Cholesterol Biosynthesis Inhibitor RO 48-8071 Suppresses Growth of Epithelial Ovarian Cancer Cells in Vitro and In Vivo
Article Information
Yayun Liang1,2, Kenneth P Nephew3, Salman M Hyder1,2*
1Dalton Cardiovascular Research Center, University of Missouri, Columbia 65211, United States
2Dept of Biomedical Sciences, University of Missouri, Columbia 65211, United States
3Indiana University School of Medicine, Bloomington, IN 47405, United States
*Corresponding Author: Dr. Salman M. Hyder, Dalton Cardiovascular Research Center, University of Missouri-Columbia, 134 Research Park Drive, Columbia, MO 65203, United States.
Received: 15 December 2022; Accepted: 30 December 2022; Published: 09 January 2023
Citation:
Yayun Liang, Kenneth P Nephew, Salman M Hyder. Cholesterol Biosynthesis Inhibitor RO 48-8071 Suppresses Growth of Epithelial Ovarian Cancer Cells in Vitro and In Vivo. Journal of Cancer Science and Clinical Therapeutics 7 (2023): 01-08.
Share at FacebookAbstract
Introduction: Epithelial Ovarian Cancer (EOC) cells express enzymes in the cholesterol biosynthetic pathway, making this pathway an attractive therapeutic target for controlling ovarian cancer. Potent small molecule inhibitors of one biosynthetic enzyme, Oxidosqualene Cyclase (OSC), have been identified, and RO 48-8071 (4′-[6-(allylmethylamino)hexyloxy]-4- bromo-2′-fluorobenzophenone fumarate) (RO), has emerged as a useful chemotherapeutic agent for breast and prostate cancer.
Methods: Cell viability assays were performed to determine effects of RO 48-8071 on growth of EOC cells. Aldehyde Dehydrogenase (ALDH) assay was conducted to determine the effects of drug on reducing stem cell like properties of EOC cells. Finally, xenograft studies were performed to assess the ability of RO 48-8071 to inhibit the growth of EOC cells in vivo.
Results: We found that short-term (24-48 h) administration of pharmacological doses of RO effectively reduced the viability of drugresistant EOC cells (SK-OV-3 and OVCAR-3), as determined with sulforhodamine B colorimetric assays. In 7-day assays, nanomolar concentrations of RO effectively inhibited the growth of EOC cells. RO also suppressed ALDH activity, a marker of stem cells. Importantly, RO significantly suppressed growth of xenografts derived from EOC cells when given to mice intraperitoneally (20-40 mg kg-1 day-1) for 27 days once tumors reached 100 mm3 (controls: 336 + 60 mm3; treated: 171 + 20 mm3) with no toxicity to the experimental animals. Mechanistically, RO induced apoptosis in tumor cells in vivo as shown with immunohistochemistry.
Conclusion: Cholesterol biosynthesis inhibitor RO 48-8071 is thus a novel and potent inhibitor of human EOC, including EOC stem cells.
Keywords
Ovarian cancer, Cholesterol biosynthesis, Growth inhibition, Xenografts
Ovarian cancer articles, Cholesterol biosynthesis articles, Growth inhibition articles, Xenografts articles
Ovarian cancer articles Ovarian cancer Research articles Ovarian cancer review articles Ovarian cancer PubMed articles Ovarian cancer PubMed Central articles Ovarian cancer 2023 articles Ovarian cancer 2024 articles Ovarian cancer Scopus articles Ovarian cancer impact factor journals Ovarian cancer Scopus journals Ovarian cancer PubMed journals Ovarian cancer medical journals Ovarian cancer free journals Ovarian cancer best journals Ovarian cancer top journals Ovarian cancer free medical journals Ovarian cancer famous journals Ovarian cancer Google Scholar indexed journals Cholesterol biosynthesis articles Cholesterol biosynthesis Research articles Cholesterol biosynthesis review articles Cholesterol biosynthesis PubMed articles Cholesterol biosynthesis PubMed Central articles Cholesterol biosynthesis 2023 articles Cholesterol biosynthesis 2024 articles Cholesterol biosynthesis Scopus articles Cholesterol biosynthesis impact factor journals Cholesterol biosynthesis Scopus journals Cholesterol biosynthesis PubMed journals Cholesterol biosynthesis medical journals Cholesterol biosynthesis free journals Cholesterol biosynthesis best journals Cholesterol biosynthesis top journals Cholesterol biosynthesis free medical journals Cholesterol biosynthesis famous journals Cholesterol biosynthesis Google Scholar indexed journals Growth inhibition articles Growth inhibition Research articles Growth inhibition review articles Growth inhibition PubMed articles Growth inhibition PubMed Central articles Growth inhibition 2023 articles Growth inhibition 2024 articles Growth inhibition Scopus articles Growth inhibition impact factor journals Growth inhibition Scopus journals Growth inhibition PubMed journals Growth inhibition medical journals Growth inhibition free journals Growth inhibition best journals Growth inhibition top journals Growth inhibition free medical journals Growth inhibition famous journals Growth inhibition Google Scholar indexed journals Xenografts articles Xenografts Research articles Xenografts review articles Xenografts PubMed articles Xenografts PubMed Central articles Xenografts 2023 articles Xenografts 2024 articles Xenografts Scopus articles Xenografts impact factor journals Xenografts Scopus journals Xenografts PubMed journals Xenografts medical journals Xenografts free journals Xenografts best journals Xenografts top journals Xenografts free medical journals Xenografts famous journals Xenografts Google Scholar indexed journals Aldehyde Dehydrogenase articles Aldehyde Dehydrogenase Research articles Aldehyde Dehydrogenase review articles Aldehyde Dehydrogenase PubMed articles Aldehyde Dehydrogenase PubMed Central articles Aldehyde Dehydrogenase 2023 articles Aldehyde Dehydrogenase 2024 articles Aldehyde Dehydrogenase Scopus articles Aldehyde Dehydrogenase impact factor journals Aldehyde Dehydrogenase Scopus journals Aldehyde Dehydrogenase PubMed journals Aldehyde Dehydrogenase medical journals Aldehyde Dehydrogenase free journals Aldehyde Dehydrogenase best journals Aldehyde Dehydrogenase top journals Aldehyde Dehydrogenase free medical journals Aldehyde Dehydrogenase famous journals Aldehyde Dehydrogenase Google Scholar indexed journals Epithelial Ovarian Cancer articles Epithelial Ovarian Cancer Research articles Epithelial Ovarian Cancer review articles Epithelial Ovarian Cancer PubMed articles Epithelial Ovarian Cancer PubMed Central articles Epithelial Ovarian Cancer 2023 articles Epithelial Ovarian Cancer 2024 articles Epithelial Ovarian Cancer Scopus articles Epithelial Ovarian Cancer impact factor journals Epithelial Ovarian Cancer Scopus journals Epithelial Ovarian Cancer PubMed journals Epithelial Ovarian Cancer medical journals Epithelial Ovarian Cancer free journals Epithelial Ovarian Cancer best journals Epithelial Ovarian Cancer top journals Epithelial Ovarian Cancer free medical journals Epithelial Ovarian Cancer famous journals Epithelial Ovarian Cancer Google Scholar indexed journals Oxidosqualene Cyclase articles Oxidosqualene Cyclase Research articles Oxidosqualene Cyclase review articles Oxidosqualene Cyclase PubMed articles Oxidosqualene Cyclase PubMed Central articles Oxidosqualene Cyclase 2023 articles Oxidosqualene Cyclase 2024 articles Oxidosqualene Cyclase Scopus articles Oxidosqualene Cyclase impact factor journals Oxidosqualene Cyclase Scopus journals Oxidosqualene Cyclase PubMed journals Oxidosqualene Cyclase medical journals Oxidosqualene Cyclase free journals Oxidosqualene Cyclase best journals Oxidosqualene Cyclase top journals Oxidosqualene Cyclase free medical journals Oxidosqualene Cyclase famous journals Oxidosqualene Cyclase Google Scholar indexed journals Phosphate-Buffered Saline articles Phosphate-Buffered Saline Research articles Phosphate-Buffered Saline review articles Phosphate-Buffered Saline PubMed articles Phosphate-Buffered Saline PubMed Central articles Phosphate-Buffered Saline 2023 articles Phosphate-Buffered Saline 2024 articles Phosphate-Buffered Saline Scopus articles Phosphate-Buffered Saline impact factor journals Phosphate-Buffered Saline Scopus journals Phosphate-Buffered Saline PubMed journals Phosphate-Buffered Saline medical journals Phosphate-Buffered Saline free journals Phosphate-Buffered Saline best journals Phosphate-Buffered Saline top journals Phosphate-Buffered Saline free medical journals Phosphate-Buffered Saline famous journals Phosphate-Buffered Saline Google Scholar indexed journals Sulforhodamine B articles Sulforhodamine B Research articles Sulforhodamine B review articles Sulforhodamine B PubMed articles Sulforhodamine B PubMed Central articles Sulforhodamine B 2023 articles Sulforhodamine B 2024 articles Sulforhodamine B Scopus articles Sulforhodamine B impact factor journals Sulforhodamine B Scopus journals Sulforhodamine B PubMed journals Sulforhodamine B medical journals Sulforhodamine B free journals Sulforhodamine B best journals Sulforhodamine B top journals Sulforhodamine B free medical journals Sulforhodamine B famous journals Sulforhodamine B Google Scholar indexed journals therapeutic target articles therapeutic target Research articles therapeutic target review articles therapeutic target PubMed articles therapeutic target PubMed Central articles therapeutic target 2023 articles therapeutic target 2024 articles therapeutic target Scopus articles therapeutic target impact factor journals therapeutic target Scopus journals therapeutic target PubMed journals therapeutic target medical journals therapeutic target free journals therapeutic target best journals therapeutic target top journals therapeutic target free medical journals therapeutic target famous journals therapeutic target Google Scholar indexed journals
Article Details
Abbreviations:
ALDH: Aldehyde Dehydrogenase; ANOVA: Analysis of Variance; DEAB: N,N-Diethylaminobenzaldehyde; EOC: Epithelial Ovarian Cancer; FACS: Fluorescence-Activated Cell Sorting; IOSE: Immortalized Ovarian Surface Cells; OSC: Oxidosqualene Cyclase; PBS: Phosphate-Buffered Saline; RO: RO 0488071 (4′-[6-(allylmethylamino)hexyloxy]-4-bromo-2′-fluorobenzophenone fumarate); SEM: Standard Error of the Mean; SRB: Sulforhodamine B; TUNEL: Terminal Deoxynucleotidyl Transferase Dutp Nick End Labeling.
1. Introduction
Epithelial ovarian cancer (EOC) is an aggressive and deadly disease and despite concerted efforts to develop new strategies for preventing and treating the disease, almost 25,000 new cases are reported each year in the United States [1]. Those afflicted with EOC have a poor prognosis due to drug resistance and metastasis, resulting in the highest mortality rate of all known gynecological malignancies [2-4]. EOC is often detected at an advanced stage, associated with multifocal intraperitoneal dissemination, and accompanied by intense neovascularization [3,4]. Patients usually die due to drug-resistant, meta-static disease [3-5]. Consequently, both treatment and prevention strategies for ovarian tumors are needed.
Cholesterol is an essential structural and functional component of cellular membranes and cellular metabolism and is vital for tumor growth [6]. Tumor cells reprogram cholesterol metabolism to their advantage for growth, and thus provide several targets that could be exploited to control cholesterol production and tumor growth [7,8]. Targeting cholesterol biosynthesis is an active area of cancer therapeutics [7-9]. Statins are used to inhibit HMG-CoA reductase, an enzyme that is essential for cholesterol biosynthesis, but statin therapy causes a number of undesirable side effects that can be attributed to reduced levels of isoprenoids, defective post-translational modification of membrane proteins, and impaired membrane structure and function. Thus, alternative approaches to inhibiting cholesterol biosynthesis are being considered. 2, 3-Oxidosqualene Cyclase (OSC), which converts 2, 3-monoepoxysqualene to lanosterol, a key step in the biosynthesis of cholesterol, has recently emerged as a possible new target for inhibiting the cholesterol biosynthetic pathway [10] and potent small molecule inhibitors of OSC have been identified. Because OSC functions downstream of HMG-CoA reductase during cholesterol biosynthesis, it is unlikely that its inhibition will cause the adverse effects associated with statins. Further-more, cholesterol is both a metabolic precursor for endogenous steroid hormones, including estradiol, and itself can be modified to 27-hydroxycholesterol to increase estrogen signaling in some tissues, including in a subset of EOC cells [11] and contribute to disease progression.
EOC cells express enzymes involved in the biosynthesis of endogenous cholesterol [12,13]. We hypothesized that inhibition of cholesterol synthesis may be an effective means by which to arrest EOC cell proliferation. In this study, we tested a potent OSC inhibitor, RO 48-8071 (RO) [10,14] to determine its effectiveness against EOC cell lines, con-ducted studies to ascertain the ability of RO to inhibit EOC stem-cell-like properties and investigated the in vivo anti-tumor effects of RO in mouse xenograft studies. We propose that RO alone or in combination with chemotherapeutic drugs may provide a novel and hitherto untested approach to combating the progression of human EOC.
2. Materials and Methods
2.1 Cell lines and cell culture
Epithelial ovarian cancer cell lines OVCAR-3 (high grade serous) and SK-OV-3 (non-high grade serous) were obtained from ATCC (Manassas, VA, USA). Human immortalized ovarian surface cells (IOSE) were acquired from Dr. K. Nephew, Indiana School of Medicine at Bloomington, IN, following previously described approaches [15]. All cells were grown in 100 mm tissue culture dishes and harvested with 0.05% trypsin-EDTA (Invitrogen Corporation & Life Technologies, Grand Island, NY, USA). Cell-specific media were obtained from invitrogen and noted in the Figure legends 1-6.
2.2 Cell viability assay by SRB
Sulforhodamine B (SRB) assays were used to measure cell viability, as previously de-scribed [16,17]. Briefly, cells were grown to 70% confluence and, harvested with 0.05% Trypsin-EDTA then seeded in 96-well plates in 100 µL 5% FBS (JRH Biosciences, Lenexa, KS, USA) growth medium and incubated overnight at 37oC with 5% CO2 (see specific details in the Figure legends 1-6). Cells were washed once with medium without FBS and incu-bated for 24 or 48 h in 100 µL growth medium containing 5% FBS in the presence of different concentrations of RO (Tocris BioScience, Bristol, United Kingdom; Catalogue number: 5389; soluble in water up to 20 mM). Surviving or adherent cells were fixed in situ by adding 100 µL 50% cold trichloroacetic acid for 1 h at 4oC. Cells were washed with ice water, dried, and stained with 50 µL 4% SRB (Sigma, St. Louis, MO, USA) for 8 min at room temperature. Unbound stain was removed by washing cells 5× with cold 1% acetic acid, after which the cells were dried at room temperature. Cell-bound stain was solubilized in 150 µL 10 mM Tris buffer and quantified at 520 nm using a SpecTRA MAX 190 microplate reader (Sunnyvale, CA, USA). Six wells were used for each dose/experimental condition, and each experiment was performed at least twice.
2.3 FACS analysis for ALDH activity
EOC cells were washed once with Phosphate-Buffered Saline (PBS; Invitrogen) and harvested using Accutase (BD Biosciences, Franklin Lakes, NJ, USA). Aldehyde Dehydrogenase (ALDH) activity was measured using an AldefluorTM kit (STEMCELL Technologies, Vancouver, BC, Canada) as described previously [18,19], and Fluorescence-Activated Cell Sorting (FACS) was performed per the manufacturer’s protocol. The ALDH inhibitor N,N-Diethylaminobenzaldehyde (DEAB) was added to the cultures as a negative control.
2.4 Animal xenograft tumor studies in nude mice
Female athymic nu/nu nude mice, 5-6 weeks old (18-22 g) were purchased from Envigo Inc. (Indianapolis, IN, USA). The mice were housed in a laminar air-flow cabinet under specific-pathogen-free conditions. All facilities were approved by the American Association for Accreditation of Laboratory Animal Care in accordance with the current regulations and standards of the United States Department of Agriculture, the Department of Health and Human Services, and the NIH. All procedures were approved by the institutional animal care and use committee.
Cells were harvested by trypsinization, washed twice with DMEM/F12, and re-suspended in 150 µL DMEM/F12 medium mixed with Matrigel (50% v/v; BD Biosciences, Bedford, MA, USA). EOC cells (SK-OV-3; 6 × 106) were injected subcutaneously into each flank of experimental mice (n = 7-8). Tumors were measured every 3 days with a digital caliper, and tumor volumes were calculated using the following formula: (L × W × H) × p/6) [20].
2.5 RO treatment of xenografts
Animals were randomly assigned to three groups of 7-8 mice each. Once the tumors reached approximately 150 mm3, animals were treated with RO (20 or 40 mg kg-1 day-1 ip), and the third group was injected with PBS, as a vehicle control as described previously [20]. A total of 27 treatments were given during treatment. Animals were weighed throughout the experiment, and no apparent toxicity was observed based on changes in body weight. Experiments were terminated by sacrificing the animals after the last injection.
2.6 TUNEL assay
The TUNEL assay and quantification was performed as described previously [20]. Briefly, an apoptotic index was calculated from tumor sections by analyzing six sections each from approximately 4-5 tumors/group (for a total of 24–30 sections/group) and approximately 300-500 cells from each section were analyzed. Tumors were taken from different animals. Necrotic areas were avoided to reduce errors in the results.
2.7 Statistical analysis
Statistical significance was measured by using Student's t-test or one-way analysis of variance (ANOVA) with repeated measures over time. When necessary, it was assumed that ANOVA was non-parametric. Values are reported as mean ± Standard Error of the Mean (SEM). For samples with a significant F-ratio (p < 0.05), the Student-Newman-Keuls multirange test was employed (SigmaStat).
3. Results
3.1 RO suppresses growth of epithelial ovarian cancer cells at pharmacological and nanomolar levels in vitro
A pharmacological dose of RO reduced the viability of both OVCAR-3 and SK-OV-3 EOC cell lines (Figure 1A). The IC50 values for inhibition of cell viability were 20.5 + 0.3 µM and 18.3 + 0.6 µM for OVCAR-3 and SK-OV-3 cells respectively, in a 24-h SRB assay; IC50 values were reduced to 11.3 + 0.3 µM and 12.7 + 0.5 µM in a 48-h SRB assay (Table 1). We also determined whether lower concentrations of RO given over a longer period might be effective. Concentrations as low as 1 nM effectively reduced cell viability in a 7-day SRB assay (Figure 2), indicating that clinically useful levels of RO could be achieved over an extended time frame to reduce the growth of ovarian cancer cells that are generally resistant to some commonly used chemotherapeutic drugs.
|
Drug |
Cell lines |
IC50 (µM) (24 hours) |
IC50 (µM) (48 hours) |
|
Ro 48-8071 |
OV-CAR-3 |
20.51 + 0.33 |
11.29 + 0.33 |
|
SK-OV-3 |
18.28 + 0.64 |
12.72 + 0.52 |
Table 1: IC50 values for inhibition of cell viability.
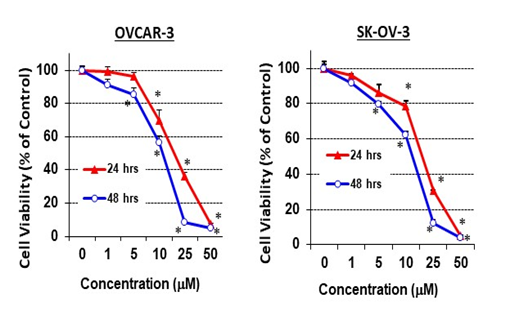
Figure 1: RO inhibits ovarian cancer cell growth in vitro.
Ovarian cancer cells (OVCAR-3 or SK-OV-3, 6 × 103/well) were seeded in 96-well plates (6 wells per concentration used) and incubated with different concentrations of RO 48-8071 for 24 or 48 h in RPMI-1640 medium + 10% FBS (OVCAR-3) or in McCoy’s 5a medium + 5% FBS (SK-OV-3). Control cells (0 µM) were incubated with vehicle (same medium + PBS). Cell viability was determined with an SRB assay. Values represent means ± SEM (n = 6). * Significant difference compared with the control group (set at 100%) (p < 0.05 using ANOVA).
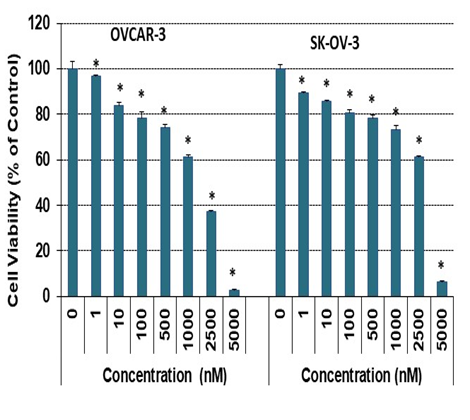
Figure 2: Low-dose RO inhibits ovarian cancer cell growth in vitro.
OVCAR-3 ovarian cancer cells (8 × 104/well) were seeded into 6-well plates in 10% FBS RPMI-1640 medium. SK-OV-3 cells (5.5 × 104/well) were seeded into 6-well plates in 5% FBS McCoy’s 5a medium. Cells were treated with the indicated concentration of RO 48-8071 for 7 days. Cells were then re-treated with the same concentration of RO 48-8071 every other day. Control cells were incubated with vehicle (same medium + PBS). Cell viability was determined by SRB assay as described in the Methods. Three or four wells were used for each concentration, and each experiment was performed twice. * Significant difference compared with the control group (0 nM). (p < 0.05 using ANOVA).
3.2 RO does not arrest growth of normal ovarian cells
In order to determine whether RO might be toxic to non-cancerous cells, we exposed normal IOSE ovarian cells to RO and compared its effects with those on SK-OV-3 ovarian cancer cells. Concentrations of RO up to 10 µM had no effect on the viability of normal IOSE cells, as determined by ANOVA (Figure 3). However, RO did affect tumor cells, 30% of which were not viable in the presence of 10 µM RO.
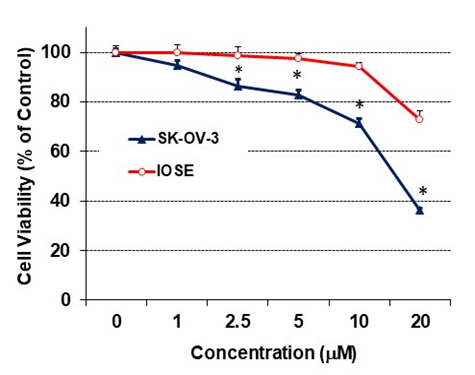
Figure 3: The effect of RO on growth of normal ovarian cells.
Normal ovarian IOSE cells (5 × 103/well) were seeded into 96-well plates and grown in NOSE medium (medium 199 and medium 105, 1:1, supplemented with 10% FBS, 10 µg/mL EGF, 200 µg/mL hydrocortisone, and 100 × penicillin-streptomycin-glutamine) overnight. The next day, the cells were washed and treated with the indicated concentration of RO 48-8071 in 5% NOSE medium for 24 hours. Control cells (concentration of 0 µM) were incubated with vehicle (same medium + PBS). Cell viability was determined with an SRB assay and each bar represents values derived from 6 wells for each concentration and controls; values represent means ± SEM (n = 6). Experiment was performed twice. *Significant difference between IOSE and SKOV-3 cells at each concentration (p < 0.05 using t-test).
3.3 RO reduces stem-cell-like properties of ovarian cancer cells
Many ovarian cancer cells display stem-cell-like properties, including high levels of the enzyme ALDH [21]. We used FACS to determine whether RO inhibits ALDH activity in SK-OV-3 cells. Cells treated overnight with 10 or 20 µM RO had substantially less ALDH than cells treated with PBS alone (controls) (Figure 4). As expected, cells treated with DEAB showed lower levels of ALDH positivity in the controls and no change when exposed to either concentration of RO.
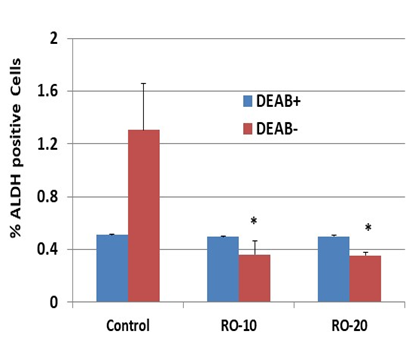
Figure 4: RO inhibits ALDH expression in SK-OV-3 ovarian cancer cells.
An Aldefluor kit was used for identification of cancer stem cells, which express high levels of ALDH. SK-OV-3 cells were grown in 100-mm culture dishes and treated with the indicated concentrations of RO 48-8071 (10 µM or 20 µM in 5% FBS McCoy’s 5a medium) for 24 hours (n=3 dishes per RO concentration). Cells (2.5 × 104/sample) were mixed with BAAA (from the Aldefluor kit) with or without DEAB (an inhibitor of ALDH) and incubated for 40 minutes before being suspended in the Aldefluor assay buffer. Fluorescence was measured using a FACSCalibur and analyzed with Summit V5.2.0.7477 software. The ALDH+ subpopulation was obtained in the absence of DEAB. The values represent % of ALDH-positive cells ± SEM. *Significant difference compared with the control DEAB– group (p < 0.05 using ANOVA).
3.4 RO suppresses growth of human epithelial ovarian cancer xenografts in vivo
We next determined whether RO suppresses the growth of EOC cells in vivo. SK-OV-3 cells were injected subcutaneously into both flanks of nude mice, which were subsequently treated daily with PBS (control) or 20 or 40 mg/kg RO ip for 27 days. RO significantly suppressed the growth of the tumor xenografts derived from SK-OV-3 ovarian cancer cells (171 ± 20 mm3 versus 336 ± 60 mm3 for the control after 27 days; p < 0.05, ANOVA; Figure 5A). The animals showed no significant weight loss during the treatment protocol (Figure 5B). Examples of tumors in control and treatment groups are shown in Figure 5C. RO similarly suppressed growth of OVCAR-3 cells in vivo (data not shown).
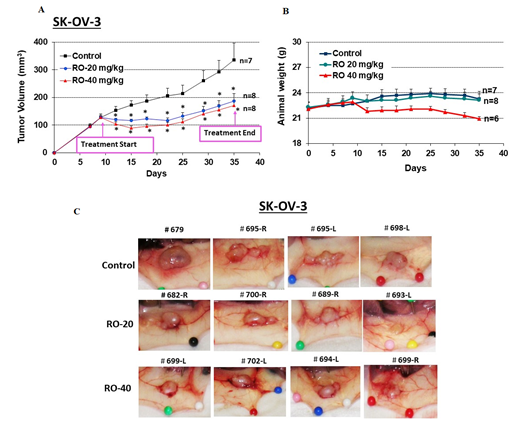
Figure 5: RO inhibits SK-OV-3 tumor xenograft growth in vivo.
SK-OV-3 ovarian cancer cells (6 × 106) in 0.15 mL of Matrigel:McCoy’s 5a medium (4:1 v/v) were injected into each flank of nude mice (two sites per mouse). When tumor volumes reached approximately 150 mm3, animals were assigned to one of three groups and injected ip daily with vehicle (PBS), RO 20 mg/kg, or RO 40 mg/kg for 27 days. (A) Tumor volumes were monitored throughout the experiment. Values represent mean ± SEM (n = 7–8 animals/group). * Significant difference compared with the control group (p < 0.05 using ANOVA). (B) Animal weight was monitored throughout the experiment. Values represent means ± SEM (n = 7–8 animals). There were no significant differences among control and treatment groups in B. (C) Representative tumors shown in each group.
3.5 RO induces apoptosis in epithelial ovarian cancer cells in vivo
To investigate the mechanism of tumor suppression, we performed TUNEL assays in tumor sections to assess apoptosis. RO induced apoptosis in EOC cells in vivo (Figure 6) which likely reduced tumor volumes. Several areas of extensive necrosis in the tumors treated with 40 mg/kg RO were not included in the analysis to avoid errors in counting apoptotic cells. While 40 mg/kg treatment led to the detection of more apoptotic cells in tumors compared with 20 mg/kg treatment, this was not reflected in any differences in tumor volume. It is our contention that this could be due to the time of tissue collection in this experiment, which did not allow for tumor volumes to shrink even though treatment with 40 mg/kg RO initiated far greater levels of apoptosis compared with the lower treatment. Little tumor volume change was seen in Figure 5C, which represents tumors in situ.
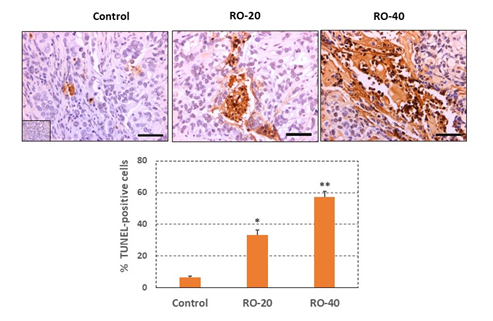
Figure 6: RO induces apoptosis in SK-OV-3 tumor cells and cell death in vivo.
Tumors were collected at the end of the experiment shown in Figure 5 and processed for immunohistochemistry to measure the TUNEL signal as described in the Methods. The upper panel shows the TUNEL signal in representative sections taken from tumors. The mean % TUNEL-positive cells per group are shown in the lower panel (bar graph). Values represent mean ± SEM of TUNEL-positive cells in 24-30 tumor sections/group in tumors from 4-5 individual tumors taken from different animals. Approximately 300-500 cells were analyzed per section. Inset represents negative staining control. Bars in the sections represent 50 mm. *Significant difference compared with the control group, p < 0.05; ** Significant difference compared with the Control and RO-20 groups, p< 0.05.
4. Discussion
EOC is an extremely aggressive form of cancer which often has a poor prognosis due to drug resistance and metastasis [2,3,22]. It is now well established that cholesterol bio-synthesis plays a major role in the growth of many different types of tumors, providing various biosynthetic enzymes that could be targeted pharmaceutically to suppress the production of cholesterol and hence reduce tumor growth [7-9]. The enzyme 2,3-Oxidosqualene Cyclase (OSC) has recently emerged as a possible new target for inhibiting this pathway [7/10], as it performs a key step in the cholesterol biosynthetic pathway, by converting 2, 3-monoepoxysqualene to lanosterol. While many studies have concentrated on the role of statins, which control HMG CoA reductase, the rate limiting enzyme in cholesterol biosynthesis, we examined whether targeting OSC might be even more beneficial.
In the studies reported herein, we observed that an inhibitor of OSC, RO, potently suppressed the growth of EOC cells, both in vitro and in vivo. Initially we studied the effects of RO in vitro on two aggressive EOC cell lines, high grade serous OVCAR-3 and non-high grade serous SK-OV-3, and established that the inhibitor reduced the viability of both cell lines. Although the SRB procedure used was designed to determine cell viability [16,17], it is our contention that RO was killing the cells directly, as also noted later in the in vivo studies. The rapid suppression of tumor cells in response to treatment with RO supports the notion that RO does indeed destroy tumor cells, though this needs to be confirmed in vitro. Inhibition occurred when cells were exposed to pharmacological levels of the drug, and also when a more therapeutically relevant dose in the nanomolar range was used in long-term assays.
Based on our observations, we could foresee the potential clinical infusion of low levels of RO to OC patients, though further preclinical testing is clearly necessary to ensure there are no toxic effects of the drug. It is encouraging to note that RO at concentrations up to 10 nM did not affect normal ovarian cells that were tested in the same cell viability assay, suggesting that it may be non-toxic when administered clinically at low doses in the nM range, which were also found to be effective over a longer time in vitro. Animals treated with RO did not exhibit changes in weight, further supporting the view that RO has no significant toxic side-effects in vivo. These results are encouraging, and we are optimistic that the anti-tumor properties of RO might be harnessed to treat EOC.
Because EOC cells are enriched with stem cells [23,24] we determined whether RO might also suppress stem cell activity. The cholesterol biosynthesis inhibitor potently suppressed ALDH activity, a marker of stem cells, in SK-OV-3 cells [25]. This suggests that treatment of existing tumors with RO could reduce the number of stem cells and thereby prevent tumor re-emergence, though this needs further confirmatory studies involving several tumor cell-lines, both in vitro and in vivo. The mechanism by which RO affects stem-cell markers is unclear and requires further study.
We extended our studies to the in vivo situation by examining the ability of RO to suppress subcutaneous tumors derived from SK-OV-3 EOC cells in a nude mouse xenograft model. Following injection of SK-OV-3 cells into both flanks, animals were given either 20 or 40 mg/kg RO for 27 days. Both doses effectively suppressed tumor growth, and tumor size remained static throughout the treatment timeframe due to tumor cells under-going apoptosis. While 40 mg/kg RO induced greater levels of apoptosis, tumor volumes in the two treatment groups remained the same, indicating that perhaps there was insufficient time for tumors to shrink in the 40 mg/kg dose group. It also appeared that following the initial response to RO, tumors became resistant and began to grow again. This suggests that combination therapy using RO in tandem with additional chemotherapeutic agents might be necessary to control progression of ovarian cancer cells in vivo.
Mechanistically, our data showed that RO induced apoptosis of ovarian tumor cells in vivo, as it does with breast and prostate tumor cells [26-28] although its mechanism of action as an anti-tumor agent, as well as its ability to suppress cholesterol biosynthesis in ovarian cancer cells requires further investigation. It also remains to be determined whether RO can potentially function as a preventive agent by reducing cholesterol synthesis in the early phases of tumor development, if the disease is detected earlier, which could result in the suppression of tumor growth beyond a very small volume.
5. Conclusions
In conclusion, our data show that the cholesterol biosynthesis inhibitor RO effectively suppresses the growth of both high grade and non-high grade EOC cells, including EOC with stem-cell like properties, and induces apoptosis in vivo. As reported previously [29] RO may also function as an anti-angiogenic agent, while also degrading steroid receptors that could be involved in EOC cell growth [26,27]. However, it appears that following treatment, some tumors begin to develop resistance to RO, as shown by increasing tumor volumes in the later stages of treatment, suggesting that combination therapy using RO together with existing or new therapeutic compounds might more effectively control this deadly disease.
Author Contributions
SMH conceived the project and SMH, YL and KPN designed the experiments. SMH and YL supervised the study. YL was involved in acquisition of most of the data and all authors were involved in its analysis. KPN provided essential material for some experiments. All authors were involved in writing and approving the final draft.
Funding
This research was supported by an Ovarian Cancer Alliance of Greater Cincinnati (to KPN) and through the IU Simon Comprehensive Cancer Center P30 Support Grant (P30CA082709-20) (KPN) and a Faculty COR award from the College of Veterinary Medicine, University of Missouri (SM and YL). SMH is the Zalk Missouri Professor of Tumor Angiogenesis.
Acknowledgments
This research was supported by an Ovarian Cancer Alliance of Greater Cincinnati (to KPN) and through the IU Simon Comprehensive Cancer Center P30 Support Grant (P30CA082709-20) (KPN) and a Faculty COR award from the College of Veterinary Medicine, University of Missouri (SM and YL). SMH is the Zalk Missouri Professor of Tumor Angiogenesis.
Institutional Review Board Statement
All facilities were approved by the American Association for Accreditation of Laboratory Animal Care in accordance with the current regulations and standards of the United States Department of Agriculture, the Department of Health and Human Services, and the NIH. All procedures were approved by the institutional animal care and use committee. Approval protocol was #9111.
Data Availability Statement
All datasets presented in this study are included in the article. Any other information can be obtained from authors on request.
Conflicts of Interest
:
The authors declare that they have no competing interests in this work.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 70 (2020): 7-30.
- Momenimovahed Z, Tiznobaik A, Taheri S, et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 11 (2019): 287-299.
- Yeung TL, Leung CS, Yip KP, et al. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol 309 (2015): C444-C456.
- Vaughan S, Coward JI, Bast RC Jr, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11 (2011): 719-725.
- Yousefi M, Dehghani S, Nosrati R, et al. Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles. Cell Oncol (Dordr) 43 (2020): 515-538.
- Kuzu OF, Noory MA, Robertson GP. The Role of Cholesterol in Cancer. Cancer Res 76 ( 2016): 2063-2070.
- Giacomini I, Gianfanti F, Desbats MA, et al. Cholesterol Metabolic Reprogramming in Cancer and Its harmacological Modulation as Therapeutic Strategy. Front Oncol 11 (2021): 682911.
- Yang J, Wang L, Jia R. Role of de novo cholesterol synthesis enzymes in cancer. J Cancer 11 (2020): 1761-1767.
- He J, Siu MKY, Ngan HYS, et al. Aberrant Cholesterol Metabolism in Ovarian Cancer: Identification of Novel Therapeutic Targets. Front Oncol 11 (2021): 738177.
- Grinter SZ, Liang Y, Huang S-Y, Hyder SM, Zou X. An inverse docking approach for identifying new potential anti-cancer targets. Molecular Graphics and Modelling 29(2011): 795-799.
- He S, Ma L, Baek AE, et al. Host CYP27A1 expression is essential for ovarian cancer progression. Endocr Relat Cancer 26 (2019): 659-675.
- Zhao J, Zhang X, Gao T, et al. SIK2 enhances synthesis of fatty acid and cholesterol in ovarian cancer cells and tumor growth through PI3K/Akt signalling pathway. Cell Death Dis (2020): 11:25.
- Zhao S, Cheng L, Shi Y, et al. MIEF2 reprograms lipid metabolism to drive progression of ovarian cancer through ROS/AKT/mTOR signaling pathway. Cell Death Dis 12 (2021): 18.
- Thoma R, Schulz-Gasch T, D'Arcy B, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 432 (2004): 118-122.
- Ahluwalia A, Hurteau JA, Bigsby RM, et al. DNA methylation in ovarian cancer II: expression of DNA methyltransferases in ovarian cancer cell lines and normal ovarian epithelial cells. Gynecol Oncol 82 (2001): 299-304.
- Rubinstein LV, Shoemaker RH, Paull KD, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst 82 (1990): 1113-1118.
- Skehan P, Storeng R, Scudiero D, et al. New Colorimetric cytotoxicity assay for anti-cancer-drug screening. J Natl Cancer Inst 82 (1990): 1107-1112.
- Cook MT, Liang Y, Besch-Williford C, et al. Luteolin Inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 4 (2015): 444.
- Goyette S, Liang Y, Mafuvadze B, et al. Natural and synthetic progestins enrich cancer stem cell-like cells in hormone-responsive human breast cancer cell populations in vitro. Breast Cancer (Dove Med Press) 9 (2017): 347-357.
- Liang Y, Mafuvadze B, Besch-Williford C, et al. A combination of p53-activating APR-246 and phosphatidylserine-targeting antibody potently inhibits tumor development in hormone- dependent mutant p53-expressing breast cancer xenografts. Breast Cancer (Dove Med Press) 10 (2018): 53-67.
- Mizuno T, Suzuki N, Makino H, et al. Cancer stem-like cells of ovarian clear cell carcinoma are enriched in the ALDH-high population associated with an accelerated scavenging system in reactive oxygen species. Gynecol Oncol 137 (2015): 299-305.
- Motohara T, Masuda K, Morotti M. et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 38 (2019): 2885-2898.
- Matei D, Nephew KP. Epigenetic Attire in Ovarian Cancer: The Emperor's New Clothes. Cancer Res 80 (2020): 3775-3785.
- Song Y, Pan S, Li K, et al. Insight into the role of multiple Signaling pathways in regulating cancer stem cells of gynecologic cancers. Semin Cancer Biol 85 (2022): 219-233.
- Silva IA, Bai S, McLean K, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res 71 (2011): 3991-4001.
- Liang Y, Besch-Williford C, Aebi JD, et al. Cholesterol biosynthesis inhibitors as potent novel anti-cancer agents: suppression of hormone-dependent breast cancer by the oxidosqualene cyclase inhibitor RO 48-8071. Breast Cancer Res Treat 146 (2014): 51-62.
- Liang Y, Mafuvadze B, Aebi JD, et al. Cholesterol biosynthesis inhibitor RO 48-8071 suppresses growth of hormone-dependent and castration-resistant prostate cancer cells. OncoTargets and Therapy 9 (2016): 3223-3232.
- Liang Y, Zou X, Hyder SM. Cholesterol biosynthesis inhibitor RO 48-8071 inhibits viability of aggressive cancer cells. Cancer Rep Rev 4 (2020): 1-4.
- Maione F, Oliaro-Bosso S, Meda C, et al. The cholesterol biosynthesis enzyme oxidosqualene cyclase is a new target to impair tumour angiogenesis and metastasis dissemination. Scientific Reports 5 (2015): 9054.
