Hence, severe COVID-19 disease is associated with increased levels of neutrophils, NLR and neutrophil granular proteins.
The absolute neutrophils counts, percentages and Neutrophil to Lymphocyte Ratio in mild (n=30) and severe (n=15) COVID-19 individuals sampled between days 15 to 60 following RT-PCR confirmation. The data are represented as scatter plots with each circle representing a single individual. p values were calculated using the Mann– Whitney U-test. (B) The levels of neutrophil granular proteins; Neutrophil Elastase (NE), Myeloperoxidase (MPO) and Proteinase-3 (PTN-3) in mild (n=30) and severe (n=15) COVID-19 sampled between days 15 to 60 following RT-PCR confirmation. The data are represented as scatter plots with each circle representing a single individual. p values were calculated using the Mann– Whitney U- test.
4.4. Association between absolute neutrophil counts and neutrophil granular proteins levels
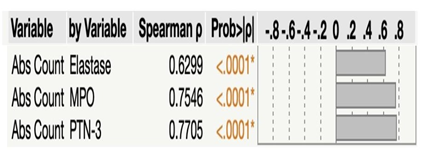
Next, we sought to ascertain the relationship between absolute neutrophil counts and neutrophil granular proteins levels in seven groups of COVID-19 patients. As illustrated in Figure 4, absolute counts of neutrophils revealed significant positive correlation with Neutrophil Elastase (r = 0.6299), MPO (r = 0.7546) and PTN-3 (r = 0.7705). Thus, absolute neutrophil counts exhibited positive correlation with neutrophil granular proteins.
Multiparametric correlation plot of absolute neutrophils counts and the levels of neutrophil granular proteins; Neutrophil Elastase (NE), Myeloperoxidase (MPO) and Proteinase-3 (PTN-3) from all seven group of convalescent COVID-19 individuals classified as groups based on days since RT-PCR confirmation. Correlation analysis was done using Spearman's correlation analysis.
5. Discussion
Recent studies suggest that during COVID-19, neutrophils play a dynamic role in inflammation, immunopathology and severe disease [17]. Neutrophils are mostly the primary responders to act up on infection and injury, also causes consequential collateral impairment [18]. Neutrophils could intensify pathological impairment or manipulate other cell subsets determined by the infection characteristics [17, 19]. In COVID-19, a possible function of neutrophils has not been well studied, similar to monocyte and macrophages [20]. Recently, significant data has started to come-up, containing the predictive value of NLR [21]. Also, the bronchoalveolar lavage of critically ill COVID-19 patients contains elevated neutrophils and immature neutrophils [22]. Various findings have determined that both in animals and human studies showed that neutrophils are dynamically conscripted to the infection site, which induces the development of lung inflammation in several viral infections [23, 24]. Longitudinal studies from hospitalized patients, determined that the severity linked with elevated neutrophil number continues over the period of time [17, 25-27]. Acute COVID-19 individuals exhibited significantly elevated counts of neutrophils than the mild and moderate COVID-19 hospitalized patients [17, 28-31]. Recent studies determined that acute COVID-19 patients exhibited higher neutrophil counts than HCs and non-ICU patients those who had mild to moderate disease [17, 31,32]. Lu et. al. described that following 14 days of hospitalization, WBCs, neutrophils, monocytes and eosinophils slowly improved [33]. Ding et. al., observed that severe COVID-19 patients exhibited elevated neutrophil counts than the non-severe disease of samples collected in days varying from 1-15 of post-hospitalisation [28]. Consistent with previous reports, our data also exhibited decreased absolute and percentages of neutrophils decreased with increased time of convalescence. In line with previous reports, the percentage and absolute counts of neutrophils were significantly increased in severe cases of COVID-19 in comparison with mild COVID-19 cases. An enhanced neutrophil: lymphocyte ratio (NLR) occurs as a results of SARS-CoV-2-stimulated apoptosis, systemic inflammation and lymphopenia alongside increased counts of neutrophils [30, 34]. Following the COVID-19 patient improvement, the ratio of neutrophil–lymphocyte ratio (NLR) gradually returned to normal [33]. Additionally, elevated NLR ratio is observed in acute COVID-19 patients [7, 35]. Similarly, we also observed that NLR started decreasing over the period of time. Also, severely infected patients exhibited increased NL ratio in comparison to mild COVID-19 patients which indicates that stimulation of neutrophil might alter immune response following SARS-CoV-2 infection [7]. Thus, our data shows that neutrophils and NL ratio act a crucial role in innate immunity, exacerbation in COVID-19 and following recovery reverts to its homeostatic level. Neutrophils act via the release of neutrophil elastase and antimicrobic particles and the development of neutrophil extracellular traps (NETs) [36, 37]. NE belongs to serine protease accumulated inside the elemental particles of neutrophils. Following SARS-CoV-2 infection, the stimulated neutrophils release the granule-derived proteins (e.g. NE, MPO) and potent degranulation occurs [17]. Upon neutrophil activation, myeloperoxidase (MPO) granular proteins are produced for dissemination [38, 39]. The acute COVID-19 patients who have ARDS and COVID-19 individuals with varying clinical spectrum ranges from mild to fatal exhibited significantly elevated levels of NE respectively [40, 41]. A recent report showed that MPO-DNA levels were enhanced in admitted COVID-19 patients than the controls and associated with their absolute neutrophil count [42]. Consistent with earlier studies, the circulating levels of NE, MPO and PTN-3 started decreasing over a period of time and then plateaued after 121 days of post COVID-19 disease. Also, the severe COVID-19 patients showed enhanced levels of NE, MPO and PTN-3 in comparison to mild COVID-19 patients. In general, in COVID-19 patients, the NE and MPO are involved in neutrophil infiltration [43, 44]. Thus, our study demonstrates that alterations in the circulating neutrophil granular proteins levels are correlated with the absolute counts of neutrophils seen in acute and convalescent COVID-19 individuals. Our study has certain limitations, we have not examined the functional impact of the above mentioned alterations of neutrophils and granular proteins and it was a cross-sectional and not a longitudinal study. Nevertheless, our study offers impetus to stimulate the study of the role of these neutrophils and granular proteins in acute and convalescent COVID-19. Our study has the benefit of a quite significant sample size, provides the dynamics of neutrophils and its granular proteins from early infection to more than 6 months post COVID-19 infection. Our data therefore correlates dynamic changes in the number, percentages of neutrophil, NLR, and neutrophil granular proteins NE, MPO and PTN-3 as a key player in COVID-19.
Supplementary Material
Supplementary Table.1. contains the value of 95% of CI for neutrophils, NLR and neutrophils granular proteins.
Table 1: 95% of CI of neutrophil, NLR and neutrophil granular proteins.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Conflict of Interest
The authors declare no competing interests.
Funding
This work was supported by NIRT-ICER and ICMR-NIE.
Acknowledgments
We thank the Director of the ICMR-NIRT for the constant support. We thank the data entry operators Mr. Jaiganesh and Mr. Vigneshwaran, and also all the staff members of the ICER department for the timely help. We thank D. Sudha Rani Scientist B, staff nurse M Beula margrete, Technical Officers C K. Sathish Kumar, Annamma Jose, D Augustine, C. Prabakaran, Technical Officers B P. Ashok Kumar and laboratory technician T. Mahesh, S. Kalaivani, M. Sheeba Mary, C. Kanagasivam, Technical Assistant R. Sivakumar, Project Assistants Y Radhakrishnaiah, R. Swapna ShindeReferences
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130 (2020): 2620-2629.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 5 (2020).
- Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26 (2020): 842-844.
- Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 5 (2020): 33.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323 (2020): 1061-1069.
- Shrivastava S, Chelluboina S, Jedge P, et al. Elevated Levels of Neutrophil Activated Proteins, Alpha-Defensins (DEFA1), Calprotectin (S100A8/A9) and Myeloperoxidase (MPO) Are Associated With Disease Severity in COVID-19 Patients. Front Cell Infect Microbiol 11 (2021): 751232.
- Henry BM, de Oliveira MHS, Benoit S. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 58 (2020): 1021-1028.
- Feng X, Li S, Sun Q, et al. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 7 (2020): 301.
- Karimi A, Shobeiri P, Kulasinghe A. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front Immunol 12 (2021): 741061.
- Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care 24 (2020): 647.
- Jenne CN, Liao S, Singh B. Neutrophils: multitasking first responders of immunity and tissue homeostasis. Cell Tissue Res 371 (2018): 395-397.
- Carmona-Rivera C, Carlucci PM, Goel RR, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 5 (2020).
- Paryzhak S, Dumych T, Mahorivska I, et al. Neutrophil-released enzymes can influence composition of circulating immune complexes in multiple sclerosis. Autoimmunity 51 (2018): 297-303.
- Rajamanickam A, Kumar NP, Nancy PA, et al. Recovery of Memory B-cell Subsets and Persistence of Antibodies in Convalescent COVID-19 Patients. Am J Trop Med Hyg 105 (2021): 1255-1260.
- Rajamanickam A, Kumar NP, Pandiarajan AN, et al. Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals. Sci Rep 11 (2021): 20254.
- COVID-19: Active Participants and Rational Therapeutic Targets. Front Immunol 12 (2021): 680134. Hazeldine J, Lord JM. Neutrophils and
- Bardoel BW, Kenny EF, Sollberger G, et al. The balancing act of neutrophils. Cell Host Microbe 15 (2014): 526-536.
- Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol 4 (2013): 1.
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20 (2020): 355-362.
- Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81 (2020): 6- 12.
- Meizlish ML, Pine AB, Bishai JD, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv 5 (2021): 1164-1177.
- Penaloza HF, Lee JS, Ray P. Neutrophils and lymphopenia, an unknown axis in severe COVID-19 disease. PLoS Pathog 17 (2021): 1009850.
- Camp JV, Jonsson CB. A Role for Neutrophils in Viral Respiratory Disease. Front Immunol 8 (2017): 550.
- Shi S, Nie B, Chen X, et al. Clinical and laboratory characteristics of severe and non-severe patients with COVID-19: A retrospective cohort study in China. J Clin Lab Anal 35 (2021): 23692.
- Wang J, Li Q, Yin Y, et al. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front Immunol 11 (2020): 2063.
- Payen D, Cravat M, Maadadi H, et al. A Longitudinal Study of Immune Cells in Severe COVID-19 Patients. Front Immunol 11 (2020): 580250.
- Chevrier S, Zurbuchen Y, Cervia C, et al. A distinct innate immune signature marks progression from mild to severe COVID-19. Cell Rep Med 2 (2021): 100166.
- Ding X, Yu Y, Lu B, et al. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin Chem Lab Med 58 (2020): 1365-1371.
- Song CY, Xu J, He JQ, et al. Immune dysfunction following COVID-19, especially in severe patients. Sci Rep 10 (2020): 15838.
- Li Q, Xie Y, Cui Z, et al. Analysis of Peripheral Blood IL-6 and Leukocyte Characteristics in 364 COVID-19 Patients of Wuhan. Front Immunol 11 (2020): 559716.
- Silvin A, Chapuis N, Dunsmore G, et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 182 (2020): 1401-1418.
- Lu G, Wang J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta 508 (2020): 98-102.
- Petito E, Falcinelli E, Paliani U, et al. Association of Neutrophil Activation, More Than Platelet Activation, With Thrombotic Complications in Coronavirus Disease 2019. J Infect Dis 223 (2021): 933-944.
- Zhang HF, Ge YL, Wang HY, et al. Neutrophil-to-Lymphocyte Ratio Improves the Accuracy and Sensitivity of Pneumonia Severity Index in Predicting 30-Day Mortality of CAP Patients. Clin Lab 65 (2019).
- Gueant JL, Gueant-Rodriguez RM, Fromonot J, et al. Elastase and exacerbation of neutrophil innate immunity are involved in multi-visceral manifestations of COVID-19. Allergy 76 (2021): 1846-1858.
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18 (2018): 134-147.
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5 (2003): 1317-1327.
- Tardif MR, Chapeton-Montes JA, Posvandzic A, Page N, et al. Secretion of S100A8, S100A9, and S100A12 by Neutrophils Involves Reactive Oxygen Species and Potassium Efflux. J Immunol Res 2015 (2015): 296149.
- Adrover JM, Aroca-Crevillen A, Crainiciuc G, et al. Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol 21 (2020): 135-144.
- Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 58 (2020): 102925.
- Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 5 (2020).
- Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 1 (2020): 290- 299.
- Iliadi V, Konstantinidou I, Aftzoglou K, et al. The Emerging Role of Neutrophils in the Pathogenesis of Thrombosis in COVID-19. Int J Mol Sci 22 (2021).