Dual Case Reports for Lipid Loss from Human Hair
Article Information
Sang-Hun Song*, Seong Kil Son, Nae-Gyu Kang*
LG Science Park in Magok, LG Household & Health Care, Gangseo-gu, Seoul, Republic of Korea
*Corresponding Authors: Dr. Sang-Hun Song, LG Science Park in Magok, LG Household & Health Care, Gangseo-gu, Seoul, 07795, Republic of Korea
Dr. Nae-Gyu Kang, LG Science Park in Magok, LG Household & Health Care, Gangseo-gu, Seoul, 07795, Republic of Korea
Received: 21 August 2019; Accepted: 07 September 2019; Published: 26 November 2019
Citation: Sang-Hun Song, Seong Kil Son, Nae-Gyu Kang. Dual Case Reports for Lipid Loss from Human Hair. Archives of Clinical and Medical Case Reports 3 (2019): 573-579.
Share at FacebookAbstract
This study aims to understand the mechanism of lipid loss from the hair upon contact with surfactants. We herein proposes that the process of lipid loss can be categorized into two mechanisms. In the first mechanism, the lipids inside the hair diffuse through the cell membrane complex (CMC) to the outermost layer of hair and are removed by a roll-up mechanism. In the second mechanism, the surfactant that penetrates hair directly emulsifies the lipids. The mechanism of lipid loss depends on the relative
Keywords
Lipid loss; Hydrophobicity; Highly hydrophobic lipids; Surfactants
Article Details
1. Introduction
Surfactants play a significant role in maintaining cleanliness but destroy the lipid barrier of human tissues [1]. Destruction of the lipid barrier leads to problems such as loss of moisture, accelerating aging. Therefore, understanding the mechanism by which surfactants destroy the lipid barrier of tissues and establishing effective solutions to prevent this destruction become more significant. Hair can be a good model for understanding how lipids are lost from tissues by surfactants. Since hair contains various lipids [2], it is advantageous to identify the effects of surfactants on various lipid types. Additionally, by virtue of the well-known structure of hair [2], the interpretation of the location at which the surfactants act is possible. Furthermore, because hair is an inactive tissue, the lipid that is lost is not naturally generated from the inside but has to be supplied from the external scarp [3]. Thus, the loss of lipids can be clearly monitored when the supply of external sebum is blocked. The composition and location of hair lipids have been revealed by several groups [4-10]. However, no studies have been conducted to directly characterize the loss of hair lipids. To identify the characteristics of the loss of hair lipids, we have characterized lipid loss by surfactants could be identified. This article shows that the hydrophobicity of lipids distinguishes the mechanisms of lipid loss in response to surfactant treatment.
2. Materials and Methods
2.1 Formulation of shampoo
Surface and internal modifications were performed using a shampoo consisting of surfactant and a cationic conditioning polymer. The shampoos that were used in all tests were formulated as a mixture of ingredients in water. As a conditioning polymer, 0.5% PQ-10 (LR30M, Dow Chemical, US), a cationic cellulose polymer, was employed. The ingredients were generated by mixing the solid that needed to be melted with water at a mixing speed of up to 400 rpm. Other ingredients, including polylysine and polycarbodiimide polymers, were also added to the cooled product.
2.2 Extraction of lipids
The following procedure applies to the extraction of lipids from human hair. A 15 cm hair swatch was cut into lengths below 1 mm with a mass of 50 mg, and the hair residue was transferred into a 15 mL vial filled with 3 mL of following solvents. Lipid was extracted merely by isolation with the solvents of chloroform and methanol. The vial was tightly covered with a Teflon cap, and the vial was incubated at 80°C for 4 days.
2.3 Preparation of keratin-based film and measurement of contact angle
White hair was used to extract keratin protein because it is difficult to determine protein concentrations in black hair by optical transmission due to melanin (20). 2 g of white hair was thoroughly washed with 95% ethanol, and rinsed with clean water. The dried hair samples were cut into snippets of a few millimeters and then placed into 30 mL of an aqueous solution containing 25 mM Tris-HCl buffer (pH 9.0), 2.6 M thiourea, 5 M urea and 5% β-mercaptoethanol for 3 days. The solution was filtered with a vacuum pump, and the concentration determined by an optical spectrometer (Nanodrop2000, Thermo Scientific, US) was 170 mg mL-1. The protein solution was then diluted 1:3 in a solution containing 1 M NaCl, and 10 mM MgCl2. After gelation for 4 h, the supernatant was removed and the film was washed several times with pure water. Thereafter, the film was dried in the air. To adsorb a cationic polymer onto the keratin-based film, the film was placed in 2 mL of a cleansing solution for 5 min. The solution was removed and discarded. The film was thoroughly washed three times with 2 mL of pure water, and air-dried. The zeta potential of the film was tested using a Zeta-potential & Particle size Analyzer ELSZ-2000. Distilled water with a refractive index of 1.3328, viscosity of 0.8878 cP, and dielectric constant of 78.3 was used as a dispersive medium.
Lipid complexes were prepared by dissolving solid lipids in an aqueous lipid at 50°C and 150 rpm for 5 min with a single step addition of each lipid. Myristic acid, palmitic acid, stearic acid, and cholesterol (1:2:1:1, w/w) were mixed in oleic acid. Static contact angle measurements using a drop volume method were recorded and analyzed at room temperature by a Sanyo camera with FTA 32 (Ver 2.0). An acrylic cube filled with 0.36 mM SLES solution was set on a surface analyzer, DSA-100 (Krüss, Germany). The concentration of 0.36 mM was the estimated value derived from rinsing solutions after shampooing. The cube has hole at the bottom, and the hole is covered with a rubber stopper. A syringe filled with the lipid mixture penetrated the rubber stopper. The contact angle was measured after 30 s of lipid loading on the keratin film.
2.4 Fluorescence dye tagging
To identify the penetration of polymer for cross-section in hair, carboxytetramethylrhodamine (TAMRA) dye was added to a 5% aqueous solution of polylysine. Then, the TAMRA-labeled polylysine was purified by filtration and dialysis for 2 d. The purified TAMRA-labeled polylysine solution to be lyophilized was frozen in liquid nitrogen and applied to a Bondiro (IlshinBio, Korea) model 8 freeze-drier at a pressure of approximately 50 m Torr and a condenser temperature of -80°C. The lyophilization was performed for 1 week. Images were acquired using a fluorescence microscope (Leica DM1RB, Leica Microsystems, US). Sample preparation and cross-section of the hairs for fluorescence measurements were prepared in the same way for samples for Raman measurements. Small hair swatches consisting of 20 fibers of 5 cm length were soaked in an aqueous solution containing 5% TAMRA-labeled polylysine or an aqueous solution containing 5% TAMRA-labeled polylysine and 5% PCI for 24 h each. The fluorescence signals of hair fibers were observed at 594 nm after excitation at 490 nm.
3. Results and Discussion
Methylation is a general method to extract lipids from hair. When the extraction is performed after methylation, there is a limitation in that the amount of wax esters and triglycerides cannot be accurately analyzed because they are transformed into fatty acid methyl esters after methylation. Therefore, to investigate the behavior of lipids in response to surfactants in detail, the extraction of lipids without the methylation process was conducted, which resulted in the extraction of noncovalently bound lipids. During the washing, the surfactant can access the covalently bound lipids, but since the bond is not substantially broken by surfactants, the methylation process is not required to analyze the loss of lipids occurring by the washing [11].
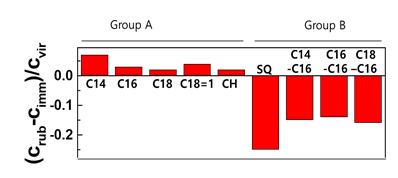
Hair swatches were immersed for various periods of time, and the other swatches were washed several times by the rubbing method so that the contact time with the surfactant was the same as the immersion time. Lipid loss by washing 10 times was conspicuously observed, as shown in Figure 1. Interestingly, the retained lipids in Group A and B are different. The concentrations by rubbing are higher than those in immersion for lipids in group A. This indicates that the immersion method for group B played the role of the extraction. Additionally, this suggests that the loss mechanism for two types of lipids are different.
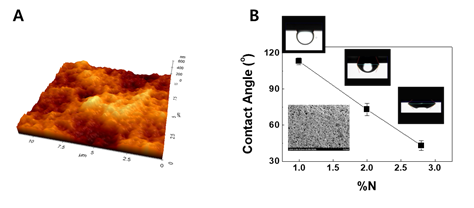
Surface coating decreases interfacial tension between hair surface and lipids in group A from hair [1]. To clarify interfacial behaviors of a lipid mixture consisting of group A lipids on hair, contact angles were measured in an SLES solution. Since the shape of hair is cylindrical, it is difficult to obtain an immobile droplet. A static contact angle on a flat surface was required, so a film composed of keratin proteins isolated from human hair was first fabricated. The fabricated film was flat enough to provide an immobile droplet. This was demonstrated by identifying a low root mean square (RMS) value of 40 nm from the AFM (atomic force microscope) image on square area of 5 × 5 μm. The keratin film treated with a shampoo containing a cationic polymer with 1.0% nitrogen content was measured, and a contact angle of 113o was obtained from the lipids. ζ-potential of the control keratin film coated with the cationic polymer having 1.0% nitrogen content was -11 mV, whereas that of film with PQ10B was 19 mV1. This increase in ζ-potential is in good agreement with the phenomenon occurred in real hairs.
As the percent of nitrogen content increases, the contact angle of lipid in group A reduces. As can be seen in Figure 2, the reduction in the contact angle on the positively charged keratin-based film means that the polymer for the surface modification blocks the roll-up mechanism. Although the retained lipids can be removed in repetitive wash, the hydrophilic nature will be able to retain some lipids in group A.
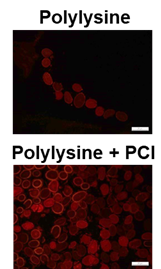
To fill polymer inside the hair, carbodiimide chemistry was performed with lysine and polycarbodiimide (PCI). The penetration of TAMRA-labeled polylysine into the hair was performed by the cross-sections in Figure 3. Tryptophan in an untreated control hair also can generate fluorescence at lower than 500 nn [12-13]. And we detected the emission at 594 nm. Thus, there was no fluorescence in the control hair. At the images in Figure 3, it is obvious that the TAMRA-labeled polylysine and PCI penetrated the hair, because the fluorescence intensity from the hair with polylysine and PCI was higher than that from the hair with polylysine only.
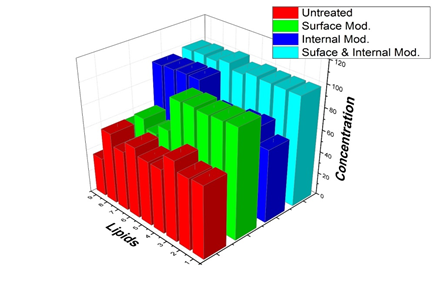
Finally, we washed hair modified by coating with lower interfacial tension (highly cationic charged film) as shown in Figure 2 and filling with a cross section as shown in Figure 3. The amounts of lipids after 10 times wash are shown in Figure 4. Compared to that in the control group, in the surface-modified group represented by green bars, most amounts of the components of group A (from 1 to 5), such as fatty acids and cholesterol, were prevented after washing with surfactants. Unlike the results of the components of group A, the surface modification did not significantly inhibit the loss of squalene and wax esters (group B; from 6 to 9). The losses of fatty acids and cholesterol (group A) were not significantly affected by whether the hair was internally modified. The lipids in group A were highly affected by surface modification, whereas the lipids in group B were influenced by internal modification as shown in blue bars. Finally, different types of lipids were successfully protected by surface and internal modifications from the surfactant treatment (cyan bars).
4. Conclusion
In this study, we used hair as a representative model of tissue and systematically demonstrated how lipids in tissues are lost by surfactant use. It has been demonstrated that the mechanism by which lipids are lost from tissues depends on the type of lipid. This scientific finding would be a good basis for maintaining the health of our external tissues, such as skin and hair.
Acknowledgments
The authors thank Dr. Jinhyun Kim and Dr. Kyungin Min for fluorescence measurement and fabrication of keratin film, respectively.
Competing Interests
All authors are employed by LG Household & Health Care, Ltd.
References
- Song SH, Lim JH, Son SK, et al. Prevention of lipid loss from hair by surface and internal modification. Scientific Reports 9 (2019): 9834.
- Robbins CR. Chemical and Physical Behavior of Human Hair. 5th edn. Springer: Heidelberg (2012).
- Scawartz JR, DeAngelis YM, Dawson T L, et al. Practical Modern Hair Science. Allured books: Carol Stream (2012).
- Masukawa Y, Narita H, Imokawa G. Characterization of the lipid composition at the proximal root regions of human hair J. Cosmet Sci 56 (2005): 1-16.
- Wu Y, Chen G, Ji C, et al. Gas chromatography-mass spectrometry and Raman imaging measurement of squalene content and distribution in human hair. Anal. Bioanal. Chem 408 (2016): 2357-2362.
- Masukawa Y, Tsujimura H, Imokawa G. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. Journal of Chromatography B 823 (2005): 131-142.
- Martí M, Barba C, Manich AM, et al. The influence of hair lipids in ethnic hair properties. Int. J. Cosmet. Sci 38 (2016): 77-84.
- Fitzgerald M, Murphy RC. Electrospray mass spectrometry of human hair wax esters. J. Lipid Res 48 (2007): 1231-1246.
- Takahashi T, Yoshida S. Distribution of glycolipid and unsaturated fatty acids in human hair. Lipids 49 (2014): 905-917.
- Masukawa Y, Tsujimura H, Narita H. Liquid chromatography-mass spectrometry for comprehensive profiling of ceramide molecules in human hair. J. Lipid Res 47 (2006): 1559-1571.
- Capablanca JS, Watt IC. Factors affecting the zeta potential at wool fiber surfaces. Textile Res. J 56 (1986): 49-55.
- Witt S, Wunder C, Paulke A, et al. Detection of oxidative hair treatment using fluorescence microscopy. Drug Test Anal 8 (2016) 826-831.
- Jachowicz J, McMullen R. Tryptophan fluorescence in hair-examination of contributing factors. J. Cosmet. Sci 62 (2011): 291-304.