Characterization of Humoral Response after COVID-19 Infection in an Unvaccinated Cohort
Article Information
Maria Martínez-Serrano1,2*, Nuria Tormo-Palop1,2, David Navalpotro-Rodríguez1,2, Marta Moreno-Córdoba1, Roberto Olmos-Arenas1, Concepción Gimeno-Cardona1,3
1Department of Microbiology, Consorcio Hospital General Universitario, Av. Tres Cruces s/n, 46014, Valencia, Spain
2These authors contributed equally to this article and share first authorship.
3University of Medicine, Av. Blasco Ibáñez 15, 46010, Valencia, Spain
*Corresponding author: Maria Martínez-Serrano, Department of Microbiology, Consorcio Hospital General Universitario, Av. Tres Cruces s/n, 46014, Valencia, Spain
Received: 15 November 2021; Accepted: 22 November 2021; Published: 09 December 2021
Citation: Martínez-Serrano M, Tormo-Palop N, Navalpotro-Rodríguez D, Moreno-Córdoba M, Olmos-Arenas O, Gimeno-Cardona C. Characterization of Humoral Response after COVID-19 Infection in an Unvaccinated Cohort. Archives of Clinical and Biomedical Research 5 (2021): 959-971.
Share at FacebookAbstract
Coronavirus disease 2019 (COVID-19), caused by a novel Sarbecovirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide since December 2019. In our region, cases were initially reported in March 2020. Nine months after COVID-19 infection, we evaluated the serostatus of a cohort of 77 patients. Anti-spike IgG (Euroimmun), both qualitative and quantitative tests, anti-nucleocapsid IgG and anti-spike IgM (Abbott), total nucleocapsid antibodies (Roche), and neutralizing antibodies (DIA.PRO) were analyzed. Anti-spike IgG was detectable in 74% of the subjects and anti-nucleocapsid IgG in 45.5%. The total anti-nucleocapsid antibodies assay was positive for all individuals. Noticeably, the percentage of subjects with detectable IgG(S) and IgG(N) was significantly higher in the age-group over 60 years than in the <60 years group (82% and 60% vs. 59.3% and 18.5%). Median quantitative IgG(S) values were also statistically different: 34.4 RU/mL for the elder group vs 9.5 RU/mL for the younger. We found 76.6% of subjects to be seropositive for neutralizing antibodies. A strong correlation was found between IgG(S) and neutralization assays. These data demonstrate that long-lasting and neutralizing humoral response can be detected in SARS-CoV-2 recovered patients as long as 9 months after infection. Quantitative assays are useful tools in order to measure and compare humoral responses. Now that vaccination programmes are largely stablished all over the country, humoral response after natural infection in naïve population could hardly be studied again in the future.
Keywords
Antibody detection; COVID-19 diagnosis; Nucleocapsid protein; SARS-CoV-2; Spike protein
Antibody detection articles; COVID-19 diagnosis articles; Nucleocapsid protein articles; SARS-CoV-2 articles; Spike protein articles
Article Details
1. Introduction
The emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus infectious disease 2019 (COVID-19), has spread worldwide since December 2019. The first cases were detected in Wuhan, China, and the WHO declared the disease a pandemic on March 2020. By October 2021, over 234 million cases have been reported and 4.8 million patients have died [1]. RNA detection by real time PCR in nasal/throat swabs, or other respiratory tract samples, is the gold standard diagnosis for COVID-19. Serological testing is a complementary tool for diagnosis. It has a key role in seroprevalence studies, contact tracing strategies, plasma donors search, and vaccines investigation. A wide range of serological assays was commercialized in a short period to detect IgG, IgM, IgA, or total SARS-CoV-2 antibodies. The Nucleocapsid (N) and the Spike (S) proteins showed great immunogenicity for the previous coronavirus and, therefore, they have been used as main target antigens. This has led to a great number of reports on COVID-19 serology but with considerable differences in terms of study design. Particularly, multitude of commercialized or existing in-house tests, antigenic protein used and various immunoglobulins detected, making it difficult to correctly understanding COVID-19 serology. In general, detection is weak during the first week after the onset of symptoms, but sensitivity improves from day eight onwards. IgA and IgM against SARS-CoV-2 spike and IgM against SARS-CoV-2 nucleocapsid were found to be the earliest markers of acute infection as they could be detected as early as 4 days after the onset of symptoms [2-5]. In the IgG assays, on the other hand, an early antibody response against nucleocapsid protein has been observed which would make IgG(N)-based assays better for early COVID-19 detection in the acute phase of the disease and IgG(S)-based assays for the late phase [6-9]. The first reinfection cases raised concerns about the humoral response duration and protection after natural infection. Long durability of humoral response was reported in some studies when sufficient time had passed since the first pandemic wave [10]. Soon after, a new scenario in COVID-19 serology began after vaccination became widespread. Characterization of natural infection response in unvaccinated population has been therefore limited to that gap of time. In this cross-sectional study we analyzed SARS-CoV-2 antibody detection results obtained with qualitative (anti-spike IgG and IgM, anti-nucleoprotein IgG and total antibodies), quantitative IgG(S), and a neutralization assay, to assess humoral response 9 months after COVID-19 infection in a high risk cohort. Test performance differences and correlation between assays were also investigated.
2. Materials and Methods
Serum samples included in the studyA. total of 77 serum specimens from 77 COVID-19 patients drawn nine months after COVID-19 infection were included. All subjects belonged to a nursing home in Valencia (Spain) and were confirmed to be infected by SARS-CoV-2 by at least one positive RNA RT-PCR result in March, 2020. Residents and staff members were included. Minimal demographic or clinical data were recorded as this was not the objective of the study. Consent to participate in the study was obtained for all individuals.
Serum Specimens Collected for Specificity Studies. 60 healthy adult serum samples collected in March, 2018, and stored at -80°C, supposed to be SARS-CoV-2 negative, were tested to estimate assays specificities.
Assays
Euroimmun Anti-SARS-CoV-2 IgG and Quanti-Vac IgG ELISA (Lübeck, Germany). The Euroimmun ELISA assays are based on a recombinant S1 domain of the SARS-CoV-2 spike protein. For the qualitative assay, index values (signal to cut-off [S/Co] ratios) of <0.8, ≥0.8 to <1.1, and ≥1.1 are interpreted as negative, borderline, and positive result, respectively. For Euroimmun Quanti-Vac IgG ELISA values are expressed in relative units per milliliter (RU/mL). Values of <8, ≥8 to <11, and ≥11 are interpreted as negative, borderline, and positive result, respectively.
Abbott Laboratories SARS-CoV-2 IgG and IgM Chemiluminescent Microparticle Immunoassay (CMIA; Abbott Park, IL). The Abbott Laboratories SARS-CoV-2 IgG (nucleocapsid protein) and IgM (spike protein) assays are a two-step qualitative CMIA performed on the ARCHITECT i2000SR automated immunoassayanalyzer. The patient sample signal is divided by the calibrator signal, with calculated signal to cut-off (S/C) values of <1.4 and ≥1.4 reported as negative and positive, respectively.
Elecsys Anti-SARS-CoV-2 (Roche Diagnostics GmbH). Elecsys Anti-SARS-CoV-2 is an electrochemiluminescence immunoassay (ECLIA) for the in vitro qualitative detection of total antibodies (including IgG) against SARS-CoV-2. It uses a recombinant protein representing the nucleocapsid (N) antigen and is intended for use on Cobas eimmunoassay analyzer. The result for a sample is given in the form of a cut-off index (signal sample/cut-off) and results are given either as reactive (COI ≥ 1.0) or non-reactive (COI < 1.0).
ACE2-RBD Neutralization Assay (Diagnostic Bioprobes Srl). The ACE2-RBD neutralization assay is an ELISA for the semi-quantitative determination of inhibition activity of RBD-ACE2 binding induced by SARS-CoV-2 antibodies. As a screening assay samples are diluted 2:3. Serial dilutions can be analyzed to give a titer value of neutralization. Results are interpreted as ratio of the Cut-Off value (Co) and of the sample OD450nm/620-630nm (S), or Co/S, and given as negative (<1) or positive (³1) for neutralizing antibodies. A value of 10 has been calculated from comparison studies to provide a correlation to the “in vivo” neutralization assay (VNT) with an acceptable approximation, despite the two systems are quite different (ELISA titer 1:4 ~ VNT titer 1:40).
Testing, for all assays, was performed according to manufacturer's instructions.
Statistical analysis. Differences between groups were calculated using SPSS 26 software. Figures were created with Statgraphics Centurion 17. A p-value <0.05 was considered statistically significant.
3. Results
Specificities for all assays were 100%. Cohort patients were mainly women (87%) with a median age of 75 years-old (range 19-95). Results obtained for all individuals, and divided by age groups of less than vs. equal to or more than 60 years old are shown in Table 1. SARS-CoV-2 IgG(S) was the most prevalent immunoglobulin, and it was detected in a higher proportion (74%) than IgG(N) (45.5%).
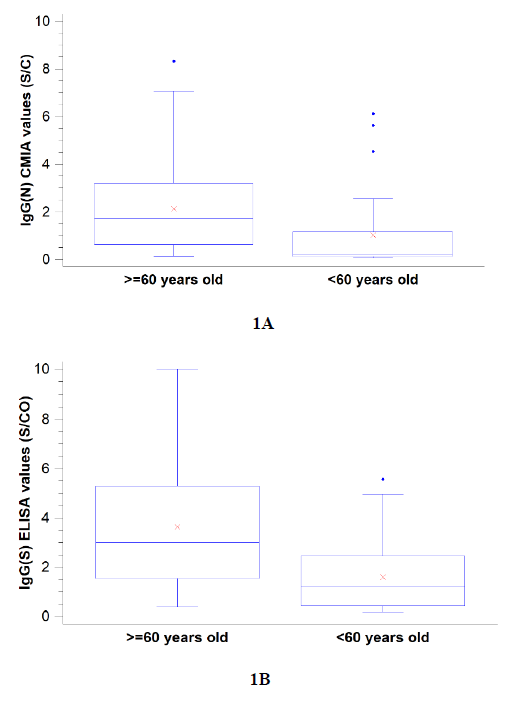
Spike-specific IgM was detectable only in a small group of patients (16.9%), with no statistically significant differences between age groups. Both IgG(S) and IgG(N) were significantly more detected in the elderly group (Figure 1). The total N antibodies assay resulted positive for all patients included in the study, whereas the IgG(N) CMIA assay resulted positive in 45.5% of the cases.
Quantitative results of IgG(S) showed that 50 out of 77 (65%) patients had values above the assay cut-off (11 RU/mL). According to age groups, there were statistically significant differences between patients under and above 60 years old as 40.7% and 78% of individuals, respectively, were found to have IgG(S) quantitative values >11 RU/ml.
|
COVID-19 patients |
||||
|
<60 years (n=27) |
³60 years (n=50) |
total (n=77) |
||
|
women/men |
26/1 |
41/9 |
67/10 |
p>0.05* |
|
IgG(N) (%) |
5 (18.5%) |
30 (60%) |
35 (45.5%) |
p=0.001* |
|
IgM(S) (%) |
5 (18.5%) |
8 (16%) |
13 (16.9%) |
p>0.05* |
|
IgG(S) (%) |
16 (59.3%) |
41 (82%) |
57 (74%) |
p=0.045* |
|
Total antibodies (N) (%) |
27 (100%) |
50 (100%) |
77 (100%) |
- |
|
Quantitative IgG(S) median RU/mL (IC 95%) |
9.5 (0-19,3) |
34.4 (22.7-46.1) |
23.3 (14.5-32.1) |
p<0.001** |
* Exact Fisher test results. **Mann-Whitney test result.
Table 1: Detectable SARS-CoV-2 antibodies nine months after infection in serum specimens of convalescent COVID-19 patients.
Figure 1: SARS-CoV-2 specific anti-spike protein (1A, Euroimmun) and anti-nucleocapsid protein (1B, Abbott) IgG detection 9 months after COVID-19 infection in 77 individuals divided by age groups (<60 vs. ³60 years). Values are signal to cut-off ratios. For both assays differences between groups were found to be statistically significant by the Mann-Whitney Test (p<0.05).
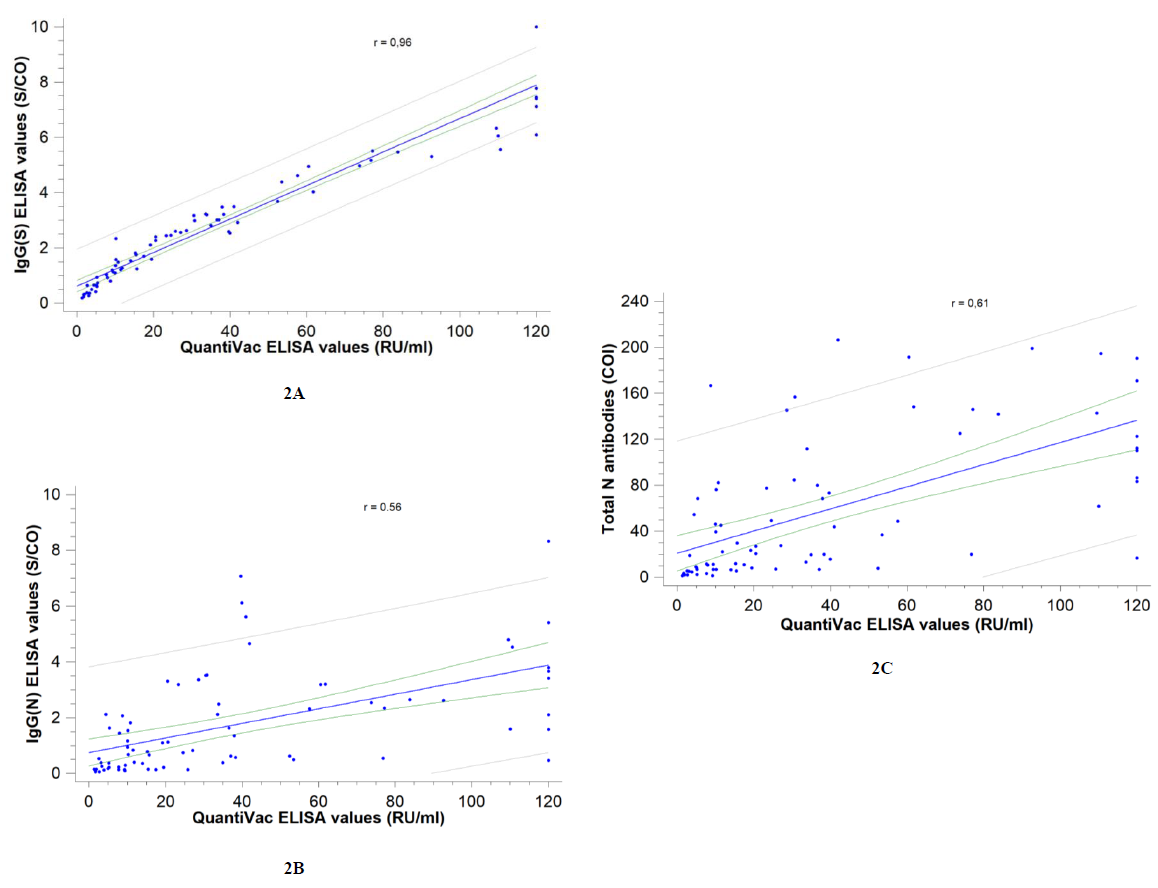
Correlation between assays was investigated: the strongest correlation was observed with the Euroimmun SARS-CoV-2 IgG(S) assay (Spearman correlation coefficient r= 0.96) and the weakest with the Abbott SARS-CoV-2 IgG(N) assay (r= 0.56) (Figure 2).
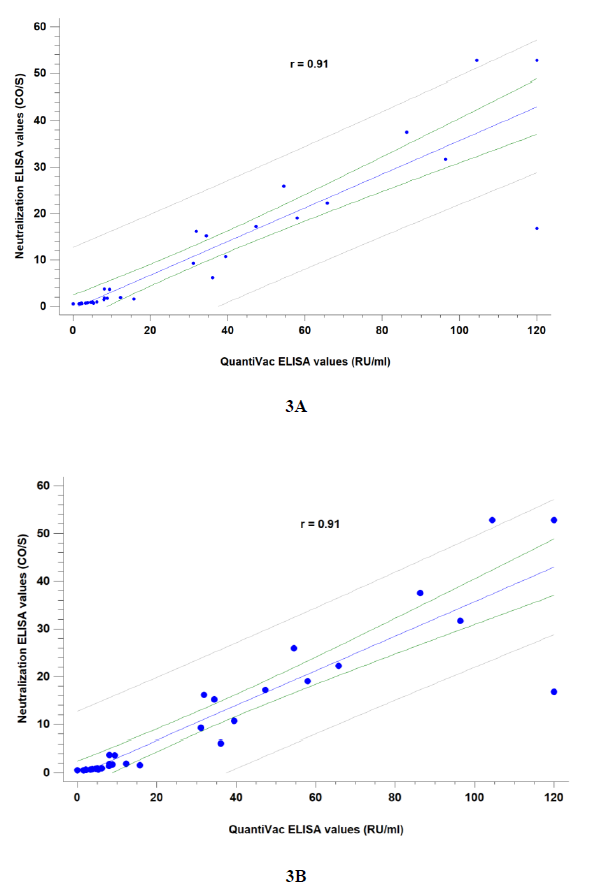
Correlation between quantitative IgG(S) and neutralization assays was good (r= 0.91). Samples were tested either at 2:3 (~ 1:15 VNT titer) and 1:4 (~ 1:40 VNT titer) dilutions (Figure 3) to provide IgG(S) correlates. A value of anti-spike IgG ³ 8 RU/mL was correlated to a positive neutralization result at a 2:3 dilution and ³ 15 RU/mL to a 1:4 dilution. According to this estimated in-house cut-offs, 59/77 (76.6%) individuals could be assumed to have a low neutralization titer (1:15) and 47/77 (61%) to have a medium neutralization titer (³ 1:40 VNT).
Figure 3: Correlation between quantitative SARS-CoV-2 IgG(S) QuantiVac (Euroimmun) and Neutralization ELISA (DIA.PRO) assays at 2:3 screening dilution (3A) and 1:4 dilution (3B). Manufacturer provides a correlation to the “in vivo” neutralization assay (VNT) as follows: 2:3 ~ 1:15 VNT titer (low)and 1:4 ~ 1:40 VNT titer (medium). Spearman correlation coefficients were calculated.
4. Discussion
As the pandemic progresses the knowledge of COVID-19 serology has improved as it has deal with different scenarios. The cases of the first wave of SARS-CoV-2 pandemic in our region were detected between March and June 2020. Vaccination programmes started on January 2021. This time lapse between both dates has given us the opportunity to assess humoral response against SARS-CoV-2 after natural infection in a selected cohort of unvaccinated population.
In this study, we could assess that IgG(S) antibodies are present in a higher proportion of patients than IgG(N) antibodies 9 months after natural infection. High IgG(S) seroprevalence and longevity after infection have been reported, from 3-5 months [11] up to 6-8 months post-infection [10]. Moreover, a significantly higher proportion of persons older than 60 years were seropositive compared to persons younger than 60. As in previous studies IgG(S) correlates well to neutralizing antibodies (nAbs) and thus to protective immunity [11-15]. Although it is not well established which IgG(S) values can be considered protective, quantitative assays are available. We aimed at correlating quantitative IgG(S) values with qualitative ratios and neutralization results.
Euroimmun IgG(S) quantitative assay results confirm the longevity of this SARS-CoV-2 antibody after a natural infection and are positively correlated with the qualitative ratios. Euroimmun QuantiVac IgG(S) assay showed perfect correlation with the Anti-SARS-CoV-2 Antibody Diagnostic Calibrant (NIBSC code: 20/162) and has established standardized units based on the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (NIBSC code: 20/136). For binding antibody assays, an arbitrary unitage of 1000 binding antibody units (BAU)/mL can be used with this standard to assist the comparison of assays detecting the same class of immunoglobulins [15,16].
Virus Neutralization Test (VNT) remains the gold standard for assessing specific immunity performance, but needs for increased biosafety level and is time-consuming. Some commercial assays, like ACE2-RBD Neutralization ELISA Assay, are simple and fast, and allow routine testing on a clinical laboratory. NIBSC Diagnostic Calibrant (code: 20/136, 1000 arbitrary Units/mL) was detected positive with this ELISA diluted up to a final concentration of 15 arbU/mL. Although it is not a substitute for the VNT titer, it could be an acceptable alternative method for detecting nAbs.
Spike-based IgG assays have previously been correlated with nAbs and specifically, the Euroimmun qualitative assay ratios had shown a high discriminative capacity for detecting high nAbs [14]. J. Dan et al.found 90% of their subjects to be seropositive for SARS-CoV-2 nAbs at 6 to 8 months after the onset of symptoms. NAbs titers correlated with their RBD and IgG(S) assays titers [10]. Although slightly lower, caused by a logical decay kinetics, our results are in accordance with previously reported, as percentages of SARS-CoV-2 recovered patients with detectable IgG(S) and nAbs matched strongly. Two-thirds of the patients in our study showed values over both assays’ cut-offs, regardless of their age, 9 months post-infection. In fact, 61% showed positive results at a higher dilution (1:40), which means that medium neutralization titers can be expected in COVID-19 recovered patients long time after infection. We can assume that values had been higher in the preceding months. Whether these would be protective enough against COVID-19 reinfection is unclear.
Surprisingly in our study, all patients had a positive result with the total N-antibodies test. The Elecsys assay (Roche) is supposed to detect a combination of immunoglobulins, including IgG, against SARS-CoV-2 nucleoprotein antigen. However, the results are very high compared to the IgG(N) detected by the Euroimmun ELISA assay, which was positive in less than half of the subjects. Experience with IgA against N antigen is scarce and therefore, its kinetics are largely unknown. IgM(N) has been reported to be an early marker of infection [3]. As IgM(S) detected by the Abbott assay is present in a very small percentage of our studied cohort, we could hypothesize that IgM(N) should be very low too, as N-antibodies are known to be shorter-lived than anti-spike ones. Differences in the recombinant N antigen used in both assays may explain a much higher sensitivity of the Cobas assay. Heterogeneous results with SARS-CoV-2 N-based assays have been found frequently before [18,19]. The manufacturer indicates that the pan-Ig N-assay is designed to detect “mature” antibodies, and we speculate that it could, in some way, detect antibodies with a stronger binding affinity than other N-based assays could, similar to how an “avidity” test would work. Muecksch F. et al. also reported persistence of positive results with the pan-Ig(N) assay while a decrease in IgG(N) detection (Abbott) at >7.5 weeks after positive PCR test in their longitudinal study [20]. Given the results, this test can be considered a good marker of past natural infection and a useful tool to serologically differentiate patients who had a previous COVID-19 infection from those who have been vaccinated. Furthermore, it could be helpful to discriminate between previously and recently infected. This last point requires, however, further research.
Our study is not free from limitations. Although low, we detected a percentage of patients with no detectable IgG(S) anti-SARS-CoV-2. Seronegative COVID-19 patients have been found previously, mainly in paucisymptomatic or asymptomatic cases, and usually in studies that included short periods after diagnosis (<30 days) [21-23]. Some of those patients who did not develop detectable IgG antibodies could have detectable nAbs [24]. We could also detect a few recovered patients with borderline IgG(S) values but seropositive for Nabs, possibly as a result of variable kinetics decay among patients, which is not surprising given the long period between the infection and follow-up samples. Unfortunately, we cannot differentiate those patients who did not produce antibodies after infection from those who experimented a drop in antibodies over time not being detected in time of our study.
Secondly, as a cross-sectional study, our results only show a static picture of COVID-19 humoral response at 9 months after infection. Thus, we could not draw any conclusions about antibody kinetics during the early-phase of COVID-19, that is whether antibodies against nucleocapsid or spike proteins were produced earlier in confirmed patients. However, our results support previous data on the longevity of IgG(S) and the wane in time of IgG(N). Fenwick C. et al reported a substantial drop of N-specific antibody responses in the post-infection phase compared to the anti-S response [19]. In the study assessing antibody detection up to 8 months after COVID-19 infection, the authors stated a longer IgG(S) half-time compared to IgG(N) (103 vs. 68 days), with a very high percentage of seropositive IgG(S) subjects at 6-8 months after symptoms onset [10]. Given the fact that IgG(N) assays could underestimate the proportion of infected/exposed individuals in a late phase, according to our results, the Roche total N-antibodies assay could be useful as a past infection marker.
Because of the study design we were also not able to investigate the reasons for the differences observed between patients under and over the age of 60 years. Other authors associated a higher and earlier antibody response in more severe COVID-19 cases [12,22,24,25]. This could be an explanation if we assume more severe infections in the elder group. If this group of patients could have produced a strong immunological response in the acute phase of infection, their decay kinetics would have taken more time until immunoglobulins became undetectable. Likewise, an asymptomatic or mild infection could produce a weaker humoral response which could wane within a shorter period of time.
5. Conclusions
In this study we asses humoral response 9 months after natural COVID-19 infection in a cohort of unvaccinated convalescent patients. IgG against SARS-CoV-2 spike protein and neutralizing antibodies were present in a high proportion of the cohort at that time point. IgG, both anti-spike and anti-nucleocapsid, were especially prevalent in subjects over 60 years. Anti-nucleocapsid IgG was detected in less than the half of individuals whereas Elecsys total N-antibodies assay was positive for all patients of the study. Thus we demonstrate here that it can detect long-lasting humoral response after natural infection. Euroimmun QuantiVac is a valuable tool for SARS-CoV-2 IgG(S) measurement.
References
- World Health Organization. Weekly epidemiological update on COVID-19 (2021).
- Beavis KG, Matushek SM, Abeleda APF, Bethel C, Hunt C, Gillen S, et al. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. Journal of Clinical Virology 129 (2020): 104468.
- Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, et al. Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 71 (2020): 1930-1934.
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 71 (2020):778-785.
- Serrano MM, Rodríguez DN, Palop NT, Arenas RO, Córdoba MM, Mochón MDO, et al. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. Journal of Clinical Virology 129 (2020): 104529.
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes and Infections. 9 (2020): 386-389.
- Tang MS, Hock KG, Logsdon NM, Hayes JE, Gronowski AM, Anderson NW, et al. Clinical Performance of Two SARS-CoV-2 Serologic Assays. Clinical chemistry 66 (2020): 1055-1062.
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology 20 (2020): 363-374.
- Theel ES, Harring J, Hilgart H, Granger D. Performance Characteristics of Four High-Throughput Immunoassays for Detection of IgG Antibodies against SARS-CoV-2. J Clin Microbiol 58 (2020): e01243.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371 (2021): eabf4063.
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 370 (2020): 1227-1230.
- Okba NMA, Müller MA, Li W, Wang C, Geurtsvankessel CH, Corman VM, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerging Infectious Diseases 26 (2020): 1478-1488.
- To KK-W, Hung IF-N, Chan K-H, Yuan S, To W-K, Tsang DN-C, et al. Serum Antibody Profile of a Patient With Coronavirus Disease 2019 Reinfection. Clinical Infectious Diseases 72 (2020): 659-662.
- Patel EU, Bloch EM, Clarke W, Hsieh YH, Boon D, Eby YJ, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. medRxiv 59 (2020): 1-10.
- Wu F, Liu M, Wang A, Lu L, Wang Q, Gu C, et al. Evaluating the Association of Clinical Characteristics with Neutralizing Antibody Levels in Patients Who Have Recovered from Mild COVID-19 in Shanghai, China. JAMA Internal Medicine 180 (2020): 1-7.
- Comparison between the EUROIMMUN Anti-SARS-CoV-2 QuantiVac ELISA ( IgG ) and the cPass SARS-CoV-2 Neutralization Antibody Detection Kit from GenScript. (2020): 23560.
- Anti-SARS-CoV-2 QuantiVac ELISA ( IgG ). 31-32.
- Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. Journal of Clinical Virology 129 (2020): 104480.
- Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. Journal of Virology 95(2020): e01828.
- Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. Journal of Infectious Diseases 223 (2021): 389-398.
- Montesinos I, Gruson D, Kabamba B, Dahma H, Van den Wijngaert S, Reza S, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. Journal of Clinical Virology 128 (2020): 104413.
- Yongchen Z, Shen H, Wang X, Shi X, Li Y, Yan J, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerging Microbes and Infections. Taylor and Francis Ltd 9 (2020): 833-836.
- Chew KL, Tan SS, Saw S, Pajarillaga A, Zaine S, Khoo C, et al. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clinical Microbiology and Infection 26 (2020): 1256.
- Marklund E, Leach S, Axelsson H, Nystrom K, Norder H, Bemark M, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE 15 (2020): 1-11.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 71 (2020): 2027-2034.