Carbamylated Haemoglobin is an Early Biomarker to Predict Chronic Kidney Disease
Article Information
Sharaban Tahora1*, Md. Majadul Islam2, Ferdous Jahan3, A. K. M Shahidur Rahman3, Abu Ahmed Golam Akbar4, Marjoa Humaira Mekhola3, Md. Mahmudul Hassan5, Md. Rezaul Alam3, Syed Mahtab ul Islam6, Md. Mustafizur Rahman7
1Resident (Nephrology), Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
2Medical Officer, Sarkari Karmachari Hospital, Dhaka, Bangladesh
3Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
4Medical Officer, Department of Nephrology, Sylhet MAG Osmani Medical College Hospital, Sylhet, Bangladesh
5Assistant Professor, Department of Nephrology, Monno Medical College and Hospital, Manikgonj, Bangladesh
6Assistant Professor, Department of Nephrology, Chittagong Medical College, Chattogram, Bangladesh
7Assistant Professor, Department of Nephrology, Sheikh Hasina Medical College, Tangail, Bangladesh
*Corresponding Author: Dr. Sharaban Tahora, Resident (Nephrology), Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
Received: 02 August 2021; Accepted: 09 August 2021; Published: 16 August 2021
Citation: Tahora S, Islam MM, Jahan F, Rahman AKMS, Akbar AAG, Mekhola MH, Hassan MM, Alam MR, Islam SMU, Rahman MM. Carbamylated Haemoglobin is an Early Biomarker to Predict Chronic Kidney Disease. Archives of Nephrology and Urology 4 (2021): 101-114.
Share at FacebookAbstract
Background: Diagnosing chronic kidney disease (CKD) in clinical practice is quite difficult for patients presenting with uremia for the first time. Carbamylated haemoglobin (CarHb) may provide a better index of chronic uraemic exposure than a single measurement of urea.
Objective: To evaluate the carbamylated haemoglobin as an early biomarker in the diagnosis of CKD.
Methodology: This prospective observational study was conducted in the Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from January 2019 to December 2019. A total of seventy (70) patients with renal impairment were enrolled. On the basis of developing CKD the study population was divided into two groups- group A (CKD) and group B (Non CKD). Relevant investigations like- urine routine microscopic examination, 24 hours urinary total protein, blood urea, serum creatinine, serum electrolytes, complete blood count, serum albumin, serum calcium, serum phosphate, serum parathyroid hormone, carbamylated haemoglobin levels and renal ultrasonography were done. These investigations were repeated in each patient after 3 months to define CKD according to KDIGO guideline. All data were analyzed and compared by statistical tests.
Results: Among total 70 study subjects, 15(21.4%) patients had CKD and their mean age was 54.47(±11.84) years. Carbamylated haemoglobin (CarHb) level was significantly higher (107.2±9.3 µgVH/g Hb versus 86.2±15.2 µgVH/g Hb; p<0.001) in subjects who had CKD.
Conclusion: In patients with chronic kidney disease (CKD), carbamylated haemoglobin (CarHb) level is raised early before appearance of other biomarkers of CKD. Carbamylated haemoglobin (CarHb) may be a good biomarker for the early prediction of CKD.
Keywords
Biomarker; Carbamylated Haemoglobin (CarHb); Chronic Kidney Disease (CKD); Renal Impairment
Biomarker articles; Carbamylated Haemoglobin (CarHb) articles; Chronic Kidney Disease (CKD) articles; Renal Impairment articles
Biomarker articles Biomarker Research articles Biomarker review articles Biomarker PubMed articles Biomarker PubMed Central articles Biomarker 2023 articles Biomarker 2024 articles Biomarker Scopus articles Biomarker impact factor journals Biomarker Scopus journals Biomarker PubMed journals Biomarker medical journals Biomarker free journals Biomarker best journals Biomarker top journals Biomarker free medical journals Biomarker famous journals Biomarker Google Scholar indexed journals Carbamylated Haemoglobin articles Carbamylated Haemoglobin Research articles Carbamylated Haemoglobin review articles Carbamylated Haemoglobin PubMed articles Carbamylated Haemoglobin PubMed Central articles Carbamylated Haemoglobin 2023 articles Carbamylated Haemoglobin 2024 articles Carbamylated Haemoglobin Scopus articles Carbamylated Haemoglobin impact factor journals Carbamylated Haemoglobin Scopus journals Carbamylated Haemoglobin PubMed journals Carbamylated Haemoglobin medical journals Carbamylated Haemoglobin free journals Carbamylated Haemoglobin best journals Carbamylated Haemoglobin top journals Carbamylated Haemoglobin free medical journals Carbamylated Haemoglobin famous journals Carbamylated Haemoglobin Google Scholar indexed journals Chronic Kidney Disease articles Chronic Kidney Disease Research articles Chronic Kidney Disease review articles Chronic Kidney Disease PubMed articles Chronic Kidney Disease PubMed Central articles Chronic Kidney Disease 2023 articles Chronic Kidney Disease 2024 articles Chronic Kidney Disease Scopus articles Chronic Kidney Disease impact factor journals Chronic Kidney Disease Scopus journals Chronic Kidney Disease PubMed journals Chronic Kidney Disease medical journals Chronic Kidney Disease free journals Chronic Kidney Disease best journals Chronic Kidney Disease top journals Chronic Kidney Disease free medical journals Chronic Kidney Disease famous journals Chronic Kidney Disease Google Scholar indexed journals Renal Impairment articles Renal Impairment Research articles Renal Impairment review articles Renal Impairment PubMed articles Renal Impairment PubMed Central articles Renal Impairment 2023 articles Renal Impairment 2024 articles Renal Impairment Scopus articles Renal Impairment impact factor journals Renal Impairment Scopus journals Renal Impairment PubMed journals Renal Impairment medical journals Renal Impairment free journals Renal Impairment best journals Renal Impairment top journals Renal Impairment free medical journals Renal Impairment famous journals Renal Impairment Google Scholar indexed journals parathyroid hormone articles parathyroid hormone Research articles parathyroid hormone review articles parathyroid hormone PubMed articles parathyroid hormone PubMed Central articles parathyroid hormone 2023 articles parathyroid hormone 2024 articles parathyroid hormone Scopus articles parathyroid hormone impact factor journals parathyroid hormone Scopus journals parathyroid hormone PubMed journals parathyroid hormone medical journals parathyroid hormone free journals parathyroid hormone best journals parathyroid hormone top journals parathyroid hormone free medical journals parathyroid hormone famous journals parathyroid hormone Google Scholar indexed journals
Article Details
1. Introduction
Accurately determining acute or chronic nature of kidney failure is frequently needed in clinical practice in patients presenting with uremia for the first time. One of the most important steps in acute nephrological emergencies is to identify renal failure either acute kidney injury (AKI) or chronic kidney disease (CKD). AKI is defined as any increase in serum creatinine by ≥0.3 mg/dl within 48 hours (≥ 26.5 μmol/L) [1]. AKI represents a substantial risk factor for CKD [1, 2]. CKD is characterized by a progressive and ongoing loss of kidney function for >3 month duration with or without decrease in glomerular filtration rate (GFR) [3].
Diagnosis of chronic kidney disease (CKD) is quite difficult for patients who present with uremia for the first time and if their kidney functions of previous 3 months are unknown.In the cases where findings are consistent with CKD, it makes the diagnosis easier. However, when the kidney size is normal as in diabetes mellitus, multiple myeloma, polycystic kidney disease, it is quite difficult to differentiate acute kidney injury (AKI) from chronic kidney disease (CKD) [4]. Patient’s history regarding renal impairment, relevant clinical examination and routine biochemical tests along with long-standing hypertension, uremic neuropathy, anemia, hypocalcaemia, hyperphosphatemia and hyperparathyroidism are consistent with CKD but still with low sensitivity and specificity [5].
Loss of kidney function is always associated with increases in blood urea level. Therefore, a new approach has been taken for its measurement that has broadened the scope of its use [6, 7]. Urea and other metabolites are retained in renal failure, which gradually increase day by day. It spontaneously rearranges under physiologic conditions to form ammonia and cyanate [7]. The protonated form of cyanate, isocyanic acid reacts with the amino groups of proteins resulting in the carbamylation of proteins like- carbamylated hemoglobin [7].
Carbamylated hemoglobin (CarHb) was first identified by Fluckiger et al. among uremic patients in the early eighties of last century [8]. It was reported that, carbamylated hemoglobin (CarHb), formed as a result of the reaction of isocyanate with N-terminal valine residues of α and β chains of hemoglobin (Hb), that was found in high levels in patients with renal failure [9]. Under physiological conditions, the level of isocyanate is approximately 1% that of urea [10].
Evaluation of carbamylated hemoglobin (CarHb) in patients with various degrees of renal function has shown that its measurement has potential clinical value [11-13]. Carbamylated haemoglobin has been found to be capable of diagnosing chronic kidney disease (CKD) and differentiating it from acute kidney injury (AKI) [14]. Carbamylated haemoglobin may provide a better index of chronic uraemic exposure than a single measurement of urea, as urea is subjected to day-to-day dietary and metabolic influences [15]. The aim of this current study was to evaluate carbamylated hemoglobin (CarHb) in patients with renal impairment and to assess its clinical value in the diagnosis of CKD.
2. Methodology
This prospective observational study was conducted at Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from January 2019 to December 2019. A total of seventy (70) patients, who admitted in the Department of Nephrology, BSMMU, Dhaka, Bangladesh with renal impairment for the first time during the study period were selected following selection criteria. Adult patients (age ≥18 years) of both sexes with renal impairment for the first time (serum creatinine >1.3 mg/dl) was included in this study. Patient with pre-existing kidney disease (acute/chronic), patient with acute or chronic infections, patient with any types of malignancy, patients with severe anaemia, hypo-calcaemia, hyper-phosphataemia, hyper-parathyroidism, patients received blood transfusion in preceding three months and pregnant/lactating subjects were excluded from the study.
The study was approved by the Ethical Review Committee, BSMMU, Dhaka, Bangladesh. The study population was divided into two groups- group A (CKD) and group B (Non CKD); group A consists of the patients admitted in the Department of Nephrology with renal impairment for the first time and subsequently developing CKD, while group B includes the patients admitted in the Department of Nephrology with renal impairment for the first time but did not develop CKD.
After taking informed written consent, the data were collected from each patient. The relevant history and clinical examination findings were recorded in a data collection sheet. All study patients were investigated for urine routine and microscopic examination (R/M/E), 24 hours urinary total protein (24- hr UTP), blood urea, serum creatinine, serum electrolytes, complete blood count (CBC), serum albumin, serum calcium, serum phosphate, serum parathyroid hormone (PTH), carbamylated haemoglobin (CarHb) and renal ultrasonography. These investigations were repeated in each patient after 3 months to define CKD according to KDIGO guideline.
2.1 Procedure of measuring carbamylated haemoglobin [16]
Following standard procedure 3 ml of venous blood was collected in a plane test tube from each patient. Then centrifugation was done at 2000-3000 rpm/minutes in room temperature (22°C - 24°C) for 15 minutes, plasma was separated by micro-pipette and discarded. Then the red blood cells (RBC) were haemolysed by adding cold distilled water. Carbamylated haemoglobin released after haemolysis of the RBC. Carbamylated haemoglobin was measured by modified Rosen’s colorimetric method [16].
2.2 Definition of Chronic Kidney Disease (CKD) [17]
According to *KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, CKD is defined as abnormalities of kidney structure or function, present for >3 months.
[*KDIGO = Kidney Disease Improving Global Outcomes].
Criteria for CKD (either of the following present for >3 months)
1. a) Markers of kidney damage (one or more)
- Albuminuria [Urine albumin excretion rate (AER)] ≥ 30 mg/24 hours; Urine albumin to creatinine ratio (ACR) ≥ 30 mg/gm
- Urine sediment abnormalities
- Electrolyte and other abnormalities due to tubular disorders
- Abnormalities detected by histology
- Structural abnormalities detected by imaging
- History of kidney transplantation
1. b) Decreased glomerular filtration rate (GFR)
Glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 (GFR categories G3a–G5)
2.3 Statistical analysis
Data analysis was performed by Statistical Packages for Social Sciences (SPSS) version- 23. Quantitative data were expressed as mean with standard deviation (±SD), while qualitative data were expressed as frequency and percentage. The statistics used to analyze the data was descriptive statistics. The correlation between carbamylated haemoglobin (CarHb) and study parameters was assessed using Pearson’s correlation test.
Association between categorical variables was examined by chi-square test and continuous variables by unpaired t-test. A p value <0.05 was considered as statistically significant.
3. Results
This study was intended to evaluate carbamylated hemoglobin (CarHb) as an early biomarker to predict CKD in patients with renal impairment. Table 1 shows the distribution of patients by study groups on the basis of developing CKD. A total of 70 patients were recruited in this study, among which 15 (21.4%) patients were developed CKD [group A (CKD)] and 55 (78.6%) patients did not develop CKD [group B (Non CKD)].
|
Study group |
Number |
Percentage (%) |
|
Group A (CKD) |
15 |
21.4 |
|
Group B (Non CKD) |
55 |
78.6 |
|
Total |
70 |
100.00 |
Table 1: Distribution of the study patients by study group (n=70).
|
Age (years) |
Total (n=70) No (%) |
Group A (CKD) (n=15)No (%) |
Group B (Non CKD) (n=55)No (%) |
p-value |
|
<30 31-40 41-50 51-60 >70 |
14 (20.0%) 18 (25.7%) 10 (14.3%) 24 (34.3%) 4 (5.7%) |
0 (0.0%) 3 (20.0%) 3 (20.0%) 9 (60.0%) 0 (0.0%) |
14 (25.5%) 15 (27.3%) 7 (12.7%) 15 (27.3%) 4 (7.3%) |
|
|
Total |
70 (100.0%) |
15 (100.0%) |
55 (100.0%) |
|
|
Mean ± SD |
45.83 ± 16.11 |
54.47 ± 11.84 |
43.47 ± 16.40 |
0.018s |
Unpaired t-test was done, s= significant
Table 2: Age distribution of the study group (n=70).
Table 2 shows the age distribution of the study subjects in both groups. The mean age of the subjects in group A was 54.47 (± 11.84) years and majority [9 (60%)] of them was in 51 to 60 year age group, while in group B mean age of the subjects was 43.47 (± 16.40) years. There was significant age difference between the groups (p=0.018).
Among 15 patients of group A, 10 (66.7%) were male and 5 (33.3%) were female; while in group B, 37 (67.3%) out of 55 patients were male and rest 18 (32.7%) patients were female, male were predominant than female in both groups, but the difference was not statistically significant (p=0.965) (Table 3).
|
Variables |
Group A (CKD) (n=15) No (%) |
Group B(Non CKD) (n=55) No (%) |
p-value |
|
Gender Male Female |
10 (66.7%) 5 (33.3%) |
37 (67.3%) 18 (32.7%) |
0.965ns |
Chi-square test was done, ns= not significant
Table 3: Distribution of the patients according to gender (n=70).
Table 4 shows different risk factors of the study patients. There were multiple responses in the participants. Among group A; 6 (40.0%) patients had glomerulonephritis (GN), 7 (46.7%) patients had diabetes mellitus, 10 (66.7%) patients had hypertension, no [0 (0.0%)] patients had obstructive nephropathy and 6 (40.0%) patients had previous history of taking non-steroidalanti-inflammatorydrugs (NSAID). On the other hand, in group B; 28 (50.9%) patients had GN, 9 (16.4%) patients had diabetes mellitus, 30 (54.5%) patients had hypertension, 4 (7.3%) patients had obstructive nephropathy and 11 (20.0%) patients had previous history of taking NSAID.
|
*Risk factors |
Total (n=70) No (%) |
Group A (CKD) (n=15) No (%) |
Group B (Non CKD) (n=55) No (%) |
p-value |
|
Glomerulonephritis (GN) |
34 (48.6%) |
6 (40.0%) |
28 (50.9%) |
0.454ns |
|
Diabetes mellitus |
16 (22.9%) |
7 (46.7%) |
9 (16.4%) |
0.013s |
|
Hypertension |
40 (57.1%) |
10 (66.7%) |
30 (54.5%) |
0.400 ns |
|
Obstructive nephropathy |
4 (5.7%) |
0 (0.0%) |
4 (7.3%) |
0.282ns |
|
History of NSAIDs |
17 (24.3%) |
6 (40.0%) |
11 (20.0%) |
0.109ns |
|
Others |
4 (5.7%) |
0 (0.0%) |
4 (7.3%) |
0.282 ns |
Unpaired t-test was done, s= significant, *Multiple response
Table 4: Distribution of the study groups according to the risk factors (n=70).
Table 5 displaying the different biochemical parameters of the study subjects among the groups. There were no statistically significant differences (p>0.005) observed between group A and group B patients in different biochemical parameters like- hemoglobin (Hb), 24 hours urinary total protein (24- hr UTP), serum creatinine, serum albumin, serum calcium,serum phosphate andserum parathyroid hormone(PTH) levels, except blood urea level (p<0.001).
|
Biochemical parameters |
Total (n=70) Mean±SD |
Group A (CKD) (n=15) Mean±SD |
GroupB (Non CKD) (n=55) Mean±SD |
p-value |
|
Hb (%) |
11.77 ± 0.84 |
11.79 ± 0.83 |
11.77 ± 0.85 |
0.917ns |
|
24- hr UTP (gm/24h) |
2.42 ± 2.19 |
2.27 ± 2.10 |
2.46 ± 2.23 |
0.761ns |
|
Blood Urea (mmol/L) |
13.66 ± 5.02 |
20.17 ± 3.71 |
11.89 ± 3.70 |
<0.001s |
|
S. creatinine (µmol/L) |
256.3 ± 136.2 |
201.1 ± 55.4 |
271.3 ± 147.8 |
0.409ns |
|
S. albumin (grm/dl) |
3.19 ± 0.73 |
3.15 ± 0.60 |
3.20 ± 0.78 |
0.830ns |
|
S. calcium (mg/dl) |
8.91 ± 0.57 |
8.89 ± 0.53 |
8.91 ± 0.58 |
0.884ns |
|
S. phosphate (mg/dl) |
3.78 ± 0.49 |
3.61 ± 0.56 |
3.82 ± 0.47 |
0.148ns |
|
S. PTH (pg/ml) |
55.37 ± 26.51 |
49.09 ± 16.33 |
57.08 ± 28.55 |
0.305ns |
Unpaired t-test was done, s= significant, ns= not significant
Table 5: Comparison of different biochemical parameters between group A (CKD) and group B (Non CKD) patients (n=70).
Among the study population; it was found that, the level of carbamylated hemoglobin (CarHb) of group A (CKD) patients was significantly higher (107.2 ± 9.3 µgVH/g Hb) than that of group B (Non CKD) patients (86.2 ± 15.2 µgVH/g Hb) (p<0.001) (Table 6).
|
Variable |
Total (n=70) Mean±SD |
Group A (CKD) (n=15) Mean±SD |
Group B (Non CKD) (n=55) Mean±SD |
p-value |
|
Carbamylated hemoglobin (µgVH/g Hb) |
90.7 ± 16.5 |
107.2 ± 9.3 |
86.2 ± 15.2 |
<0.001s |
Unpaired t-test was done, s= significant
Table 6: Comparison of carbamylated hemoglobin (CarHb) level between the group A (CKD) and group B (Non CKD) patients (n=70).
Figure 1 displaying the receiver operated curve (ROC) analysis, which showed the best cut off point of carbamylated hemoglobin (CarHb) (blue color) was 101.5 for CKD. It revealed the area under the curve (AUC) of CarHb was 0.950 (95% confidence interval 0.903-0.997) (p<001).
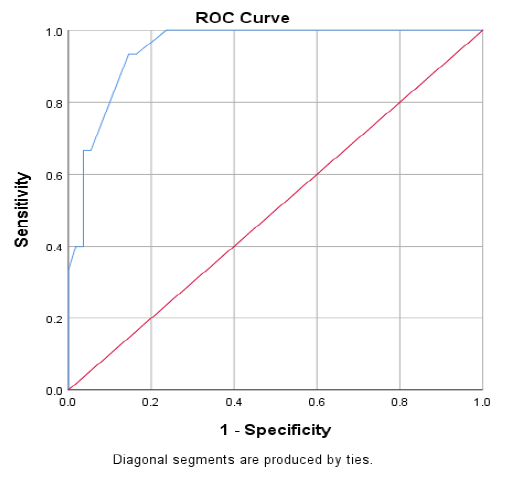
Figure 1: Receiver operated curve (ROC) analysis of carbamylated hemoglobin (CarHb) levels in CKD versus Non-CKD.
|
Area Under the Curve (AUC) |
||||||
|
Test Result Variable(s) |
Cutoff value |
AUC |
Std. Error |
p-value |
95% CI |
|
|
Lower |
Upper |
|||||
|
CarHb |
101.5 |
.950 |
.034 |
.001 |
.903 |
.997 |
Table 7 shows effectiveness of carbamylated hemoglobin (CarHb) to predict chronic kidney disease (CKD). It shows that, a cut-off value of carbamylated hemoglobin (CarHb) was 101.5 µg VH/g Hb, the sensitivity was 93.33%, specificity was 85.45%, positive predictive value was 63.64%, negative predictive value was 97.92% and the accuracy of the test was 87.14%.
|
Value |
95% CI |
|
|
Sensitivity |
93.33% |
68.05% to 99.83% |
|
Specificity |
85.45% |
73.34% to 93.50% |
|
Positive Predictive Value |
63.64% |
47.62% to 77.11% |
|
Negative Predictive Value |
97.92% |
87.58% to 99.68% |
|
Accuracy |
87.14% |
76.99% to 93.95% |
Table 7: Diagnostic validity test.
Figure 2 shows that there was weak positive correlation between carbamylated hemoglobin (CarHb) and serum creatinine in scatter graph. The Pearson’s correlation coefficient was +0.121 and p-value was 0.319 (Figure- 2).
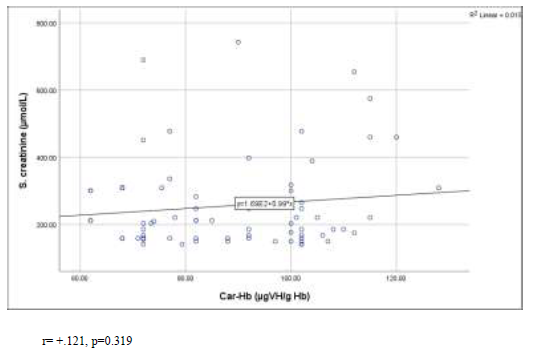
Figure 2: Correlation of carbamylated hemoglobin (CarHb) with serum creatinine levels in the study population.
r= +.121, p=0.319
The scatter graph in Figure 3 displaying that, there was a significant linear relationship between blood urea and carbamylated hemoglobin (CarHb) in case of CKD patients and non CKD patients (p<0.001). But the Pearson’s correlation coefficient was greater (r=+0.865) in group A (CKD) patients than group B (Non CKD) patients (r=+0.674).
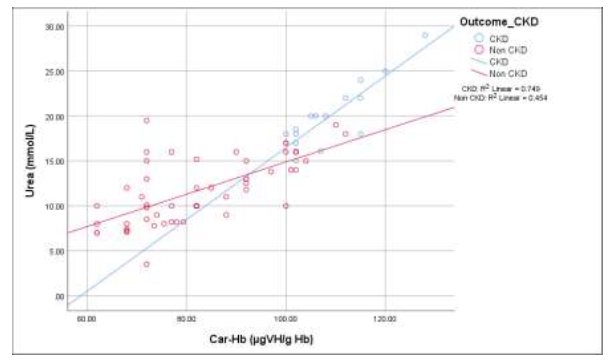
Figure 3: Correlation of carbamylated hemoglobin (CarHb) with blood urea levels among the study groups.
Group A (CKD): r= +.865, p<0.001; Group B (Non CKD): r=+.674, p <0.001
4. Discussion
It is difficult in clinical practice to diagnose chronic kidney disease (CKD) among patients presenting with renal impairment for the first time with unknown kidney function in preceding 3 months and normal renal imaging [18]. The purpose of this current study was to evaluate the carbamylated haemoglobin (CarHb) as an early biomarker in the diagnosis of CKD in similar type of patients.
A total of 70 study subjects were enrolled in this study and out of which 21.4% patients had CKD which was consistent with the study conducted by Hasan et al. where overall prevalence of CKD among Bangladeshi adults was 17% [19]. In accordance another previous study reported that prevalence of CKD in Bangladeshi population was 26.0% [20].
In our study the mean age of the CKD patients was 54.47 years which was similar to the study conducted by Anand et al. where the mean age of CKD group was 49.50 years [20]. Almost similar finding was demonstrated in a related previous study where mean age of CKD group was 53.00 years [21]. This may be explained by as a part of the normal physiologic process; renal function (GFR) starts to decline even in a healthy individual after 30 to 40 years of age, which might deteriorate after 50–60 years of age due to structural changes in kidneys [22]. This increased prevalence of CKD among elderly individuals also can be explained by the higher prevalence of co-morbidities specially diabetes mellitus and hypertension among this group of people that are considered as important risk factors for developing CKD [23].
Among the CKD patients of this study; 40.0% patients had glomerulonephritis (GN), 46.7% patients had diabetes mellitus and 66.7% patients had hypertension. Similar findings were observed in a related previous study [24]. This might be explained by the fact that the patients with diabetes mellitus and hypertension had intra-renal atherosclerosis and arteriosclerosis [25]. This microvascular damage significantly affects the kidney in the short- and the long-term [25].
In this study, biochemical parameters of CKD and non-CKD patients revealed that there was no significant difference between these two groups and this may be due to patients with complications of CKD were excluded from our study. But there was significant difference between the groups in case of blood urea. This could be due to retention of nitrogenous waste products occurs as an early sign observed in patients with renal impairment and usually occurs before the appearance of other symptoms. Urea is one of the first worsening renal dysfunction nitrogenous waste substances that accumulate in the blood in renal disease and urea levels increase progressively with degree and duration of worsening renal function [21].
In this study we selected the patients with renal impairment along with normal serum calcium, serum phosphate, serum parathyroid hormone (PTH) levels and having no significant anaemia. Because these parameters are deranged from stage 3 CKD [17]. We included this type of subjects in our study to observe that carbamylated haaemoglobin level rises earlier than other marker of CKD. Patients with mild anaemia were accepted as most of our study populations were mildly anaemic without any organic dysfunction.
It was found that carbamylated hemoglobin level of CKD patients at first presentation was significantly higher (107.2 ± 9.3 µgVH/g Hb) than that of non CKD patients (86.2 ± 15.2 µgVH/g Hb). Similar result was observed in the study conducted by Okaka et al. where carbamylated hemoglobin level of CKD patients detected by colorimetric method and that was 107.5 ± 16.2 µgVH/g Hb [16]. A couple of similar previous studies also revealed significant difference of cabamylated haaemoglobin (CarHb) level between the CKD patients and non CKD patients [9, 13]. The plausible explanation for this could be; the higher the duration of exposure of reactive protein such as haaemoglobin (Hb) to urea concentration, the higher the amount of carbamulated haaemoglobin (CarHb) obtained [13]. It results from non-enzymatic post-translational modification of haaemoglobin by isocyanic acid, the reactive form of cyanate derived from the spontaneous dissociation of urea [9].
The current study showed that, cabamylated haaemoglobin (CarHb) has a weak positive relationship with serum creatinine level but strong positive correlation with blood urea. This type of correlation was found in the related previous studies [13, 16, 21, 26]. It may be explained by the fact that urea is the first nitrogenous product that is retained in blood in renal disease and urea level increases progressively with duration and worsening of renal function [26]. It was reported that, constant exposure of hemoglobin to increased urea levels results in the increased level of carbamylated hemoglobin (CarHb) [21].
In this study, we depicted the receiver operating characteristics (ROC) curve of carbamylated haaemoglobin (CarHb) for the diagnosis of CKD. The cut off level of CarHb was taken 101.5 µgVH/g Hb, the sensitivity level was 93.33%, the specificity was 85.45% and the area under curve (AUC) was 0.950. It was comparable to the study conducted by Naresh et al. where a cut off value for CarHb was taken 98.33 µgVH/g Hb, the sensitivity was 85%, the specificity was 75% and the AUC was 0.840 [21]. In another previous study it was showed that, cut off value of CarHb was 100 µgVH/g Hb with sensitivity 95% and specificity was 80 % [16].
According to KDIGO guideline; to define a case as chronic kidney disease (CKD), it needs to be persistence of any of the marker of chronic kidney damage or GFR<60 ml/min/1.73 m2 at least for 3 months [17]. Following these criteria to level CKD, it may be delayed in diagnosis and specific management of CKD. But in our study it was found that, the patients with first presentation of renal impairment whose other parameters revealing CKD were not significant but level of carbamylated hemoglobin (CarHb) was significantly raised among the patients with CKD.
The current study demonstrated a strong and positive association between carbamylated haaemoglobin (CarHb) and chronic kidney disease (CKD) among the study patients with first presentation of renal impairment. Early diagnosis of CKD is important for specific management. Sometimes there is delay in diagnosis of CKD by conventional markers, hence early biomarkers are preferred. It was shown that carbamylated haaemoglobin (CarHb) can predict chronic kidney disease much earlier than the conventionally used biomarkers for CKD which need not to wait 3 months. Therefore, carbamylated haaemoglobin (CarHb) may be used as an early biomarker for detecting CKD.
5. Conclusions
This study concluded that the patients with CKD had significant higher level of carbamylated haaemoglobin (CarHb) than patients without CKD, before appearance of other biomarkers of CKD. Therefore, carbamylated haaemoglobin (CarHb) can predict chronic kidney disease (CKD) much earlier than the conventionally used biomarkers for CKD. Further studies will be warranted to elucidate the fact.
Limitations
It was a single centre study with a relatively small sample size and the study was done in a limited time span.
Recommendations
Further multi-centered prospective study with large sample size should be done to establish the carbamylated haaemoglobin (CarHb) as an early biomarker of CKD.
Competing Interest
The authors declare that they have no competing interests regarding the publication of this article.
References
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney international 81 (2012): 442-448.
- Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney international 74 (2008): 101-107.
- Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. American journal of kidney diseases 37 (2001): S66-S70.
- Floege J, Johnson RJ, Feehally J. Comprehensive clinical nephrology E-book. Elsevier Health Sciences (2010): 52-53.
- Davison AM, editor. Oxford Textbook of Clinical Nephrology Volume 3. Oxford University Press, USA (2005): 1717-1769.
- Dirnhuber P, Schütz F. The isomeric transformation of urea into ammonium cyanate in aqueous solutions. Biochemical Journal 42 (1948): 628-632.
- Lee JA, Lee HA, Sadler PJ. Uraemia: is urea more important than we think?. The Lancet 338 (1991): 1438-1440.
- Flückiger R, Harmon W, Meier W, et al. Hemoglobin carbamylation in uremia. New England Journal of Medicine 304 (1981): 823-827.
- Wynckel A, Randoux C, Millart H, et al. Kinetics of carbamylated haemoglobin in acute renal failure. Nephrology Dialysis Transplantation 15 (2000): 1183-1188.
- Stark GR, Stein WH, Moore S. Reactions of the cyanate present in aqueous urea with amino acids and proteins. Journal of Biological Chemistry 235 (1960): 3177-3181.
- Kraus AP, Soni P, Stephens MC, et al. Carbamylated hemoglobin in uremia: Continuous ambulatory peritoneal dialysis (CAPD) versus hemodialysis. Blood 62 (1983): 47a.
- Kwan JT, Carr EC, Neal AD, et al. Carbamylated haemoglobin, urea kinetic modelling and adequacy of dialysis in haemodialysis patients. Nephrology Dialysis Transplantation 6 (1991): 38-43.
- Stim J, Shaykh M, Anwar F, et al. Factors determining hemoglobin carbamylation in renal failure. Kidney international 48 (1995): 1605-1610.
- Davenport A, Jones S, Goel S, et al. Carbamylated hemoglobin: a potential marker for the adequacy of hemodialysis therapy in end-stage renal failure. Kidney international 50 (1996): 1344-1351.
- Kwan JT, Carr EC, Barron JL, et al. Carbamylated haemoglobin in normal, diabetic and uraemic patients. Annals of clinical biochemistry 29 (1992): 206-209.
- Okaka EI, Oforofuo IAO, Momoh SM. A colorimetric method for measurement of carbamylated haemoglobin in patients with chronic kidney disease using a spectrophotometer. Journal of Medicine and Medical Science 8 (2012): 494-498.
- Wheeler DC, Winkelmayer WC. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) foreword. Kidney International Supplements 7 (2017):1-59.
- Akin D, Ozmen S, Yilmaz ME. Hyaluronic acid as a new biomarker to differentiate acute kidney injury from chronic kidney disease. Iranian journal of kidney diseases 11 (2017): 409.
- Hasan M, Sutradhar I, Gupta RD, et al. Prevalence of chronic kidney disease in South Asia: a systematic review. BMC nephrology 19 (2018): 1-2.
- Anand S, Khanam MA, Saquib J, et al. High prevalence of chronic kidney disease in a community survey of urban Bangladeshis: a cross-sectional study. Globalization and health 10 (2014): 1-7.
- Naresh Y, Srinivas N, Vinapamula KS, et al. Carbamylated hemoglobin can differentiate acute kidney injury from chronic kidney disease. Indian journal of nephrology 28 (2018): 187.
- Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Transactions of the American Clinical and Climatological Association 120 (2009): 419-428.
- Anand S, Shivashankar R, Ali MK, et al. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney international 88 (2015): 178-185.
- Biswas RS, Kashem MA. Etiological survey of chronic kidney disease patients on maintenance hemodialysis in different centers of Chittagong, Bangladesh. Journal of Integrative Nephrology and Andrology 3 (2016): 118-120.
- Legrand M, De Berardinis B, Gaggin HK, et al. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One 9 (2014): e112313.
- Frazao JM, Barth RH, Berlyne GM. Carbamylated hemoglobin in prerenal azotemia. Nephron 71 (1995): 153-155.
